Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases
Abstract
:1. Introduction
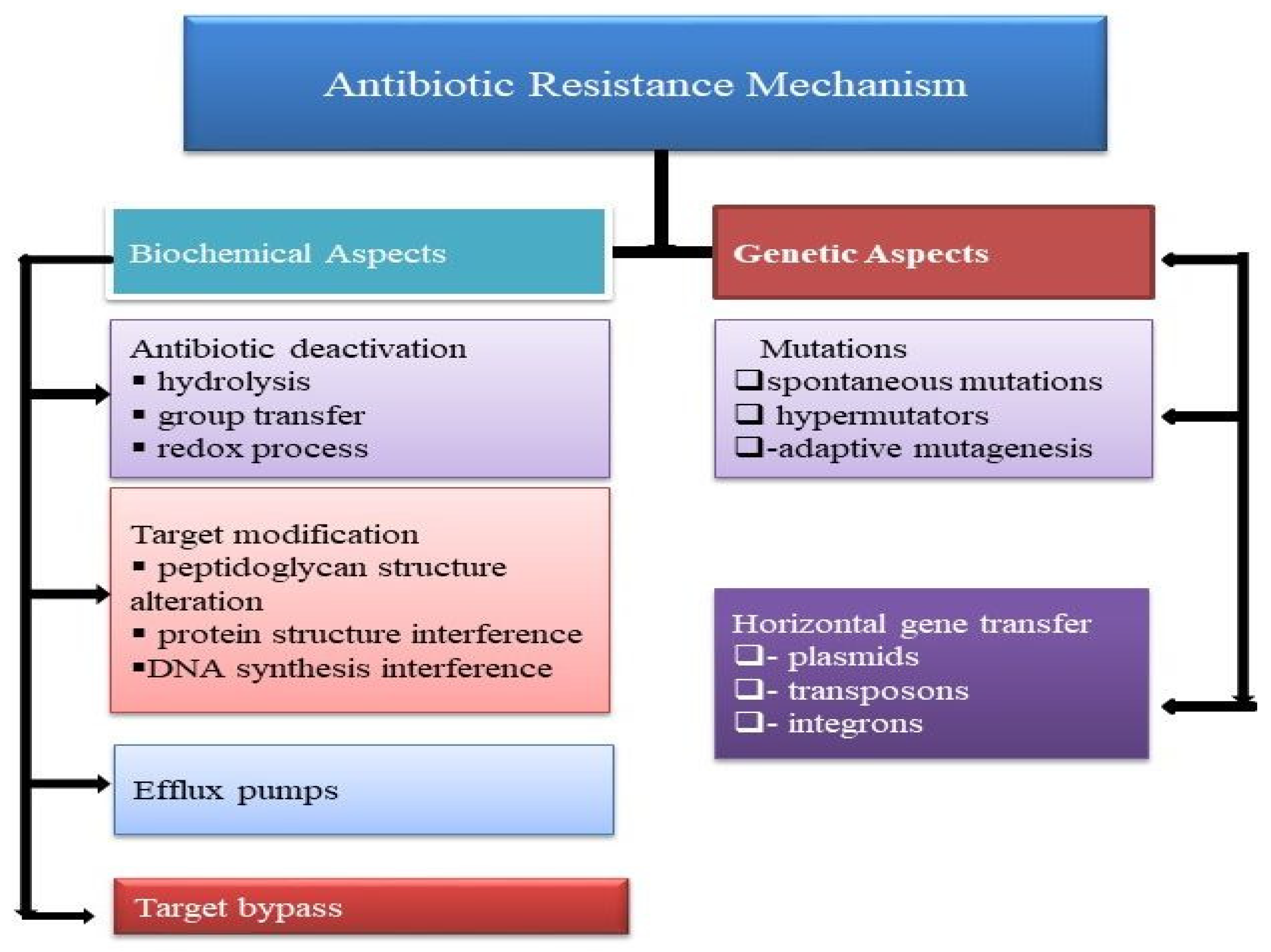
2. Antibacterial-Resistance Mechanism
2.1. Modification of Antibacterial Configuration
2.2. Modification of the Antibacterial Target Site
2.3. Antibacterial Efflux Pump and Reduced Permeability
2.4. Antibacterial Deactivation by Group Transfer
2.5. Rehabilitation of Cell Wall
3. ESBL Definition and Categorization
3.1. Type SHV
3.2. Type TEM
3.3. Type CTX
3.4. Type OXA
3.5. PER Type
3.6. Type GES
3.7. VEB-1, BES-1 and Other ESBL Types
4. Detection
4.1. Phenotypic Identification
4.2. Genotypic Identification
5. Role of Plant Secondary Metabolites as Antimicrobial Agents
5.1. Antimicrobial Activity of Plants
5.2. Essential Oils
5.3. Alkaloids
5.4. Phenolics
6. Plant Extracts against the ESBL-Producing Multidrug Resistant (MDR) Gram-Negative Bacteria
7. Mode of Action of Plant-Derived Drugs
8. Plant-Oriented Drug Resistance
9. Reversal of Antibacterial Resistance through Synergism
10. Future Prospects
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, C.; Zhu, G.; Li, T.; Zhao, T. Chemical Composition, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Activities of Essential Oils from the Leaves and Rhizomes of Acorusmacrospadiceus (Yamamoto) FN Wei et YK Li. J. Essent. Oil Bear. 2021, 24, 1323–1332. [Google Scholar] [CrossRef]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awari, A.; Nighute, S.; Khatoon, M. Study of urinary isolates with reference to extended spectrum beta lactamases detection and antibiogram. J. Evol. Med. Dent. Sci. 2013, 2, 1049–1056. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid. Based Complementary Altern. Med. 2019, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: http://www.who.int/drugresistance/en (accessed on 21 June 2022).
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.A.D.S.; Pereira, R.L.S.; Santos, A.T.L.D.; Coutinho, H.D.M.; Morais-Braga, M.F.B.; da Silva, V.B.; Costa, A.R.; Generino, M.E.M.; de Oliveira, M.G.; de Menezes, S.A.; et al. Phytochemical Analysis, Antibacterial Activity and Modulating Effect of Essential Oil from Syzygiumcumini (L.) Skeels. Molecules 2022, 27, 3281. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.W.A.; Rodrigues, F.C.; Pereira da Cruz, R.; Silva, L.E.D.; do Amaral, W.; Andrade Rebelo, R.; Begnini, I.M.; Fonseca Bezerra, C.; Iriti, M.; Varoni, E.M.; et al. Antibiotic potential and chemical composition of the essential oil of Piper caldense C. DC. (Piperaceae). Appl. Sci. 2020, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Valli, M.; Pivatto, M.; Danuello, A.; Castro-Gamboa, I.; Silva, D.H.S.; Cavalheiro, A.J.; Araújo, Â.R.; Furlan, M.; Lopes, M.N.; Bolzani, V.D.S. Tropical biodiversity: Has it been a potential source of secondary metabolites useful for medicinal chemistry? Química Nova 2012, 35, 2278–2287. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; de Freitas, M.A.; Rodrigues, F.C.; de Souza, M.A.; da Silva, A.R.P.; Dos Santos, A.T.L.; Linhares, K.V.; Coutinho, H.D.M.; de Lima Silva, J.R.; et al. Chemical composition, antimicrobial, modulator and antioxidant activity of essential oil of Dysphaniaambrosioides (L.) Mosyakin&Clemants. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 58–64. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Fisher, J.F.; Mobashery, S. Enzymology of Bacterial Resistance. Comprehensive Natural Products II Chemistry and Biology. Enzym. Enzym. Mech. 2010, 8, 443–487. [Google Scholar]
- Bonnet, R. Growing group of extended-spectrum β-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drawz, S.M.; Bonomo, R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loder, B.; Newton, G.G.F.; Abraham, E.P. The cephalosporin C nucleus (7-aminocephalosporanic acid) and some of its derivatives. Biochem. J. 1961, 79, 408. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Chakravarty, I.; Ojha, S.; Kundu, K. Design and Development of Antibiotic Fermentation Using Different Processing Strategies: Challenges and Perspectives. Appl. Microbiol. Bioeng. Interdiscip. Approach 2019, 163–183. [Google Scholar] [CrossRef]
- Morin, R.B.; Jackson, B.G.; Flynn, E.H.; Roeske, R.W.; Andrews, S.L. Chemistry of cephalosporin antibiotics. XIV. Reaction of cephalosporin C with nitrosyl chloride. J. Am. Chem. Soc. 1969, 91, 1396–1400. [Google Scholar] [CrossRef]
- Goffin, C.; Ghuysen, J.M. Multimodular penicillin-binding proteins: An enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 1998, 62, 1079–1093. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. Resistance to β-lactam antibiotics. Cell. Mol. Life Sci. 2004, 61, 2200–2223. [Google Scholar] [CrossRef]
- Macheboeuf, P.; Di Guilmi, A.M.; Job, V.; Vernet, T.; Dideberg, O.; Dessen, A. Active site restructuring regulates ligand recognition in class A penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Sacco, E.; Cortes, M.; Josseaume, N.; Rice, L.B.; Mainardi, J.L.; Arthur, M. Serine/threonine protein phosphatase-mediated control of the peptidoglycan cross-linking L, D-transpeptidase pathway in Enterococcus faecium. mBio 2014, 5, e01446-14. [Google Scholar] [CrossRef] [Green Version]
- Goswami, M.; Subramanian, M.; Kumar, R.; Jass, J.; Jawali, N. Involvement of antibiotic efflux machinery in glutathione-mediated decreased ciprofloxacin activity in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 4369–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, K.; Gotoh, N.; Nishino, T. Alterations of susceptibility of Pseudomonas aeruginosa by overproduction of multidrug efflux systems, MexAB-OprM, MexCD-OprJ, and MexXY/OprM to carbapenems: Substrate specificities of the efflux systems. J. Infect. Chemother. 2002, 8, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Viveiros, M.; Rosato, A.E.; Melo-Cristino, J.; Couto, I. Impact of efflux in the development of multidrug resistance phenotypes in Staphylococcus aureus. BMC Microbiol. 2015, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melano, R.; Corso, A.; Petroni, A.; Centrón, D.; Orman, B.; Pereyra, A.; Moreno, N.; Galas, M. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum β-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 2003, 52, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetting, M.W.; Magnet, S.; Nieves, E.; Roderick, S.L.; Blanchard, J.S. A bacterial acetyltransferase capable of regioselective N-acetylation of antibiotics and histones. Chem. Biol. 2004, 11, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Miyakozawa, I.; Nakazawa, K.; O’Hara, K.; Sawai, T. Detection and characterization of a macrolide 2′-phosphotransferase from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 2000, 44, 3241–3242. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Sasaki, T. Inactivation of macrolides by producers and pathogens. Curr. Drug Targets Infect Disord. 2004, 4, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Houang, E.T.; Chu, Y.W.; Lo, W.S.; Chu, K.Y.; Cheng, A.F. Epidemiology of rifampin ADP-ribosyltransferase (arr-2) and metallo-β-lactamase (bla IMP-4) gene cassettes in class 1 integrons in Acinetobacter strains isolated from blood cultures in 1997 to 2000. Antimicrob. Agents Chemother. 2003, 47, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Benton, B.; Breukink, E.; Visscher, I.; Debabov, D.; Lunde, C.; Janc, J.; Mammen, M.; Humphrey, P. O258 Telavancin inhibits peptidoglycan biosynthesis through preferential targeting of transglycosylation: Evidence for a multivalent interaction between telavancin and lipid II. Int. J. Antimicrob. Agents 2007, 29, S51–S52. [Google Scholar] [CrossRef]
- Meziane-Cherif, D.; Stogios, P.J.; Evdokimova, E.; Egorova, O.; Savchenko, A.; Courvalin, P. Structural and functional adaptation of vancomycin resistance VanT serine racemases. mBio 2015, 6, e00806-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, M.; Molinas, C.; Bugg, T.D.; Wright, G.D.; Walsh, C.T.; Courvalin, P. Evidence for in vivo incorporation of D-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 1992, 36, 867–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugg, T.D.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef]
- Park, I.S.; Lin, C.H.; Walsh, C.T. Gain of D-alanyl-D-lactate or D-lactyl-D-alanine synthetase activities in three active-site mutants of the Escherichia coli D-alanyl-D-alanine ligase B. Biochemistry 1996, 35, 10464–10471. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum β-lactamases: A clinical update. Clinic. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [Green Version]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annual Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Ambler, R.P.; Coulson, A.F.; Frère, J.M.; Ghuysen, J.M.; Joris, B.; Forsman, M.; Levesque, R.C.; Tiraby, G.; Waley, S.G. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 1991, 276, 269. [Google Scholar] [CrossRef]
- Livermore, D.M.; Struelens, M.; Amorim, J.; Baquero, F.; Bille, J.; Canton, R.; Henning, S.; Gatermann, S.; Marchese, A.; Mittermayer, H.; et al. Multicentre evaluation of the VITEK 2 Advanced Expert System for interpretive reading of antimicrobial resistance tests. J. Antimicrob. Chemother. 2002, 49, 289–300. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Bonomo, R.A. SHV-type beta-lactamases. Curr. Pharm. Des. 1999, 5, 847–864. [Google Scholar]
- Datta, N.; Kontomichalou, P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 1965, 208, 239–241. [Google Scholar] [CrossRef]
- Bois, S.D.; Marriott, M.S.; Amyes, S.G.B. TEM-and SHV-derived extended-spectrum β-lactamases: Relationship between selection, structure and function. J. Antimicrob. Chemother. 1995, 35, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Gazouli, M.; Tzelepi, E.; Markogiannakis, A.; Legakis, N.J.; Tzouvelekis, L.S. Two novel plasmid-mediated cefotaxime-hydrolyzing β-lactamases (CTX-M-5 and CTX-M-6) from Salmonella typhimurium. FEMS Microbiol. Lett. 1998, 165, 289–293. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Tzelepi, E.; Tassios, P.T.; Legakis, N.J. CTX-M-type β-lactamases: An emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 2000, 14, 137–142. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Humeniuk, C.; Arlet, G.; Gautier, V.; Grimont, P.; Labia, R.; Philippon, A. β-Lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob. Agents Chemother. 2002, 46, 3045–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, A.B.; Silverman, M.; Boyd, D.A.; McGeer, A.; Willey, B.M.; Pong-Porter, V.; Daneman, N.; Mulvey, M.R. Identification of a progenitor of the CTX-M-9 group of extended-spectrum β-lactamases from Kluyvera georgiana isolated in Guyana. Antimicrob. Agents Chemother. 2005, 49, 2112–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, K.; Jacoby, G.A.; Medeiros, A.A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 1995, 39, 1211–1233. [Google Scholar] [CrossRef] [Green Version]
- Weldhagen, G.F.; Poirel, L.; Nordmann, P. Ambler class A extended-spectrum β-lactamases in Pseudomonas aeruginosa: Novel developments and clinical impact. Antimicrob. Agents Chemother. 2003, 47, 2385–2392. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Le Thomas, I.; Naas, T.; Karim, A.; Nordmann, P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2000, 44, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Philippon, L.N.; Naas, T.; Bouthors, A.T.; Barakett, V.; Nordmann, P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum beta-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1997, 41, 2188–2195. [Google Scholar] [CrossRef] [Green Version]
- Bauernfeind, A.; Stemplinger, I.; Jungwirth, R.; Ernst, S.; Casellas, J.M. Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrobial Antimicrob. Agents Chemother. 1996, 40, 509–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhauser, M.M.; Weinstein, R.A.; Rydman, R.; Danziger, L.H.; Karam, G.; Quinn, J.P. Antibiotic resistance among gram-negative bacilli in US intensive care units: Implications for fluoroquinolone use. JAMA 2003, 289, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Vahaboglu, H.; Coskunkan, F.; Tansel, O.; Ozturk, R.; Sahin, N.; Koksal, İ.; Kocazeybek, B.; Tatman-Otkun, M.; Leblebicioglu, H.; Ozinel, M.A.; et al. Clinical importance of extended-spectrum β-lactamase (PER-1-type)-producing Acinetobacter spp. and Pseudomonas aeruginosa strains. J. Med. Microb. 2001, 50, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microb. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naas, T.; Oxacelay, C.; Nordmann, P. Identification of CTX-M-type extended-spectrum-β-lactamase genes using real-time PCR and pyrosequencing. Antimicrob. Agents Chemother. 2007, 51, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Management of multidrug-resistant organisms in healthcare settings. Am. J. Infect. Control 2006, 35, 165–193. [Google Scholar] [CrossRef]
- Kahlmeter, G. Breakpoints for intravenously used cephalosporins in Enterobacteriaceae—EUCAST and CLSI breakpoints. Clin. Microbiol. Infect. 2008, 14, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, Approved Standard, 2nd ed.; M31-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002. [Google Scholar]
- Health Protection Agency. National Standard Methods, (15 July 2008). Available online: www.gov.uk/government/publications/health-protection-agency-annual-report-and-accounts-2008 (accessed on 21 June 2022).
- Spanu, T.; Sanguinetti, M.; Tumbarello, M.; D’Inzeo, T.; Fiori, B.; Posteraro, B.; Santangelo, R.; Cauda, R.; Fadda, G. Evaluation of the new VITEK 2 extended-spectrum beta-lactamase (ESBL) test for rapid detection of ESBL production in Enterobacteriaceae isolates. J. Clin. Microbiol. 2006, 44, 3257–3262. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Geiss, H.K.; Mack, D.; Sturenburg, E.; Seifert, H. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J. Clin. Microbiol. 2007, 45, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Pitout, J.D.D.; Hamilton, N.; Church, D.L.; Nordmann, P.; Poirel, L. Development and clinical validation of a molecular diagnostic assay to detect CTX-M-type β-lactamases in Enterobacteriaceae. Clin. Microb. Infect. 2007, 13, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, M.; Hopkins, K.; Threlfall, E.J.; Clifton-Hadley, F.A.; Stallwood, A.D.; Davies, R.H.; Liebana, E. bla CTX-M genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrob. Agents Chemother. 2005, 49, 1319–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef] [Green Version]
- Birkett, C.I.; Ludlam, H.A.; Woodford, N.; Brown, D.F.; Brown, N.M.; Roberts, M.T.; Milner, N.; Curran, M.D. Real-time TaqMan PCR for rapid detection and typing of genes encoding CTX-M extended-spectrum β-lactamases. J. Med. Microbiol. 2007, 56, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensor, V.M.; Livermore, D.M.; Hawkey, P.M. A novel reverse-line hybridization assay for identifying genotypes of CTX-M-type extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2007, 59, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissman, T.A. Flavonoid compounds, tannins, lignins and, related compounds. Comp. Biochem. 1963, 9, 213–250. [Google Scholar]
- Schultes, R.E. The kingdom of plants. Med. Earth. 1978, 9, 213–250. [Google Scholar] [CrossRef]
- Dejonghe, W.; Russinova, E. Plant chemical genetics: From phenotype-based screens to synthetic biology. Plant Physiol. 2017, 174, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019, 7, 8748253. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.J.; Betancourt, J.; Alonso-Gonzalez, N.; Jauregui, A. Screening of some Cuban medicinal plants for antimicrobial activity. J. Ethnopharmacol. 1996, 52, 171–174. [Google Scholar] [CrossRef]
- Hunter, M.D.; Hull, L.A. Variation in concentrations of phloridzin and phloretin in apple foliage. Phytochemistry 1993, 34, 1251–1254. [Google Scholar] [CrossRef]
- Wan, J.; Wilcock, A.; Coventry, M.J. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. J. Appl. Microbiol. 1998, 84, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Prasad, B.K.; Lakshmi, V. Antiamoebic activity of Piper longum fruits against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1996, 50, 167–170. [Google Scholar] [CrossRef]
- Ofek, I.; Goldhar, J.; Sharon, N. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. Towar. Anti-Adhes. Ther. Microb. Dis. 1996, 324, 179–183. [Google Scholar]
- Vijaya, K.; Ananthan, S.; Nalini, R. Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis) and Euphorbia hirta on Shigella spp.—A cell culture study. J. Ethnopharmacol. 1995, 49, 115–118. [Google Scholar] [CrossRef]
- Apisariyakul, A.; Vanittanakom, N.; Buddhasukh, D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J. Ethnopharmacol. 1995, 49, 163–169. [Google Scholar] [CrossRef]
- Hufford, C.D.; Jia, Y.; Croom, E.M., Jr.; Muhammed, I.; Okunade, A.L.; Clark, A.M.; Rogers, R.D. Antimicrobial compounds from Petalostemum purpureum. J. Nat. Prod. 1993, 56, 1878–1889. [Google Scholar] [CrossRef]
- Vohora, S.B.; Rizwan, M.; Khan, J.A. Medicinal uses of common Indian vegetables. Planta Med. 1973, 23, 381–393. [Google Scholar] [CrossRef]
- Freiburghaus, F.; Kaminsky, R.; Nkunya, M.H.H.; Brun, R. Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol. 1996, 55, 1–11. [Google Scholar] [CrossRef]
- Peres, M.T.; Delle Monache, F.; Cruz, A.B.; Pizzolatti, M.G.; Yunes, R.A. Chemical composition and antimicrobial activity of Croton urucurana Baillon (Euphorbiaceae). J. Ethnopharmacol. 1997, 56, 223–226. [Google Scholar] [CrossRef]
- Toda, M.; Okubo, S.; Ikigai, H.; Suzuki, T.; Suzuki, Y.; Hara, Y.; Shimamura, T. The protective activity of tea catechins against experimental infection by Vibrio cholerae O1. Microbiol. Immunol. 1992, 36, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.A.; Garcia, M.D.; Saenz, M.T. Antibacterial activity of the phenolic acids fractions of Scrophulariafrutescens and Scrophulariasambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef]
- Duke, J.A. CRC Handbook of Medicinal Herbs; CRC Press Inc.: Boca Raton, FL, USA, 1985; Volume 677. [Google Scholar]
- Perrett, S.; Whitfield, P.J.; Sanderson, L.; Bartlett, A. The plant molluscicide Millettiathonningii (Leguminosae) as a topical antischistosomal agent. J. Ethnopharmacol. 1995, 47, 49–54. [Google Scholar] [CrossRef]
- Brinkworth, R.I.; Stoermer, M.J.; Fairlie, D.P. Flavones are inhibitors of HIV-1 proteinase. Biochem. Biophy. Res. Commun. 1992, 188, 631–637. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Combination effects of antifungal nagilactones against Candida albicans and two other fungi with phenylpropanoids. J. Nat. Prod. 1993, 56, 220–226. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.J.; O’kennedy, R. The chemistry and occurrence of coumarins. Coumarins Biol. Appl. Mode Action 1997, 4, 23–66. [Google Scholar]
- Cichewicz, R.H.; Thorpe, P.A. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J. Ethnopharmacol. 1996, 52, 61–70. [Google Scholar] [CrossRef]
- Rahman, A.; Choudhary, M.I. Diterpenoid and steroidal alkaloids. Nat. Prod. Rep. 1995, 12, 361–379. [Google Scholar] [CrossRef]
- Meyer, J.J.M.; Afolayan, A.J.; Taylor, M.B.; Erasmus, D. Antiviral activity of galangin isolated from the aerial parts of Helichrysumaureonitens. J. Ethnopharmacol. 1997, 56, 165–169. [Google Scholar] [CrossRef]
- Estevez-Braun, A.; Estevez-Reyes, R.; Moujir, L.M.; Ravelo, A.G.; Gonzalez, A.G. Antibiotic activity and absolute configuration of 8S-heptadeca-2 (Z), 9 (Z)-diene-4,6-diyne-1,8-diol from Bupleurumsalicifolium. J. Nat. Prod. 1994, 57, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic, R.Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [PubMed]
- Saleem, M.; Nazir, M.; Shaiq, A.M.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, O.D. Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study Against Gram-Positive and GramNegative Bacteria. Bact. Pathog. Antibact. Control. 2018, 23, 23–48. [Google Scholar]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and components from Cinnamomumosmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Imai, H.; Osawa, K.; Yasuda, H.; Hamashima, H.; Arai, T.; Sasatsu, M. Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios 2001, 106, 31–39. [Google Scholar] [PubMed]
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Protect. 2001, 64, 1019–1024. [Google Scholar] [CrossRef]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Su, C.H.; Wang, J.T.; Hsiung, C.A.; Chien, L.J.; Chi, C.L.; Yu, H.T. Increase of Carbapenem-Resistant Acinetobacter baumannii Infection in Acute Care Hospitals in Taiwan: Association with Hospital Antimicrobial Usage. PLoS ONE 2012, 7, e37788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luqman, S.; Dwivedi, G.R.; Darokar, M.P.; Kalra, A.; Khanuja, S.P. Potential of rosemary oil to be used in drug-resistant infections. Altern. Ther. Health Med. 2007, 13, 54–59. [Google Scholar] [PubMed]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complemen. Altern. Med. 2006, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Krishnamurti, C.; Rao, S.C. The isolation of morphine by Serturner. Indian J. Anaesth. 2016, 60, 861. [Google Scholar] [CrossRef] [PubMed]
- Jeruto, P.; Nyangacha, R.M.; Mutai, C. In vitro and in vivo antiplasmodial activity of extracts of selected Kenyan medicinal plants. Afr. J. Pharm. Pharmacol. 2015, 9, 505. [Google Scholar]
- McMahon, J.B.; Currens, M.J.; Gulakowski, R.J.; Buckheit, R.W., Jr.; Lackman-Smith, C.; Hallock, Y.F.; Boyd, M.R. Michellamine B, a novel plant alkaloid, inhibits human immunodeficiency virus-induced cell killing by at least two distinct mechanisms. Antimicrob. Agents Chemother. 1995, 39, 484–488. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, J.T.; Schneider, D.M.; Katiyar, S.K.; Edlind, T.D. Berberine: A candidate for the treatment of diarrhea in AIDS patients, abstr. 175. In Proceedings of the Program and Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, LO, USA, 15–18 September 1996; American Society for Microbiology: Washington, DC, USA, 1996. [Google Scholar]
- Ohanu, E.C.; Inyang-Etoh, P.C. The efficacy of plant extracts on cecalamebiasis in rats. Vet. Sci. Dev. 2015, 5. [Google Scholar] [CrossRef]
- Omulokoli, E.; Khan, B.; Chhabra, S.C. Antiplasmodial activity of four Kenyan medicinal plants. J. Ethnopharmacol. 1997, 56, 133–137. [Google Scholar] [CrossRef]
- Dahanukar, S.A.; Kulkarni, R.A.; Rege, N.N. Pharmacology of Medicinal Plants and Natural Prod ucts. Indian J. Pharmacol. 2000, 32, S81–S118. [Google Scholar]
- Barghash, S.M. Evaluation of in vitro and in vivo activities of some medicinal plants against trypanosomiasis. Int. J. Adv. Res. 2016, 4, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Kim, M.S.; Hayat, F.; Shin, D. Recent advances in the discovery of novel antiprotozoal agents. Molecules 2019, 24, 3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacumofficinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. Inhibitory properties of phenolic compounds against enzymes linked with human diseases. In Phenolic Compounds-Biological Activity; Intech Open: London, UK, 2019; pp. 99–118. [Google Scholar]
- Rasheed, M.U.; Thajuddin, N.; Ahamed, P.; Teklemariam, Z.; Jamil, K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev. Ins. Med. Trop. Pau. 2014, 56, 341–346. [Google Scholar] [CrossRef]
- Pandit, R.; Awal, B.; Shrestha, S.S.; Joshi, G.; Rijal, B.P.; Parajuli, N.P. Extended-spectrum β-lactamase (ESBL) genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdiscip. Perspect. Infect. Dis. 2020, 4, 6525826. [Google Scholar] [CrossRef] [Green Version]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, H.S.; Yadav, J.; Shrivastava, A.R.; Singh, S.; Singh, A.K.; Gopalan, N. Antibacterial activity of actinomycetes isolated from different soil samples of Sheopur (A city of central India). J. Adv. Pharm. Tech. Res. 2013, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Gilani, S.A.; Kikuchi, A.; Shinwari, Z.K.; Khattak, Z.I.; Watanabe, K.N. Phytochemical, pharmacological and ethnobotanical studies of RhazyastrictaDecne. Phytother. Res. Int. J. Dev. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 301–307. [Google Scholar]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.N. Glossary of Indian Medicinal Plants; FAO: Rome, Italy, 1956. [Google Scholar]
- Fleming, A. Penicillin. Nobel Lecture. 11 December 1945. Available online: https://www.nobelprize.org/nobelprizes/medicine/laureates/1945/fleming-lecture.pdf (accessed on 21 June 2022).
- Rabe, T.; Van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Agarwal, S.S. Clinically Useful Herbal Drugs; Ahuja Book Company Pvt. Ltd.: Delhi, India, 2005. [Google Scholar]
- Kamboj, V.P. Herbal medicine. Curr. Sci. 2000, 78, 35–38. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Moore-Neibel, K.; Gerber, C.; Patel, J.; Friedman, M.; Ravishankar, S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J. Appl. Microbiol. 2012, 112, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtuscommunis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Morphostructural damage in food-spoiling bacteria due to the lemon grass oil and its vapour: SEM, TEM, and AFM investigations. Evid. Based Complementary Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gallardo-Moreno, A.M.; Pinzon-Arango, P.A.; Reynolds, Y.; Rodriguez, G.; Camesano, T.A. Cranberry changes the physicochemical surface properties of E. coli and adhesion with uroepithelial cells. Colloids Surf. B 2008, 65, 35–42. [Google Scholar] [CrossRef]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinusofficinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopuseuropaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef]
- Yang, Z.; Niu, Y.; Le, Y.; Ma, X.; Qiao, C. Beta-lactamase inhibitory component from the roots of Fissistigmacavaleriei. Phytomedicine 2010, 17, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Petra, O.N.; Franklin, C.K.; Wilfred, N.O. Cardiospermum grandiflorum leaf extract potentiates amoxicillin activity on Staphylococcus aureus. J. Med. Plants Res. 2012, 6, 901–905. [Google Scholar]
- Coutinho, H.D.; Costa, J.G.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P.; Lima, E.O. Effect of Momordicacharantia L. in the resistance to aminoglycosides in methicilin-resistant Staphylococcus aureus. Com. Immuno. Micro. Infec. Dis. 2010, 33, 467–471. [Google Scholar] [CrossRef]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; SiqueiraJúnior, J.P. Herbal therapy associated with antibiotic therapy: Potentiation of the antibiotic activity against methicillin–resistant Staphylococcus aureus by Turneraulmifolia L. BMC Comp. Alt. Med. 2009, 9, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-hebshi, N.; Al-haroni, M.; Skaug, N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Arch. Oral Biol. 2006, 51, 183–188. [Google Scholar] [CrossRef]
- Braga, L.C.; Leite, A.A.; Xavier, K.G.; Takahashi, J.A.; Bemquerer, M.P.; Chartone-Souza, E.; Nascimento, A.M. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 2005, 51, 541–547. [Google Scholar] [CrossRef]
- Singh, B.R.; Singh, V.; Singh, R.K.; Ebibeni, N. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int. Res. J. Pharm. Pharmacol. 2011, 1, 228–236. [Google Scholar]
- Singh, B.R.; Singh, V.; Ebibeni, N.; Singh, R.K. Antimicrobial and herbal drug resistance in enteric bacteria isolated from faecal droppings of common house lizard/gecko (Hemidactylusfrenatus). Int. J. Microbiol. 2013, 2013, 340848. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Jiang, X. Prevalence of antibiotic-resistant bacteria in herbal products. J. Food Prot. 2008, 71, 1486–1490. [Google Scholar] [CrossRef]
- Ujam, N.T.; Oli, A.N.; Ikegbunam, M.N.; Adikwu, M.U.; Esimone, C.O. Antimicrobial resistance evaluation of organisms isolated from liquid herbal products manufactured and marketed in South Eastern Nigeria. Br. J. Pharm. Res. 2013, 3, 548. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Rafiq, Z.; Narasimhan, S.; Haridoss, M.; Vennila, R.; Vaidyanathan, R. Punicagranatum rind extract: Antibiotic potentiator and efflux pump inhibitor of multidrug resistant Klebsiella pneumoniae clinical isolates. Asian J. Pharm. Clin. Res. 2017, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Cristo, J.S.; Matias, E.F.; Figueredo, F.G.; Santos, J.F.; Pereira, N.L.; Junior, J.G.; Aquino, P.E.; Nogueira, M.N.; Ribeiro-Filho, J.; Cunha, F.A.; et al. HPLC profile and antibiotic-modifying activity of Azadirachtaindica A. Juss (Meliaceae). Indus. Crops Prod. 2016, 94, 903–908. [Google Scholar] [CrossRef]
- Inui, T.; Wang, Y.; Deng, S.; Smith, D.C.; Franzblau, S.G.; Pauli, G.F. Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. J. Chromatogr. A 2007, 1151, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 1–14. [Google Scholar] [CrossRef]
- Morita, Y.; Nakashima, K.I.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Y. Berberine is a novel type efflux inhibitor which attenuates the MexXY-mediated aminoglycoside resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 1223. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies–methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [Green Version]
- Neu, H.C.; Fu, K.P. Clavulanic acid, a novel inhibitor of β-lactamases. Antimicrob. Agents Chemother. 1978, 14, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, R.; Speciale, A.; Costanzo, R.; Annino, A.; Ragusa, S.; Rapisarda, A.; Pappalardo, M.S.; Iauk, L. Berberis aetnensis C. Presl. extracts: Antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents 2003, 22, 48–53. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.X.; Krishnan, T.; Yiap, B.C.; Hu, C.P.; Chan, K.G.; Lim, S.H.E. Membrane disruption and anti-quorum sensing effects of synergistic interaction between L avandulaangustifolia (lavender oil) in combination with antibiotic against plasmid conferred multidrug resistant Escherichia coli. J. Appl. Microbiol. 2014, 116, 1119–1128. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Krishnan, T.; Chan, K.G.; Lim, S.H.E. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and Synergy of Clavine Alkaloid Lysergol and its Derivatives Against Nalidixic Acid Resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.; Taylor, P.W. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar] [CrossRef]
- Oh, E.; Jeon, B. Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds. Front. Microbiol. 2015, 6, 1129. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zeng, W.C.; Xu, P.Y.; Lan, Y.J.; Zhu, R.X.; Zhong, K.; Huang, Y.N.; Gao, H. Chemical composition and antimicrobial activity of the essential oil of Kumquat (Fortunellacrassifolia Swingle) Peel. Int. J. Mol. Sci. 2012, 13, 3382–3393. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobialaction of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated fromsmaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar]
- Jassim, S.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterialactivity and mode of action of ferulic and gallic acids againstpathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Hao, X.; Ma, K.; Tan, X.; Wang, Z.; Li, N. Pharmacologic study of colchicine-amide. Chin. Med. J. 1980, 93, 188–190. [Google Scholar]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese herbal garden. Cell 2011, 146, 855–858. [Google Scholar]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotech. 2006, 24, 1504–1507. [Google Scholar]
- Segal, E.; Fondufe-Mittendorf, Y.; Chen, L.; Thåström, A.; Field, Y.; Moore, I.K.; Wang, J.P.Z.; Widom, J. A genomic code for nucleosome positioning. Nature 2006, 442, 772–778. [Google Scholar] [CrossRef] [PubMed]


| Ambler Class | Bush Group | Characteristics of β-Lactamases | Number of Enzymes |
|---|---|---|---|
| C | 1 | Often chromosomal enzymes in Gram-negative but some are plasmid coded. Not inhibited by clavulanic acid. | 51 |
| A | 2a | Staphylococcal and enterococcal penicillinases. | 23 |
| 2b | Broad-spectrum β-lactamases including TEM-1 and SHV-1, mainly occurring in Gram-negative. | 16 | |
| 2be | Extended-spectrum β-lactamases (ESBL). | 200 | |
| 2br | Inhibitor-resistant TEM (IRT) β-lactamases. | 24 | |
| 2c | Carbenicillin-hydrolyzing enzymes. | 19 | |
| 2d | Cloxacillin (oxacillin)-hydrolyzing enzymes. | 31 | |
| 2e | Cephalosporinases inhibited by clavulanic acid. | 20 | |
| 2f | Carbapenem-hydrolyzing enzyme inhibited by clavulanic acid. | 4 | |
| B | 3 | Metallo-enzymes that hydrolyze carbapenems and other β-lactams except monobactams. Not inhibited by clavulanic acid. | 24 |
| D | 4 | Miscellaneous enzymes that do not fit into other groups. | 9 |
| Common Name | Scientific Name | Compound | Class | Activity | RELATIVE TOXICITY | References |
|---|---|---|---|---|---|---|
| Aloe | Aloe barbadensis, Aloe vera | Latex | Complex mixture | Salmonella | 2.7 | [73] |
| Apple | Malus sylvestris | Phloretin | Flavonoid derivative | General | 3.0 | [74] |
| Ashwagandha | Withaniasomniferum | Withafarin A | Lactone | Bacteria, fungi | 0.0 | |
| Basil | Ocimum basilicum | Essential oils | Terpenoids | Salmonella, bacteria | 2.5 | [75] |
| Black pepper | Piper nigrum | Piperine | Alkaloid | Fungi, Lactobacillus, Micrococcus, E. coli, E. faecalis | 1.0 | [76] |
| Blueberry | Vaccinium spp. | Fructose | Monosaccharide | E. coli | [77] | |
| Coca | Erythroxylum coca | Cocaine | Alkaloid | Gram-negative and-positive cocci | 0.5 | |
| Green tea | Camellia sinensis | Catechin | Flavonoid | General Shigella | 2.0 | [78] |
| Turmeric | Curcuma longa | Curcumin | Terpenoids | Bacteria, protozoa | [79] | |
| Potato | Solanum tuberosum | - | Solanum tuberosum | 2.0 | [80] | |
| Onion | Allium cepa | Allicin | Sulfoxide | Bacteria, Candida | [81] | |
| Goldenseal | Hydrastis canadensis | Berberine, hydrastine | Alkaloids | Bacteria, Giardia duodenale, trypanosomes | 2.0 | [82] |
| Class | Subclass | Example(s) | Mechanism | References |
|---|---|---|---|---|
| Phenolics | Simple phenols | Catechol | Substrate deprivation | [83] |
| Epicatechin | Membrane disruption | [84] | ||
| Phenolic acids | Cinnamic acid | Hydrogen atom transfer, sequential proton loss electron transfer. | [85] | |
| Quinones | Hypericin | Bind to adhesins, complex with cell wall, inactivate enzymes | [86] | |
| Flavonoids | Chrysin | Bind to adhesins | [87] | |
| Flavones | Complex with cell wall | |||
| Abyssinone | Inactivate enzymes Inhibit HIV reverse transcriptase | [88] | ||
| Flavonols | Totarol | Control the accumulation of reactive oxygen species | [89] | |
| Tannins | Ellagitannin | Bind to proteins Bind to adhesins Enzyme inhibition Substrate deprivation Complex with cell wall Membrane disruption Metal ion complexation | [90] | |
| Coumarins | Warfarin | Interaction with eukaryotic DNA (antiviral activity) | [91] | |
| Terpenoids, essential oils | Capsaicin | Membrane disruption | [92] | |
| Alkaloids | Berberine | Intercalate into cell wall and/or DNA | [93] | |
| Lectins and polypeptides | Mannose-specific agglutinin Fabatin | Block viral fusion or adsorption Form disulfide bridges | [94] | |
| Polyacetylenes | 8S-Heptadeca-2(Z),9(Z)-diene- 4,6-diyne-1,8-diol | Pleiotropic profile of bioactivity | [95] |
| Plant Species | a | b | c | d | e | f | g | h | i | |
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | ||||||||||
| Cichoriumintybus | E | 5 | 10 | 10 | 10 | 20 | 20 | 20 | 5 | 2.5 |
| Et | 2.18 | 2.18 | 2.18 | 8.75 | 8.75 | 2.18 | 2.18 | 2.18 | 1.09 | |
| Ac | 2.5 | 2.5 | 2.5 | 5 | 5 | 2.5 | 2.5 | 2.5 | 2.5 | |
| Salvia officinalis | E | 5 | 5 | 5 | >20 | 10 | >20 | >20 | >20 | 2.5 |
| Et | 10 | 20 | 20 | >20 | >20 | >20 | >20 | >20 | 2.5 | |
| Ac | 0.03 | 0.15 | 0.31 | 20 | 1.25 | 20 | 0.31 | 0.156 | 0.019 | |
| Clinopodium vulgare | E | 1.25 | >20 | >20 | >20 | 20 | >20 | 20 | 2.5 | 20 |
| Et | 0.625 | 20 | 10 | 10 | 10 | 10 | 10 | 2.5 | 10 | |
| Ac | 1.25 | 20 | 10 | 10 | 10 | 20 | 10 | 0.625 | 10 | |
| Cytisus nigricans | E | 2.5 | 20 | 20 | 10 | 10 | 20 | 20 | 5 | 1.25 |
| Et | 5 | 20 | 20 | 20 | 20 | 20 | 20 | 5 | 5 | |
| Ac | 2.5 | 20 | 20 | 10 | 20 | >20 | 20 | 2.5 | 10 | |
| Dorycniumpentaphyllum | E | 5 | 10 | 20 | 10 | 5 | 20 | 20 | 2.5 | 10 |
| Et | 1.25 | 20 | 20 | 10 | 10 | >20 | 20 | 1.25 | 20 | |
| Ac | 1.25 | 20 | 20 | 5 | 5 | 20 | 10 | 1.25 | 10 | |
| Plant Scientific Name | Part Used | Microorganisms | Modulation of Resistance | Method of Study | References |
|---|---|---|---|---|---|
| Rosmarinus officinalis | Aerial part | S. aureus | MDR efflux inhibition | Ethidium bromide efflux assay | [139] |
| Lycopus europaeus | N/A | S. aureus | - | - | [140] |
| Fissistigma cavaleriei | Root | P. aeruginosa | β-lactamase inhibition | β-lactamase inhibitory assay | [141] |
| Cardiospermum grandiflorum | Leaves | S. aureus | - | - | [142] |
| Momordica charantia L. | Leaves | MRSA | Efflux pump inhibition | Efflux pump inhibitory assay | [143] |
| Mentha arvensis L. | Leaves | E. coli | - | - | [144] |
| Turnera ulmifolia L. | Leaves | MRSA | - | - | [145] |
| Catha edulis | Leaves | Streptococcus oralis, Streptococcus sanguis, Fusobacterium nucleatum | - | - | [146] |
| Punica granatum | Fruit | MRSA | Efflux pump inhibition | Time–kill assay, β-lactamase production detection, ethidium bromide efflux assay | [147] |
| Targets | Mode of Study | Substances | References |
|---|---|---|---|
| Cell morphology: Alteration of cell shape or surface structure of the cell | Scanning electron micrograph (SEM) | Cudrania tricuspidata EO; Allium sativum EO;oregano EO; eugenol; epigallocatechin gallate | [170] |
| - | Transmission electron micrograph (TEM) | Tea tree oil; Fortunella crassifolia EO | [171] |
| Cytoplasmic membrane: Alteration of integrity and permeability | K+ leakage assay | Tea tree oil | [172] |
| - | Respiration assay | Tea tree oil | [172] |
| - | Propidium iodide uptake assay | Ferulic and gallic acids | [172] |
| Cell wall | OM permeability test | Ceratotoxin A; luteolin; flavonoids isolated from smaller galangal | [173] |
| - | Cell lysis assay | Oregano, thyme, clove EOs | [174] |
| Cell surface charge | Zeta potential measurement | Ferulic and gallic acids; lipids | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics 2022, 11, 855. https://doi.org/10.3390/antibiotics11070855
Alam M, Bano N, Ahmad T, Sharangi AB, Upadhyay TK, Alraey Y, Alabdallah NM, Rauf MA, Saeed M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics. 2022; 11(7):855. https://doi.org/10.3390/antibiotics11070855
Chicago/Turabian StyleAlam, Manzar, Nilofer Bano, Taufeeq Ahmad, Amit Baran Sharangi, Tarun Kumar Upadhyay, Yasser Alraey, Nadiyah M. Alabdallah, Mohd Ahmar Rauf, and Mohd Saeed. 2022. "Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases" Antibiotics 11, no. 7: 855. https://doi.org/10.3390/antibiotics11070855









