Occurrence of Serratia marcescens Carrying blaIMP-26 and mcr-9 in Southern China: New Insights in the Evolution of Megaplasmid IMP-26
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Profiles
2.2. Genome Sequencing of S. marcescens YL4
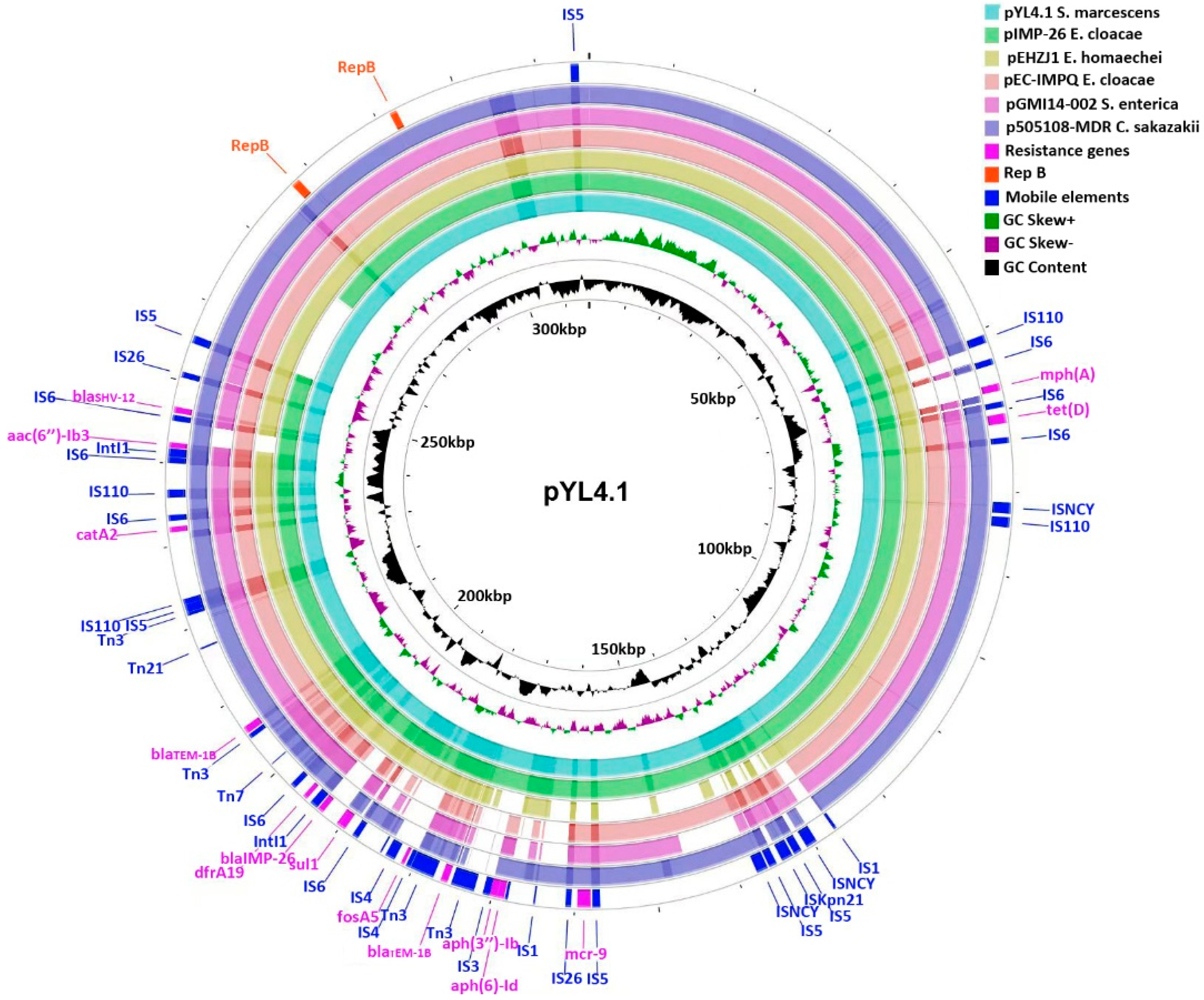
2.3. Characteristics of the IncHI2/2A Plasmid pYL4.1
2.4. Gene Environments of blaIMP-26
2.5. Gene Environments of Mcr-9
2.6. Evolutionary Pathway of Megaplasmids IMP-26
2.7. A Phage-Like Plasmid pYL4.2 Carrying blaTEM-1B
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Clinical Data
4.2. Bacterial Identification and Antimicrobial Susceptibility Testing
4.3. Whole-Genome Sequencing and Bioinformatics Analysis
4.4. GenBank Accession Numbers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hejazi, A.; Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleisch, F.; Zimmermann-Baer, U.; Zbinden, R.; Bischoff, G.; Arlettaz, R.; Waldvogel, K.; Nadal, D.; Ruef, C. Three consecutive outbreaks of Serratia marcescens in a neonatal intensive care unit. Clin. Infect. Dis. 2002, 34, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Jarousha, A.M.; El Qouqa, I.A.; El Jadba, A.H.; Al Afifi, A.S. An outbreak of Serratia marcescens septicaemia in neonatal intensive care unit in Gaza City, Palestine. J. Hosp. Infect. 2008, 70, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, A.; Benaoui, F.; El Idrissi Slitine, N.; Soraa, N.; Rabou Maoulainine, F.M. An Outbreak of Serratia marcescens in a Moroccan Neonatal Intensive Care Unit. Adv. Med. 2018, 2018, 4867134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristina, M.L.; Sartini, M.; Spagnolo, A.M. Serratia marcescens Infections in Neonatal Intensive Care Units (NICUs). Int. J. Environ. Res. Public Health 2019, 16, 610. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Bravo, L.; Gutiérrez-González, E.; San Juan-Sanz, I.; Fernández-Jiménez, I.; Ruiz-Carrascoso, G.; Gallego-Lombardo, S.; Sánchez-García, L.; Elorza-Fernández, D.; Pellicer-Martínez, A.; Omeñaca, F.; et al. Serratia marcescens outbreak in a neonatology unit of a Spanish tertiary hospital: Risk factors and control measures. Am. J. Infect. Control. 2019, 47, 271–279. [Google Scholar] [CrossRef]
- da Silva, K.E.; Rossato, L.; Jorge, S.; de Oliveira, N.R.; Kremer, F.S.; Campos, V.F.; da Silva Pinto, L.; Dellagostin, O.A.; Simionatto, S. Three challenging cases of infections by multidrug-resistant Serratia marcescens in patients admitted to intensive care units. Braz. J. Microbiol. 2021, 52, 1341–1345. [Google Scholar] [CrossRef]
- Bes, T.; Nagano, D.; Martins, R.; Marchi, A.P.; Perdigao-Neto, L.; Higashino, H.; Prado, G.; Guimaraes, T.; Levin, A.S.; Costa, S. Bloodstream Infections caused by Klebsiella pneumoniae and Serratia marcescens isolates co-harboring NDM-1 and KPC-2. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 57. [Google Scholar] [CrossRef]
- Stewart, A.G.; Paterson, D.L.; Young, B.; Lye, D.C.; Davis, J.S.; Schneider, K.; Yilmaz, M.; Dinleyici, R.; Runnegar, N.; Henderson, A.; et al. Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC beta-Lactamase-Producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2). Open Forum. Infect. Dis. 2021, 8, ofab387. [Google Scholar] [CrossRef]
- Madide, A.; Smith, J. Intracranial complications of Serratia marcescens infection in neonates. S. Afr. Med. J. 2016, 106, 36–38. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.P.; Holanda, A.R.; Lustosa, G.P.; Nóbrega, S.M.; Santana, W.J.; Souza, L.B.; Coutinho, H.D. Virulence factors and resistance mechanisms of Serratia marcescens. A short review. Acta Microbiol. Immunol. Hung. 2006, 53, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Merkier, A.K.; Rodriguez, M.C.; Togneri, A.; Brengi, S.; Osuna, C.; Pichel, M.; Cassini, M.H.; Serratia marcescens Argentinean Collaborative, G.; Centron, D. Outbreak of a cluster with epidemic behavior due to Serratia marcescens after colistin administration in a hospital setting. J. Clin. Microbiol. 2013, 51, 2295–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Shen, S.; Shi, Q.; Ding, L.; Wu, S.; Han, R.; Zhou, X.; Yu, H.; Hu, F. First Report of bla IMP-4 and bla SRT-2 Coproducing Serratia marcescens Clinical Isolate in China. Front. Microbiol. 2021, 12, 743312. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.; Rezende, G.S.; Damas, M.S.F.; Oliveira-Silva, M.; Pitondo-Silva, A.; Brito, M.C.A.; Leonardecz, E.; de Goes, F.R.; Campanini, E.B.; Malavazi, I.; et al. Characterization of KPC-Producing Serratia marcescens in an Intensive Care Unit of a Brazilian Tertiary Hospital. Front. Microbiol. 2020, 11, 956. [Google Scholar] [CrossRef]
- Batah, R.; Loucif, L.; Olaitan, A.O.; Boutefnouchet, N.; Allag, H.; Rolain, J.M. Outbreak of Serratia marcescens Coproducing ArmA and CTX-M-15 Mediated High Levels of Resistance to Aminoglycoside and Extended-Spectrum Beta-Lactamases, Algeria. Microb. Drug Resist. 2015, 21, 470–476. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [Green Version]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum beta-lactamases and carbapenemases, and antimicrobial resistance. J. Intensive Care 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Koh, T.H.; Khoo, C.T.; Tan, T.T.; Arshad, M.A.; Ang, L.P.; Lau, L.J.; Hsu, L.Y.; Ooi, E.E. Multilocus sequence types of carbapenem-resistant Pseudomonas aeruginosa in Singapore carrying metallo-beta-lactamase genes, including the novel bla(IMP-26) gene. J. Clin. Microbiol. 2010, 48, 2563–2564. [Google Scholar] [CrossRef] [Green Version]
- Tada, T.; Nhung, P.H.; Miyoshi-Akiyama, T.; Shimada, K.; Tsuchiya, M.; Phuong, D.M.; Anh, N.Q.; Ohmagari, N.; Kirikae, T. Multidrug-Resistant Sequence Type 235 Pseudomonas aeruginosa Clinical Isolates Producing IMP-26 with Increased Carbapenem-Hydrolyzing Activities in Vietnam. Antimicrob. Agents Chemother. 2016, 60, 6853–6858. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Liang, Z.; Su, X.; Xiong, Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in Chongqing, China. Ann. Lab. Med. 2012, 32, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Beyrouthy, R.; Barets, M.; Marion, E.; Dananché, C.; Dauwalder, O.; Robin, F.; Gauthier, L.; Jousset, A.; Dortet, L.; Guérin, F.; et al. Novel Enterobacter Lineage as Leading Cause of Nosocomial Outbreak Involving Carbapenemase-Producing Strains. Emerg. Infect. Dis. 2018, 24, 1505–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Bae, I.K.; Jeong, S.H.; Kim, S.H.; Song, J.H.; Choi, J.Y.; Yoon, S.S.; Thamlikitkul, V.; Hsueh, P.R.; Yasin, R.M.; et al. Dissemination of metallo-β-lactamase-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J. Antimicrob. Chemother. 2013, 68, 2820–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lascols, C.; Peirano, G.; Hackel, M.; Laupland, K.B.; Pitout, J.D. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: First report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 2013, 57, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Peirano, G.; Bradford, P.A.; Motyl, M.R.; DeVinney, R.; Pitout, J.D.D. Genomic characterization of IMP and VIM carbapenemase-encoding transferable plasmids of Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3034–3038. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, K.; Xiao, S.; Xie, L.; Gu, F.; Li, X.; Ni, Y.; Sun, J.; Han, L. A Multidrug Resistance Plasmid pIMP26, Carrying blaIMP-26, fosA5, blaDHA-1, and qnrB4 in Enterobacter cloacae. Sci. Rep. 2019, 9, 10212. [Google Scholar] [CrossRef] [Green Version]
- Gou, J.J.; Liu, N.; Guo, L.H.; Xu, H.; Lv, T.; Yu, X.; Chen, Y.B.; Guo, X.B.; Rao, Y.T.; Zheng, B.W. Carbapenem-Resistant Enterobacter hormaechei ST1103 with IMP-26 Carbapenemase and ESBL Gene bla (SHV-178). Infect. Drug Resist. 2020, 13, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Cheng, J.; Li, A.; Yu, R.; Zhao, W.; Qin, S.; Du, X.D. Molecular Characterization of an IncFII(k) Plasmid Co-harboring bla (IMP-26) and tet(A) Variant in a Clinical Klebsiella pneumoniae Isolate. Front. Microbiol. 2020, 11, 1610. [Google Scholar] [CrossRef]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Haq, Q.M.R. Current Update on Intrinsic and Acquired Colistin Resistance Mechanisms in Bacteria. Front. Med. 2021, 8, 677720. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Ling, Z.; Yin, W.; Shen, Z.; Wang, Y.; Shen, J.; Walsh, T.R. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemother. 2020, 75, 3087–3095. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Liu, Y.-H.; Feng, Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes. Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. mBio 2019, 10, e00853-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dai, X.; Zeng, J.; Gao, Y.; Zhang, Z.; Zhang, L. Characterization of the global distribution and diversified plasmid reservoirs of the colistin resistance gene mcr-9. Sci. Rep. 2020, 10, 8113. [Google Scholar] [CrossRef]
- Romaniuk, K.; Golec, P.; Dziewit, L. Insight Into the Diversity and Possible Role of Plasmids in the Adaptation of Psychrotolerant and Metalotolerant Arthrobacter spp. to Extreme Antarctic Environments. Front. Microbiol. 2018, 9, 3144. [Google Scholar] [CrossRef]
- Wein, T.; Dagan, T. Plasmid evolution. Curr. Biol. 2020, 30, R1158–R1163. [Google Scholar] [CrossRef]
- Porse, A.; Schønning, K.; Munck, C.; Sommer, M.O. Survival and Evolution of a Large Multidrug Resistance Plasmid in New Clinical Bacterial Hosts. Mol. Biol. Evol. 2016, 33, 2860–2873. [Google Scholar] [CrossRef] [Green Version]
- Harmer, C.J.; Moran, R.A.; Hall, R.M. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 2014, 5, e01801–e01814. [Google Scholar] [CrossRef] [Green Version]
- Harmer, C.J.; Hall, R.M. Targeted conservative formation of cointegrates between two DNA molecules containing IS26 occurs via strand exchange at either IS end. Mol. Microbiol. 2017, 106, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Szuplewska, M.; Czarnecki, J.; Bartosik, D. Autonomous and non-autonomous Tn3-family transposons and their role in the evolution of mobile genetic elements. Mob. Genet. Elem. 2014, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, E.; Lambin, M.; Dandoy, D.; Galloy, C.; Nguyen, N.; Oger, C.A.; Hallet, B. The Tn3-family of Replicative Transposons. Microbiol. Spectr. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Liao, T.L.; Liu, Y.M.; Lauderdale, T.L.; Yan, J.J.; Tsai, S.F. Mobilization of qnrB2 and ISCR1 in plasmids. Antimicrob. Agents Chemother. 2009, 53, 1235–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, W.; Zhou, Y.; Wang, B.; Zhan, Q.; Hu, L.; Xu, Y.; Guo, Y.; Wang, L.; Yu, F.; Li, X. First Report of Coexistence of bla (SFO-1) and bla (NDM-1) β-Lactamase Genes as Well as Colistin Resistance Gene mcr-9 in a Transferrable Plasmid of a Clinical Isolate of Enterobacter hormaechei. Front. Microbiol. 2021, 12, 676113. [Google Scholar] [CrossRef] [PubMed]
- Simoni, S.; Mingoia, M.; Brenciani, A.; Carelli, M.; Lleò, M.M.; Malerba, G.; Vignaroli, C. First IncHI2 Plasmid Carrying mcr-9.1, bla(VIM-1), and Double Copies of bla(KPC-3) in a Multidrug-Resistant Escherichia coli Human Isolate. mSphere 2021, 6, e0030221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hang, X.; Xiao, X.; Chu, W.; Li, X.; Liu, Y.; Li, X.; Zhou, Q.; Li, J. Co-occurrence of bla (NDM-1) and mcr-9 in a Conjugative IncHI2/HI2A Plasmid From a Bloodstream Infection-Causing Carbapenem-Resistant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 756201. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.H.; Li, C.; Hsu, C.H.; Ayers, S.; Borenstein, S.; Mukherjee, S.; Tran, T.T.; McDermott, P.F.; Zhao, S. The mcr-9 Gene of Salmonella and Escherichia coli Is Not Associated with Colistin Resistance in the United States. Antimicrob. Agents Chemother. 2020, 64, e00573-20. [Google Scholar] [CrossRef]
- Chavda, K.D.; Westblade, L.F.; Satlin, M.J.; Hemmert, A.C.; Castanheira, M.; Jenkins, S.G.; Chen, L.; Kreiswirth, B.N. First Report of bla (VIM-4)- and mcr-9-Coharboring Enterobacter Species Isolated from a Pediatric Patient. mSphere 2019, 4, e00629-19. [Google Scholar] [CrossRef] [Green Version]
- Shirshikova, T.V.; Sierra-Bakhshi, C.G.; Kamaletdinova, L.K.; Matrosova, L.E.; Khabipova, N.N.; Evtugyn, V.G.; Khilyas, I.V.; Danilova, I.V.; Mardanova, A.M.; Sharipova, M.R.; et al. The ABC-Type Efflux Pump MacAB Is Involved in Protection of Serratia marcescens against Aminoglycoside Antibiotics, Polymyxins, and Oxidative Stress. mSphere 2021, 6, e00033-21. [Google Scholar] [CrossRef]
- Dalvi, S.D.; Worobec, E.A. Gene expression analysis of the SdeAB multidrug efflux pump in antibiotic-resistant clinical isolates of Serratia marcescens. Indian J. Med. Microbiol. 2012, 30, 302–307. [Google Scholar] [CrossRef]
- Sandner-Miranda, L.; Vinuesa, P.; Cravioto, A.; Morales-Espinosa, R. The Genomic Basis of Intrinsic and Acquired Antibiotic Resistance in the Genus Serratia. Front. Microbiol. 2018, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Soo, P.C.; Wei, J.R.; Horng, Y.T.; Hsieh, S.C.; Ho, S.W.; Lai, H.C. Characterization of the dapA-nlpB genetic locus involved in regulation of swarming motility, cell envelope architecture, hemolysin production, and cell attachment ability in Serratia marcescens. Infect. Immun. 2005, 73, 6075–6084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlen, S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.Y.; Tsai, Y.L.; Liu, M.C.; Lin, W.C.; Hsueh, P.R.; Liaw, S.J. Serratia marcescens arn, a PhoP-regulated locus necessary for polymyxin B resistance. Antimicrob. Agents Chemother. 2014, 58, 5181–5190. [Google Scholar] [CrossRef] [Green Version]
- Mariscotti, J.F.; Garcia Vescovi, E. Serratia marcescens RamA Expression Is under PhoP-Dependent Control and Modulates Lipid A-Related Gene Transcription and Antibiotic Resistance Phenotypes. J. Bacteriol. 2021, 203, e0052320. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.T.M.; Mason, J.; Roberts, P.; Parry, C.M.; Corless, C.; van Aartsen, J.; Howard, A.; Bulgasim, I.; Fraser, A.J.; Adams, E.R.; et al. Piperacillin/tazobactam resistance in a clinical isolate of Escherichia coli due to IS26-mediated amplification of blaTEM-1B. Nat. Commun. 2020, 11, 4915. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.H.; Andreasen, M.R.; Pedersen, M.S.; Westh, H.; Jelsbak, L.; Schønning, K. Resistance to piperacillin/tazobactam in Escherichia coli resulting from extensive IS26-associated gene amplification of blaTEM-1. J. Antimicrob. Chemother. 2019, 74, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Mazel, D. Integrons: Agents of bacterial evolution. Nat. Rev. Microbiol. 2006, 4, 608–620. [Google Scholar] [CrossRef]
- Harms, K.; Starikova, I.; Johnsen, P.J. Costly Class-1 integrons and the domestication of the the functional integrase. Mob. Genet. Elem. 2013, 3, e24774. [Google Scholar] [CrossRef] [Green Version]
- Karimi Dehkordi, M.; Halaji, M.; Nouri, S. Prevalence of class 1 integron in Escherichia coli isolated from animal sources in Iran: A systematic review and meta-analysis. Trop. Med. Health 2020, 48, 16. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.; Sun, X.; Liu, M.; Wang, H. Antimicrobial Resistance and Molecular Characterization of Gene Cassettes from Class 1 Integrons in Escherichia coli Strains. Microb. Drug Resist. 2022, 28, 413–418. [Google Scholar] [CrossRef]




| Antimicrobial Class | Antimicrobial Agents | MIC (μg/mL) | S | I | R |
|---|---|---|---|---|---|
| Cephalosporins | Cefepime | 16 | ≤2 | 4–8 | ≥16 |
| Ceftazidime | ≥64 | ≤4 | 8 | ≥16 | |
| Cefatriaxone | ≥64 | ≤1 | 2 | ≥4 | |
| β-lactam inhibitor combinations | Ticarcillin/clavulanate | ≥128/2 | ≤16/2 | 32/2–64/2 | ≥128/2 |
| Carbapenems | Imipenem | ≥16 | ≤1 | 2 | ≥4 |
| Meropenem | ≥16 | ≤1 | 2 | ≥4 | |
| Ertapenem | ≥8 | ≤0.5 | 1 | ≥2 | |
| Aminoglycosides | Tobramycin | ≥16 | ≤4 | 8 | ≥16 |
| Amikacin | 16 | ≤16 | 32 | ≥64 | |
| Fluorquinolones | Levofloxacin | 4 | ≤0.5 | 1 | ≥2 |
| Ciprofloxacin | 2 | ≤0.25 | 0.5 | ≥1 | |
| Sulfanilamides | Trimethoprim/sulfamethoxazole | ≥16/304 | ≤2/38 | - | ≥4/76 |
| Glycylcycline | Tigecycline | ≥8 | ≤0.5 | - | - |
| Monobactams | Aztreonam | ≥64 | ≤4 | 8 | ≥16 |
| Tetracyclines | Minocycline | ≥16 | ≤4 | 8 | ≥16 |
| Doxycycline | ≥16 | ≤4 | 8 | ≥16 |
| Location | Antimicrobial Agents | Resistant Genes | Identity | Alignment Length/Gene Length | Start | End |
|---|---|---|---|---|---|---|
| Chromosome of YL4 strain (access No. CP083754) | Beta-lactam | blaSRT-1 | 96.22 | 1137/1137 | 775,741 | 776,877 |
| Tetracyline | tet(41) | 93.57 | 1151/1182 | 1,054,107 | 1,055,257 | |
| Aminoglycoside | aac(6′)-Ic | 94.33 | 441/441 | 2,918,180 | 2,918,620 | |
| Plasmid pYL4.1 (access No. CP083755) | Aminoglycoside | aph(6)-Id | 100.0 | 837/837 | 168,510 | 169,346 |
| aph(3″)-Ib | 99.88 | 804/804 | 169,346 | 170,149 | ||
| aac(6′)-Ib3 | 100.0 | 555/555 | 241,940 | 242,494 | ||
| Polymyxin | mcr-9 | 100.0 | 1620/1620 | 158,061 | 159,680 | |
| Fosfomycin | fosA5 | 100.0 | 420/420 | 181,153 | 181,572 | |
| Macrolide | mph(A) | 100.0 | 906/906 | 66,782 | 67,687 | |
| Folate pathway antagonist | sul1 | 100.0 | 840/840 | 189,399 | 190,238 | |
| Tetracycline | dfrA19 | 100.0 | 570/570 | 195,200 | 195,769 | |
| tet(D) | 100.0 | 1185/1185 | 70,462 | 71,646 | ||
| Beta-lactam | blaTEM-1B | 100.0 | 861/861 | 175,551 | 176,411 | |
| blaIMP-26 | 100.0 | 741/741 | 192,691 | 193,431 | ||
| blaTEM-1B | 100.0 | 861/861 | 205,714 | 206,574 | ||
| blaSHV-12 | 100.0 | 861/861 | 245,958 | 246,818 | ||
| Quaternary ammonium compound | qacE | 100.0 | 282/333 | 190,298 | 190,579 | |
| Amphenicol | catA2 | 96.11 | 642/642 | 231,688 | 232,329 | |
| Phage-like plasmid pYL4.2 (access No. CP083756) | Beta-lactam | blaTEM-1B | 100.0 | 861/861 | 23,202 | 24,062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Liu, W.; Yuan, P.; Yang, L.; Xu, Z.; Chen, D. Occurrence of Serratia marcescens Carrying blaIMP-26 and mcr-9 in Southern China: New Insights in the Evolution of Megaplasmid IMP-26. Antibiotics 2022, 11, 869. https://doi.org/10.3390/antibiotics11070869
Zhong Y, Liu W, Yuan P, Yang L, Xu Z, Chen D. Occurrence of Serratia marcescens Carrying blaIMP-26 and mcr-9 in Southern China: New Insights in the Evolution of Megaplasmid IMP-26. Antibiotics. 2022; 11(7):869. https://doi.org/10.3390/antibiotics11070869
Chicago/Turabian StyleZhong, Yuxia, Wanting Liu, Peibo Yuan, Ling Yang, Zhenbo Xu, and Dingqiang Chen. 2022. "Occurrence of Serratia marcescens Carrying blaIMP-26 and mcr-9 in Southern China: New Insights in the Evolution of Megaplasmid IMP-26" Antibiotics 11, no. 7: 869. https://doi.org/10.3390/antibiotics11070869
APA StyleZhong, Y., Liu, W., Yuan, P., Yang, L., Xu, Z., & Chen, D. (2022). Occurrence of Serratia marcescens Carrying blaIMP-26 and mcr-9 in Southern China: New Insights in the Evolution of Megaplasmid IMP-26. Antibiotics, 11(7), 869. https://doi.org/10.3390/antibiotics11070869








