Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America
Abstract
:1. Introduction
2. Epidemiology
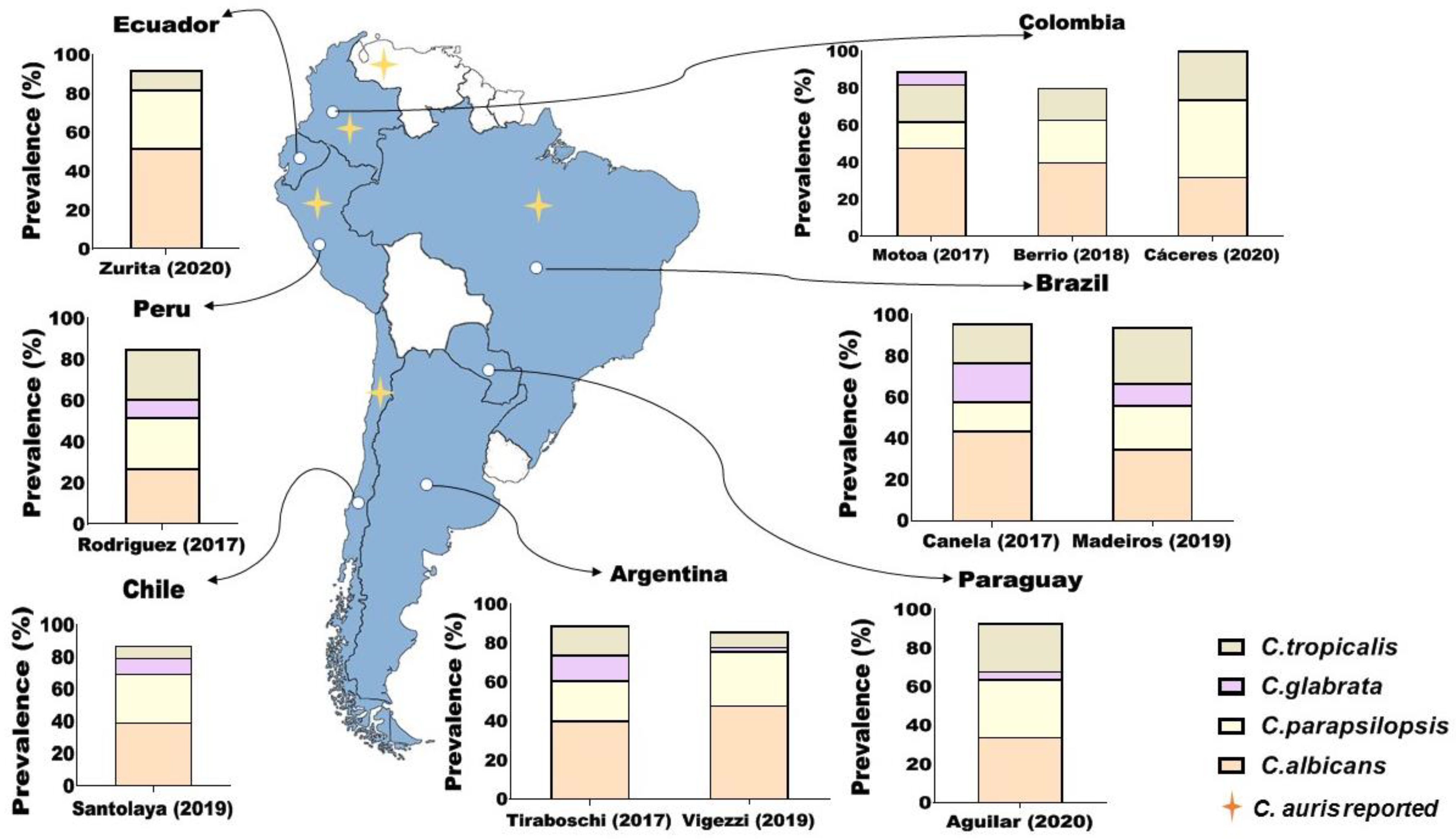
3. Risk Factors
4. Pathogenesis of Invasive Candidiasis
Host–Pathogen Interaction: Virulence Factors and Host Response
5. Treatment
- Presence of infectious complications in organs: The occurrence of endophthalmitis, osteomyelitis, endocarditis and chronic disseminated candidiasis are good examples of clinical conditions for which antifungal therapy should be extended up to 1–6 months. If prolonged therapy is needed, oral drugs should be chosen.
- Severity of the clinical presentation: This issue is controversial; fungicidal drugs are usually selected for initial treatment in patients with organ failure, and FLZ is generally saved for a second event after the initial clinical response and identification of the Candida species.
- Determination of Candida species: Non-albicans species may exhibit lower susceptibility to FLZ, requiring dose adjustment or a therapeutic switch.
- Risk of renal toxicity when using conventional Amp B: The occurrence of acute renal failure in patients with renal dysfunction in ICUs, elderly patients, and those receiving other nephrotoxic drugs.
- Previous exposure to antifungal prophylaxis regimens and/or empirical therapy: In the case of breakthrough infections in patients exposed to the determined antifungal agent, a change in therapeutic group is indicated until the Candida species and its susceptibility profile are determined.
- Central venous catheter: The clinical management of this aspect must be discussed, considering the individual conditions of the patients.
- Surgical removal of the infectious focus: Cases of osteomyelitis and endocarditis are good examples of clinical situations in which surgical cleaning (or valve replacement) should be considered.
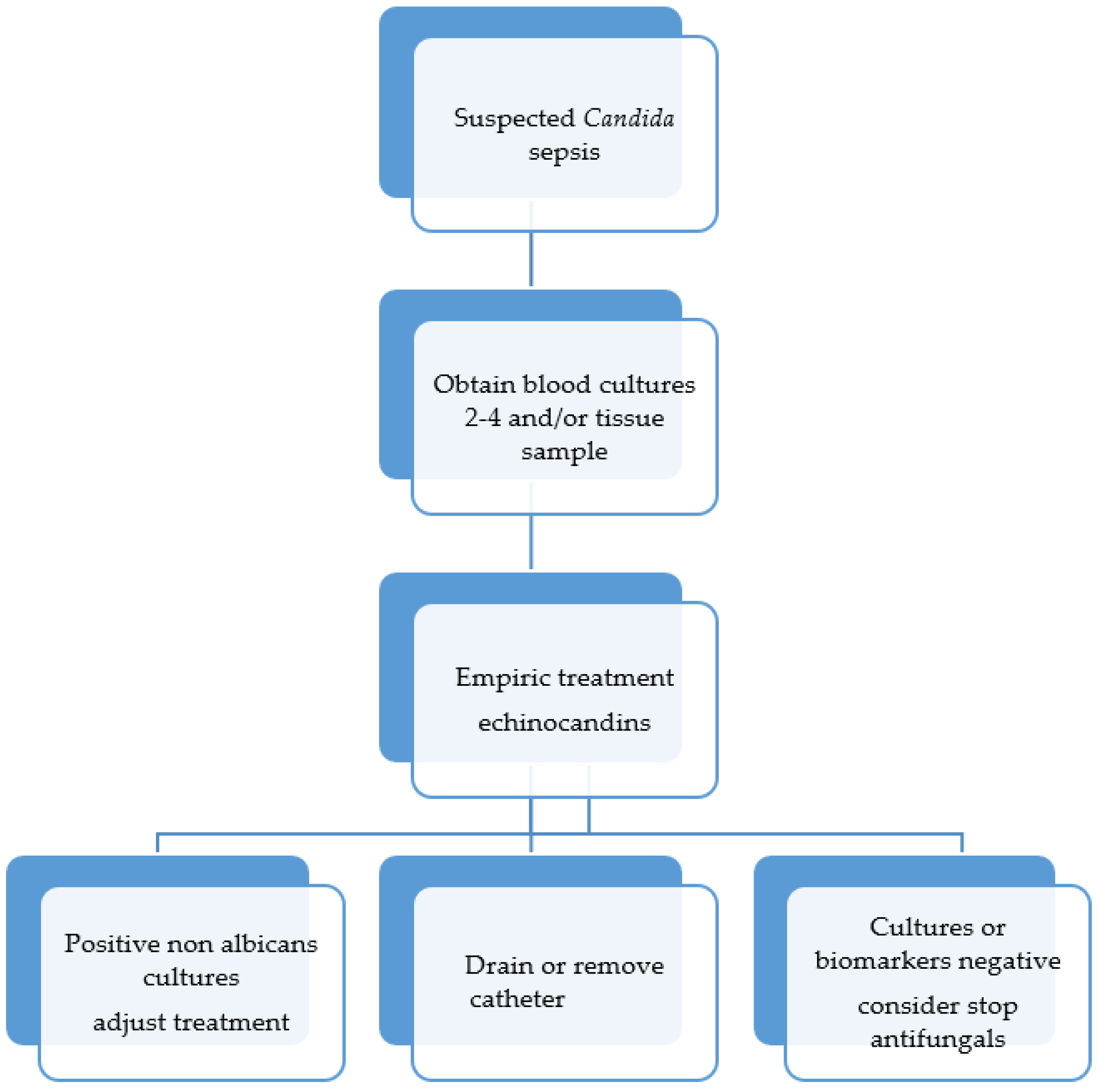
5.1. Candida Sepsis
5.2. Ocular Candidiasis
5.3. CNS Candidiasis
6. New Antifungal Drugs
7. Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bassetti, M.; Azoulay, E.; Kullberg, B.-J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T.; Bassetti, M.; Azoulay, E.; et al. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021, 72, S121–S127. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W.; Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Qin, J.; Yang, H.; Shan, Z.; Jiang, L.; Zhang, Q.; Qin, J.; Yang, H.; Shan, Z.; Jiang, L.; Zhang, Q. Clinical efficacy and safety of antifungal drugs for the treatment of Candida parapsilosis infections: A systematic review and network meta-analysis. J. Med. Microbiol. 2021, 70, 001434. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [Green Version]
- da Matta, D.A.; Souza, A.C.R.; Colombo, A.L. Revisiting Species Distribution and Antifungal Susceptibility of Candida Bloodstream Isolates from Latin American Medical Centers. J. Fungi 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia en Colombia. Biomédica 2020, 40, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2018 to 2019. J. Clin. Microbiol. 2021, 59, e01811-20. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, M.; Qiao, D.; Shen, H.; Wang, L.; Wang, D.; Li, L.; Liu, Y.; Lu, H.; Wang, C.; et al. Prevalence and Antifungal Susceptibility of Candida parapsilosis Species Complex in Eastern China: A 15-Year Retrospective Study by ECIFIG. Front. Microbiol. 2021, 12, 644000. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; de Almeida, J.N.; Lima, G.M.E.; de Nunes, M.O.; Camargo, C.H.; de Grenfell, R.C.; Benard, G.; Del Negro, G.M.B. An Azole-Resistant Candida parapsilosis Outbreak: Clonal Persistence in the Intensive Care Unit of a Brazilian Teaching Hospital. Front. Microbiol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Thomaz, D.Y.; de Almeida, J.N.; Sejas, O.N.E.; Del Negro, G.M.B.; Carvalho, G.O.M.H.; Gimenes, V.M.F.; de Souza, M.E.B.; Arastehfar, A.; Camargo, C.H.; Motta, A.L.; et al. Environmental Clonal Spread of Azole-Resistant Candida parapsilosis with Erg11-Y132F Mutation Causing a Large Candidemia Outbreak in a Brazilian Cancer Referral Center. J. Fungi 2021, 7, 259. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Vigezzi, C.; Icely, P.A.; Dudiuk, C.; Rodríguez, E.; Miró, M.S.; Castillo, G.D.V.; Azcurra, A.I.; Abiega, C.; Caeiro, J.P.; Riera, F.O.; et al. Frequency, virulence factors and antifungal susceptibility of Candida parapsilosis species complex isolated from patients with candidemia in the central region of Argentina. J. Mycol. Médicale 2019, 29, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Tiraboschi, I.N.; Pozzi, N.C.; Farías, L.; García, S.; Fernández, N.B. Epidemiología, especies, resistencia antifúngica y evolución de las candidemias en un hospital universitario de Buenos Aires, Argentina, durante 16 años. Rev. Chil. Infectología 2017, 34, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santolaya, M.E.; Thompson, L.; Benadof, D.; Tapia, C.; Legarraga, P.; Cortés, C.; Rabello, M.; Valenzuela, R.; Rojas, P.; Rabagliati, R. A prospective, multi-center study of Candida bloodstream infections in Chile. PLoS ONE 2019, 14, e0212924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Medeiros, M.A.P.; de Melo, A.P.V.; de Bento, A.O.; de Souza, L.B.F.C.; de Neto, F.A.B.; Garcia, J.B.-L.; Zuza-Alves, D.L.; Francisco, E.C.; de Melo, A.S.A.; Chaves, G.M. Epidemiology and prognostic factors of nosocomial candidemia in Northeast Brazil: A six-year retrospective study. PLoS ONE 2019, 14, e0221033. [Google Scholar] [CrossRef]
- Canela, H.M.S.; Cardoso, B.; Vitali, L.H.; Coelho, H.C.; Martinez, R.; Ferreira, M.E.d.S. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses 2018, 61, 11–21. [Google Scholar] [CrossRef]
- Aguilar, G.; Araujo, P.; Lird, G.; Insaurralde, S.; Kawabata, A.; Ayala, E.; Irala, J.; Argüello, R. Identificación y perfil de sensibilidad de Candida spp. aisladas de hemocultivos en hospitales de Paraguay. Rev. Panam. De Salud Pública 2020, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Rivera, S.M.; Armstrong, P.A.; Escandon, P.; Chow, N.A.; Ovalle, M.V.; Díaz, J.; Derado, G.; Salcedo, S.; Berrio, I.; et al. Case–Case Comparison of Candida auris Versus Other Candida Species Bloodstream Infections: Results of an Outbreak Investigation in Colombia. Mycopathologia 2020, 185, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Motoa, G.; Muñoz, J.S.; Oñate, J.; Pallares, C.J.; Hernández, C.; Villegas, M.V. Epidemiology of Candida isolates from Intensive Care Units in Colombia from 2010 to 2013. Rev. Iberoam. Micol. 2017, 34, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Berrio, I.; Maldonado, N.; De Bedout, C.; Arango, K.; Cano, L.E.; Valencia, Y.; Jiménez-Ortigosa, C.; Perlin, D.S.; Gómez, B.L.; Robledo, C.; et al. Comparative study of Candida spp. isolates: Identification and echinocandin susceptibility in isolates obtained from blood cultures in 15 hospitals in Medellín, Colombia. J. Glob. Antimicrob. Resist. 2018, 13, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gil-Alonso, S.; Quindós, G.; Cantón, E.; Eraso, E.; Jauregizar, N.; Gil-Alonso, S.; Quindós, G.; Cantón, E.; Eraso, E.; Jauregizar, N. Killing kinetics of anidulafungin, caspofungin and micafungin against Candida parapsilosis species complex: Evaluation of the fungicidal activity. Rev. Iberoam. Micol. 2019, 36, 24–29. [Google Scholar] [CrossRef]
- Ziccardi, M.; Souza, L.O.P.; Gandra, R.M.; Galdino, A.C.M.; Baptista, A.R.S.; Nunes, A.P.F.; Ribeiro, M.A.; Branquinha, M.H.; Santos, A.L.S. Candida parapsilosis (sensu lato) isolated from hospitals located in the Southeast of Brazil: Species distribution, antifungal susceptibility and virulence attributes. Int. J. Med. Microbiol. 2015, 305, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, B.; Martins, M.A.; Bonfietti, L.X.; Szeszs, M.W.; Jacobs, J.; Garcia, C.; Melhem, M.S. Species distribution and antifungal susceptibility profile of Candida isolates from bloodstream infections in Lima, Peru. J. Med. Microbiol. 2014, 63, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, X.; Reviakina, V.; Panizo, M.M.; Ferrara, G.; García, N.; Alarcón, V.; Garcés, M.F.; Dolande, M. Identificación molecular y sensibilidad a los antifúngicos de aislamientos de sangre del complejo Candida parapsilosis en Venezuela. Rev. Iberoam. Micol. 2017, 34, 165–170. [Google Scholar] [CrossRef]
- Kean, R.; Brown, J.; Gulmez, D.; Ware, A.; Ramage, G. Candida auris: A Decade of Understanding of an Enigmatic Pathogenic Yeast. J. Fungi 2020, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Allert, S.; Schulz, D.; Kämmer, P.; Großmann, P.; Wolf, T.; Schäuble, S.; Panagiotou, G.; Brunke, S.; Hube, B. From environmental adaptation to host survival: Attributes that mediate pathogenicity of Candida auris. Virulence 2022, 13, 191–214. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. Tratamiento de Las Enfermedades Infecciosas 2020–2022; Organización Panamericana de la Salud: Washington, DC, USA, 2019. [Google Scholar]
- Llert, S.; Förster, T.M.; Svensson, C.-M.; Richardson, J.P.; Pawlik, T.; Hebecker, B.; Rudolphi, S.; Juraschitz, M.; Schaller, M.; Blagojevic, M.; et al. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. mBio 2018, 9, e00915-18. [Google Scholar] [CrossRef] [Green Version]
- Lionakis, M.S.; Netea, M.G. Candida and Host Determinants of Susceptibility to Invasive Candidiasis. PLoS Pathog. 2013, 9, e1003079. [Google Scholar] [CrossRef] [Green Version]
- d’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Castanheira, M. Nosocomial Candidiasis: Antifungal Stewardship and the Importance of Rapid Diagnosis. Med. Mycol. 2015, 54, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.; Drummond, R. The Hidden Cost of Modern Medical Interventions: How Medical Advances Have Shaped the Prevalence of Human Fungal Disease. Pathogens 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.C.; Psaltikidis, E.M.; de Lima, T.C.; Fagnani, R.; Schreiber, A.Z.; de Conterno, L.O.; Kamei, K.; Watanabe, A.; Trabasso, P.; Resende, M.R.; et al. COVID-19 and invasive fungal coinfections: A case series at a Brazilian referral hospital. J. Med. Mycol. 2021, 31, 101175. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M.; Canetti, D.; Castiglioni, B.; Oltolini, C.; et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clin. Infect. Dis. 2021, 73, e2838–e2839. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Tam, J.M.; Reedy, J.L.; Lukason, D.P.; Kuna, S.G.; Acharya, M.; Khan, N.S.; Negoro, P.E.; Xu, S.; Ward, R.A.; Feldman, M.B.; et al. Tetraspanin CD82 Organizes Dectin-1 into Signaling Domains to Mediate Cellular Responses to Candida albicans. J. Immunol. 2019, 202, 3256–3266. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Levitz, S.M. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annu. Rev. Immunol. 2018, 36, 157–191. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Johnson, M.D.; Scott, W.K.; van de Vosse, E.; Velez Edwards, D.R.; Smith, P.B.; Alexander, B.D.; Yang, J.C.; Kremer, D.; Laird, G.M.; et al. Toll-like Receptor 1 Polymorphisms Increase Susceptibility to Candidemia. J. Infect. Dis. 2012, 205, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; Ng, A.; Kumar, V.; Johnson, M.D.; Plantinga, T.S.; van Diemen, C.; Arts, P.; Verwiel, E.T.P.; Gresnigt, M.S.; Fransen, K.; et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat. Commun. 2013, 4, 1342. [Google Scholar] [CrossRef] [Green Version]
- Naik, B.; Ahmed, S.M.Q.; Laha, S.; Das, S.P. Genetic Susceptibility to Fungal Infections and Links to Human Ancestry. Front. Genet. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Merkhofer, R.M.; Klein, B.S. Advances in Understanding Human Genetic Variations That Influence Innate Immunity to Fungi. Front. Cell. Infect. Microbiol. 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Matzaraki, V.; Gresnigt, M.S.; Jaeger, M.; Ricaño-Ponce, I.; Johnson, M.D.; Oosting, M.; Franke, L.; Withoff, S.; Perfect, J.R.; Joosten, L.A.B.; et al. An integrative genomics approach identifies novel pathways that influence candidaemia susceptibility. PLoS ONE 2017, 12, e0180824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Icely, P.A.; García, L.N.; Peinetti, N.; Maldonado, C.A.; Riera, F.O.; Caeiro, J.P.; Sotomayor, C.E. Corrigendum: Contribution of TLR2 pathway in the pathogenesis of vulvovaginal. Pathog. Dis. 2018, 76, fty050. [Google Scholar] [CrossRef]
- Icely, P.A.; Vigezzi, C.; Rodriguez, E.; Miró, M.S.; Renteri-Salido, B.; Sotomayor, C.E. Candida albicans Activation of Human Monocytes Toward M2 Profile is Reversed by Amphotericin B and Fluconazole. J. Bacteriol. Mycol. 2021, 8. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Canton, E.; Pemán, J.; Dilger, A.; Romá, E.; Perlin, D.S. Assessment of two new molecular methods for identification of Candida parapsilosis sensu lato species. J. Clin. Microbiol. 2011, 49, 3257–3261. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.C.; McCloskey, J.J.; Knauer, K.A. Pathobiologic features of human candidiasis. A common deep mycosis of the brin, heart and kidney in the altered host. Am. J. Clin. Pathol. 1976, 65, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Moyes, D.L.; Wächtler, B.; Hube, B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011, 13, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikou, S.-A.; Kichik, N.; Brown, R.; Ponde, N.; Ho, J.; Naglik, J.; Richardson, J. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a Multifunctional Adhesin and Invasin. Eukaryot. Cell 2011, 10, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yan, L.; Wu, C.; Zhao, X.; Tang, J. Fungal invasion of epithelial cells. Microbiol. Res. 2014, 169, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Moran, G.P.; Jacobsen, I.D.; Heyken, A.; Domey, J.; Sullivan, D.J.; Kurzai, O.; Hube, B. The Candida albicans-Specific Gene EED1 Encodes a Key Regulator of Hyphal Extension. PLoS ONE 2011, 6, e18394. [Google Scholar] [CrossRef] [Green Version]
- Strickland, A.B.; Shi, M. Mechanisms of fungal dissemination. Cell. Mol. Life Sci. 2021, 78, 3219–3238. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Tejera, E.; Machado, A. Prevalence of biofilms in Candida spp. bloodstream infections: A meta-analysis. PLoS ONE 2022, 17, e0263522. [Google Scholar] [CrossRef]
- Ferwerda, B.; Ferwerda, G.; Plantinga, T.S.; Willment, J.A.; van Spriel, A.B.; Venselaar, H.; Elbers, C.C.; Johnson, M.D.; Cambi, A.; Huysamen, C.; et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009, 361, 1760–1767. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Joosten, L.A.B.; van der Meer, J.W.M.; Kullberg, B.-J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Vigezzi, C.; Riera, F.O.; Rodriguez, E.; Icely, P.A.; Miró, M.S.; Figueredo, C.M.; Caeiro, J.P.; Sotomayor, C.E. Candidiasis invasora: Un enfoque a la infección en el sistema nervioso central. Rev. Argent. Microbiol. 2021, 53, 171–178. [Google Scholar] [CrossRef]
- Saijo, S.; Fujikado, N.; Furuta, T.; Chung, S.H.; Kotaki, H.; Seki, K.; Sudo, K.; Akira, S.; Adachi, Y.; Ohno, N.; et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007, 8, 39–46. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Hennigs, A.; Nabavi, M.; Schäffer, A.A.; Woellner, C.; Salzer, U.; Pfeifer, D.; Veelken, H.; Warnatz, K.; Tahami, F.; et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009, 361, 1727–1735. [Google Scholar] [CrossRef] [Green Version]
- Smeekens, S.P.; Gresnigt, M.S.; Becker, K.L.; Cheng, S.C.; Netea, S.A.; Jacobs, L.; Jansen, T.; van de Veerdonk, F.L.; Williams, D.L.; Joosten, L.A.B.; et al. An anti-inflammatory property of Candida albicans β-glucan: Induction of high levels of interleukin-1 receptor antagonist via a Dectin-1/CR3 independent mechanism. Cytokine 2015, 71, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, R.A.; Lionakis, M.S. Candidiasis of the Central Nervous System in Neonates and Children With Primary Immunodeficiencies. Curr. Fungal Infect. Rep. 2018, 12, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Whibley, N.; Jaycox, J.R.; Reid, D.; Garg, A.V.; Taylor, J.A.; Clancy, C.J.; Nguyen, M.H.; Biswas, P.S.; McGeachy, M.J.; Brown, G.D.; et al. Delinking CARD9 and IL-17: CARD9 Protects against Candida tropicalis Infection through a TNF-α–Dependent, IL-17–Independent Mechanism. J. Immunol. 2015, 195, 3781–3792. [Google Scholar] [CrossRef] [Green Version]
- Desai, J.V.; Lionakis, M.S. The Role of Neutrophils in Host Defense Against Invasive Fungal Infections. Curr. Clin. Microbiol. Rep. 2018, 5, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lim, J.K.; Lee, C.-C.R.; Murphy, P.M. Organ-Specific Innate Immune Responses in a Mouse Model of Invasive Candidiasis. J. Innate Immun. 2011, 3, 180–199. [Google Scholar] [CrossRef] [Green Version]
- Urban, C.F.; Nett, J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef]
- Uzun, O.; Ascioglu, S.; Anaissie, E.J.; Rex, J.H. Risk Factors and Predictors of Outcome in Patients with Cancer and Breakthrough Candidemia. Clin. Infect. Dis. 2001, 32, 1713–1717. [Google Scholar] [CrossRef]
- Heung, L.J. Monocytes and the Host Response to Fungal Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 34. [Google Scholar] [CrossRef]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A.R. Candida albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. mBio 2017, 8, e01820-16. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A Prospective Observational Study of Candidemia: Epidemiology, Therapy, and Influences on Mortality in Hospitalized Adult and Pediatric Patients. Clin. Infect. Dis. 2003, 37, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.; Meltzer, M.I.; Plikaytis, B.D.; Sofair, A.N.; Huie-White, S.; Wilcox, S.; Harrison, L.H.; Seaberg, E.C.; Hajjeh, R.A.; Teutsch, S.M. Excess Mortality, Hospital Stay, and Cost Due to Candidemia: A Case-Control Study Using Data From Population-Based Candidemia Surveillance. Infect. Control Hosp. Epidemiol. 2005, 26, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Roussos, N.; Vardakas, K.Z. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int. J. Infect. Dis. 2010, 14, e954–e966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-Year Analysis of Susceptibilities of Candida Species to Fluconazole and Voriconazole as Determined by CLSI Standardized Disk Diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Bitar, D.; Lortholary, O.; Le Strat, Y.; Nicolau, J.; Coignard, B.; Tattevin, P.; Che, D.; Dromer, F. Population-Based Analysis of Invasive Fungal Infections, France, 2001–2010. Emerg. Infect. Dis. 2014, 20, 1163–1169. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Clancy, C.J.; Nguyen, M.H. Finding the “Missing 50%” of Invasive Candidiasis: How Nonculture Diagnostics Will Improve Understanding of Disease Spectrum and Transform Patient Care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Bassetti, M.; Taramasso, L.; Nicco, E.; Molinari, M.P.; Mussap, M.; Viscoli, C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS ONE 2011, 6, e24198. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Marra, A.R.; Schweizer, M.L.; Ten Eyck, P.; Wu, C.; Alzunitan, M.; Salinas, J.L.; Siegel, M.; Farmakiotis, D.; Auwaerter, P.G.; et al. Impact of Infectious Disease Consultation in Patients With Candidemia: A Retrospective Study, Systematic Literature Review, and Meta-analysis. Open Forum Infect. Dis. 2020, 7, ofaa270. [Google Scholar] [CrossRef]
- Colombo, A.L.; Guimarães, T.; Camargo, L.F.A.; Richtmann, R.; de Queiroz-Telles, F.; Salles, M.J.C.; da Cunha, C.A.; Yasuda, M.A.S.; Moretti, M.L.; Nucci, M. Brazilian guidelines for the management of candidiasis—A joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz. J. Infect. Dis. 2013, 17, 283–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caeiro, J.P.; Riera, F. Candida Sepsis: A New Entity? Curr. Fungal Infect. Rep. 2014, 8, 95–101. [Google Scholar] [CrossRef]
- Thompson, G.R.; Boulware, D.R.; Bahr, N.C.; Clancy, C.J.; Harrison, T.S.; Kauffman, C.A.; Le, T.; Miceli, M.H.; Mylonakis, E.; Nguyen, M.H.; et al. Noninvasive Testing and Surrogate Markers in Invasive Fungal Diseases. Open Forum Infect. Dis. 2022, 9, ofac112. [Google Scholar] [CrossRef]
- Keighley, C.; Cooley, L.; Morris, A.J.; Ritchie, D.; Clark, J.E.; Boan, P.; Worth, L.J.; Slavin, M.A.; Thursky, K.A.; Roberts, J.A.; et al. Consensus guidelines for the diagnosis and management of invasive candidiasis in haematology, oncology and intensive care settings, 2021. Intern. Med. J. 2021, 51, 89–117. [Google Scholar] [CrossRef]
- O’Brien, D.; Stevens, N.T.; Lim, C.H.; O’Brien, D.F.; Smyth, E.; Fitzpatrick, F.; Humphreys, H. Candida infection of the central nervous system following neurosurgery: A 12-year review. Acta Neurochir. 2011, 153, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Katragkou, A.; Chen, T.; Salvatore, C.; Roilides, E. Invasive Candidiasis in Infants and Children: Recent Advances in Epidemiology, Diagnosis, and Treatment. J. Fungi 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C. Candida and candidaemia. Susceptibility and epidemiology. Dan. Med. J. 2013, 60, B4698. [Google Scholar]
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 2010, 16, 445–452. [Google Scholar] [CrossRef]
- Rodrigues, D.K.B.; Bonfietti, L.X.; Garcia, R.A.; Araujo, M.R.; Rodrigues, J.S.; Gimenes, V.M.F.; Melhem, M.S.C. Antifungal susceptibility profile of Candida clinical isolates from 22 hospitals of São Paulo State, Brazil. Braz. J. Med. Biol. Res. 2021, 54, e10928. [Google Scholar] [CrossRef]
- Escandón, P.; Cáceres, D.H.; Lizarazo, D.; Lockhart, S.R.; Lyman, M.; Duarte, C. Laboratory-based surveillance of Candida auris in Colombia, 2016–2020. Mycoses 2022, 65, 222–225. [Google Scholar] [CrossRef]
- Seiler, G.T.; Ostrosky-Zeichner, L. Investigational Agents for the Treatment of Resistant Yeasts and Molds. Curr. Fungal Infect. Rep. 2021, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef] [PubMed]


| Candidemia Characteristic | Treatments | ||
|---|---|---|---|
| Primary | Alternative | New Drugs | |
| Non-Neutropenic patients | Caspofungin Anidulafungin Micafungin | LF AmB Fluconazole * Isavuconazole Voriconazole | Ibrexafungerp Rezafungin Osteaconazole Fosmanogepix |
| Neutropenic Patients | Caspofungin Anidulafungin Micafungin | AmB Liposomal Fluconazole * Isavuconazole Voriconazole | |
| Ocular Compromise + | Fluconazole Voriconazole | AmB Liposomal | |
| CNS Compromise + | AmB Liposomal | Fluconazole | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. https://doi.org/10.3390/antibiotics11070877
Riera FO, Caeiro JP, Angiolini SC, Vigezzi C, Rodriguez E, Icely PA, Sotomayor CE. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics. 2022; 11(7):877. https://doi.org/10.3390/antibiotics11070877
Chicago/Turabian StyleRiera, Fernando Oscar, Juan Pablo Caeiro, Sofia Carla Angiolini, Cecilia Vigezzi, Emilse Rodriguez, Paula Alejandra Icely, and Claudia Elena Sotomayor. 2022. "Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America" Antibiotics 11, no. 7: 877. https://doi.org/10.3390/antibiotics11070877
APA StyleRiera, F. O., Caeiro, J. P., Angiolini, S. C., Vigezzi, C., Rodriguez, E., Icely, P. A., & Sotomayor, C. E. (2022). Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics, 11(7), 877. https://doi.org/10.3390/antibiotics11070877







