Abstract
Candida auris is a recently found Candida species, mainly associated with nosocomial outbreaks in intensive care hospital settings, and unlike other Candida species, it can be transmitted through person-to-person or by contact with surfaces. C. auris is described as resistant to first-line antifungals and, consequently, associated with high mortality. Nowadays, essential oils (EOs) are known to be effective against fungal and bacterial infections. This work aimed to evaluate the effect of four EOs (tea tree, niaouli, white thyme and cajeput) against C. auris. The EO’s effect on C. auris planktonic growth was evaluated by the minimum inhibitory concentration determination and by the agar disc diffusion method. Then, the same effect was evaluated on biofilm by colony-forming units’ enumeration. The results showed that EOs were able to inhibit the C. auris planktonic growth, with an MIC50 between 0.78 and 1.56% and halos of 20–21 mm for white thyme and tea tree and 13–14 mm for cajeput and niaouli. In addition, the EOs were also able to completely inhibit biofilm formation. Moreover, white thyme and cajeput completely eradicate pre-formed biofilms, while tea tree and niaouli significantly reduce it. Thus, this work demonstrates that EOs are a possible therapeutic alternative and a future perspective for the hard fight against C. auris.
1. Introduction
Candida auris is a new Candida species recently found after being isolated from the external auditory canal of a Japanese patient, and since then, it has been found and reported in more than 6 continents and 30 countries [1,2,3]. C. auris is mainly associated with nosocomial outbreaks in intensive care settings, where it has been recovered from the skin of healthcare workers and patients, areas that have been in contact with infected patients and a wide variety of surfaces [4]. C. auris has shown high colonisation and survival capabilities and, unlike the majority of the other Candida species, can be transmitted through person-to-person or through contact with surfaces or contaminated equipment [4]. This yeast causes highly invasive infections with a high mortality rate in hospitalised patients, with immunosuppression and underlying chronic disease being the main risk factors [5]. In fact, several virulence factors commonly associated with Candida albicans (considered the most virulent member of the genus) have been identified in C. auris, such as phospholipase and proteinase activities and biofilm formation, although C. auris isolates produce only rudimentary pseudohyphae [6]. In addition, C. auris has already been described as resistant to the first-line antifungal agent, fluconazole (FLC), exhibiting variable susceptibility to other azoles and echinocandins [1]. Thus, the search for alternative therapies from natural sources featuring new mechanisms of action combined with new strategies has become crucial. Nowadays, it is well known that some plant products, such as essential oils (EOs), are effective against fungal and bacterial infections, presenting good bioactivity and low toxicity [7]. The strong antimicrobial activities of EOs and their components are described in several studies [8,9,10,11]. EOs have the ability to hinder the growth of pathogens, increasing membrane permeability, disrupting the cell membrane, inducing leakage of vital intracellular constituents and interrupting cell metabolism and enzymatic kinetics [10]. Tran et al. [11] demonstrated for the first time the potential of EO from Cinnamomum zeylanicum to exert antifungal activity against planktonic C. auris cells, highlighting their ability to damage the membranous structures of fungal cells and haemolytic activities. In addition, it was also demonstrated that EO arborvitae led to a significant decrease in the intrinsic planktonic growth rate of C. auris. However, in general, there are very few studies on the effect of EOs on C. auris, especially on C. auris biofilm. In this study, four EOs were evaluated; the choice of these four specific EOs was based on clinical evidence provided by a clinic (Costa Raquel, Aromas Aqua Spa–Health Clinic) and due to their characteristics. Tea tree EO is recognised for its antifungal, antiseptic, anti-inflammatory and anti-infective properties; it also stimulates the immune system and has a calming effect. Cajeput and white thyme EOs are recognised for their respiratory tract cleansing properties and are used for their calming properties. Niaouli EO is an expectorant and decongestant that strengthens the immune system and can be used for skin problems such as acne, itching, dermatitis or sunburn [12].
Therefore, the aim of this work was to evaluate the effect of tea tree, cajeput, niaouli and white thyme EOs against C. auris species.
2. Results
2.1. Essential Oils Composition
The identified compounds and their relative contents are listed in Table 1 according to their percentage ascending on a non-polar column. The major compounds of the EOs are cineole (65%), α-terpineol (11%) and linalool (3%) in cajeput oil; p-cymene, limonene and 1,8-cineole (57%) in niaouli oil; terpinen-4-ol (42%), γ-terpinene (21%) and α-terpinene (10%) in tea tree oil; and borneol (32%), α-terpineol (16%) and carvacrol (9%) in white thyme oil.

Table 1.
Identification of the components of tea tree, cajeput, niaouli and white thyme essential oils expressed as a percentage (%) of individual compounds in the total essential oil sample.
2.2. Antifungal Susceptibility Testing
2.2.1. Fluconazole
To verify C. auris resistance to FLC, both minimum inhibitory concentrations (MIC50) and minimum fungicidal concentrations (MFC) were firstly evaluated. The results showed an MIC50 of 125 µg/mL and an MFC of ≥125 µg/mL.
2.2.2. Essential Oils
Planktonic Antimicrobial Susceptibilities
The MIC50 and MFC of white thyme, tea tree, cajeput and niaouli EOs against planktonic populations of C. auris were determined. A lower MIC50 was observed for tea tree EO (0.78% (v/v)) in comparison to the other EOs tested, with an MIC50 value of 1.56% (v/v). Similarly, the MFC recorded for the tea tree EO was lower (1.56% (w/v)) than for white thyme, cajeput and niaouli EOs (3.12% (w/v)).
The antifungal activity of EOs (white thyme, tea tree, cajeput and niaouli) against C. auris was also evaluated using the agar disc diffusion method, and the results are summa-rised in Figure 1. As a negative control, 70% ethanol (v/v) was used, resulting in a 9 mm halo. Regarding the effect of EOs, both white thyme (21.4 ± 0.5 mm) and tea tree (20.0 ± 1.9 mm) oils demonstrated stronger antifungal activity against the tested strains when compared with cajeput (13.8 ± 1.1 mm) and niaouli (13.3 ± 1.1 mm) oils. Furthermore, despite similar diameters between the tea tree and white thyme oil, there was a marked decrease in the C. auris biomass all over the petri dish with the application of white thyme EOs (Figure 1).

Figure 1.
Antifungal activity of essential oils on C. auris NCPF 8971 evaluated using the disk diffusion assay.
Biofilms Antimicrobial Susceptibilities
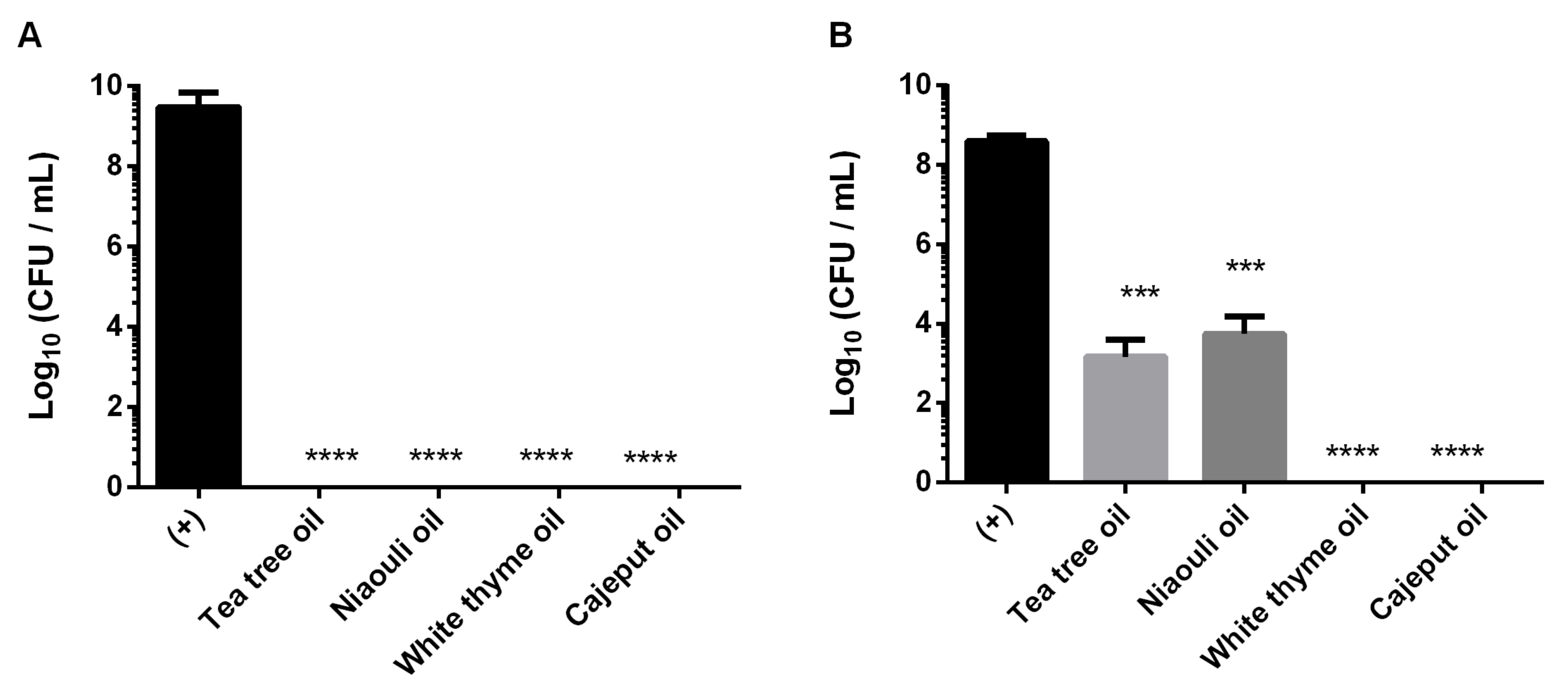
The antifungal effect of white thyme, tea tree, cajeput and niaouli EOs in biofilm formation and 24 h old biofilms of C. auris was evaluated (Figure 2). Direct application of two drops (2.4% (v/v)) of EOs on biofilm formation (Figure 2A) led to a total inhibition of biofilm growth. Moreover, the oils were also applied in pre-formed biofilms (Figure 2B), and it was observed that the application of two drops (2.4% (v/v)) of white thyme and cajeput oils induced a total eradication of the 24 h old biofilms. In addition, tea tree and niaouli oils significantly reduced viable cells of those pre-formed biofilms (p < 0.0001), with reductions of 5 Log10 CFU/mL and 4 Log10 CFU/mL (p < 0.001), respectively.
Figure 2.
Antifungal effect of four essential oils (tea tree, niaouli, white thyme and cajeput) in biofilm formation (A) and 24 h old biofilms (B) of C. auris. *** p < 0.001, **** p < 0.0001 indicates a statistically different reduction in comparison with the control (+).
3. Discussion
The general resistance of Candida spp. to most available antifungal drugs and the emergence of multidrug-resistant species, such as C. auris, make it urgent to develop new drugs or more effective therapies. Therefore, this study aimed to evaluate the antifungal activity of four EOs against C. auris in both planktonic cells and biofilms (on its formation and on pre-formed biofilms).
The results of antifungal susceptibility test (MIC50 of 125 µg/mL and a MFC of ≥ 125 µ/mL) confirm, that, as expected, C. auris is highly resistant to FLC (sensitive < 32 µg/mL; resistant ≥ 32 µg/mL) [13]. Recently, genetic analyses of the C. auris species have reported mutations in genes related to the development of triazole resistance. In addition, the presence of genes encoding proteins involved in the acquisition of resistance, such as protein kinases, efflux pumps and major facilitator superfamilies, have also been reported [14]. Thus, considering the still increasing problem of C. auris drug resistance, the antifungal properties of the EOs can be considered a promising alternative.
Regarding the antifungal activity of EOs against planktonic C. auris cells, all the EOs were able to inhibit the growth of C. auris, with MIC50 values between 0.78% (v/v)−1.56% (v/v) and EOs MFC was 2 × MIC (Figure 1). The four EOs tested showed inhibitory and fungicidal properties at low concentrations. Tran et al. [11], one of the few studies that present the MIC and MFC values of EOs in C. auris, also verified very low MIC and MFC values for cinnamon EO of < 0.03–0.13% (v/v) and 0.25% (v/v), respectively.
In the agar disc diffusion method, white thyme and tea tree oils demonstrated stronger antifungal activity when compared with cajeput and niaouli oils (Figure 1). Our results are in line with several reports of high antifungal activity of thyme and tea tree oils against both susceptible and drug-resistant strains of various Candida species [15,16]. Indeed, EOs and their constituents have been used against a wide range of fungal pathogens since they have the ability to hinder the growth and development of a diverse range of pathogens [10]. In fact, according to a previous study, although the efficiency of EOs differs substantially between Thymus species, EOs from Thymus species can be pointed out as a great contribution to the treatment of C. auris infections [17].
After these good preliminary results, the antifungal effect of EOs in biofilm formation and 24 h old biofilms of C. auris was evaluated (Figure 2). Our results confirm the high ability of C. auris to form biofilms (Figure 2—(+) control), as previously described by Horton et al. [18]. These authors demonstrated that the growth in synthetic sweat medium and in the skin of pigs allows C. auris to form dense biofilms that resist desiccation and thrive in conditions of evaporation. In addition, the draft genome that identifies several proteins involved in biofilm formation and recent descriptions of aggregative and non-aggregative phenotypes indicate the possibility of heterogeneous C. auris biofilm formation [6].
In relation to the application of EOs on biofilm formation (Figure 2A), it was possible to verify that the application of only two drops (2.4% (v/v)) of oil (white thyme, tea tree, cajeput or niaouli) induced to a total inhibition of biofilm growth after 24 h. Furthermore, the application of the same volume of white thyme and cajeput oils led to the total eradication of 24 h old biofilms (pre-formed biofilms) (Figure 2B), showcasing their great efficacy. Besides, tea tree and niaouli oils significantly reduce viable cells of those pre-formed biofilms (p < 0.001). In fact, the good anti-Candida activity of thyme was also verified in many reports [17,19]. Similar to the results obtained in this study, Asdadi et al. showed that EO of Thymus satureiodes (white thyme oil) was able to inhibit the growth of non-Candida albicans species (Candida krusei, Candida dubliniensis and Candida glabrata) resistant to conventional antifungals, such as FLC and amphotericine B [19].
In this study, the major compounds of the white thyme oil are borneol, α-terpineol and carvacrol. It has been demonstrated that oxygenated terpene compounds, such as carvacrol, are often considered the main compounds responsible for modifying membrane permeability by chemical reaction with amino and hydroxylamine groups of membrane proteins [8]. Indeed, carvacrol has the highest antifungal activity, inhibiting the formation of hyphae and biofilms in Candida species [5,20]. Furthermore, terpinen-4-ol and α-terpineol are recognised as potent compounds with a fungicidal effect [16]. Thus, the very good outcomes obtained in this work are justified by the important antifungal role of these compounds.
In turn, the major compounds of the tea tree EO are terpinen-4-ol, γ-terpinene and α-terpinene. Tea tree oil and its components increase yeast cell permeability and membrane fluidity and become embedded in the lipid bilayer membrane, eventually disrupting its structure [21]. Besides that, tea tree oil also inhibits the formation of germ tubes or mycelial conversion and inhibits respiration in C. albicans [21]. In addition, Mondello et al. [16] suggested that terpinen-4-ol is a likely mediator of the in vitro and in vivo activity of tea tree oil and that it could control C. albicans vaginal infection. The EO of Melaleuca quinquenervia (niaouli oil) was found to be effective against bacteria as well as a range of fungi, including C. albicans [22]. Additionally, Keereedach et al. found that Melaleuca cajuputi (cajeput oil) had potent antifungal activity against clinical isolates of C. albicans resistant to FLC, where it was able to reduce the MIC of FLC and reduce the expression level of MDR1 (an important gene that plays a role in resistance to azole drugs in C. albicans) [23]. However, so far, there are few studies regarding the effect of niaouli and cajeput oils in Candida species, with this being the first study to use both oils in Candida biofilms. The major compounds of these two oils are cineole, α-terpineol and linalool in cajeput oil and ρ-cymene, limonene and 1,8-cineole in niaouli oil. 1,8-Cineole showed some anti-Candida activity with a fungicidal effect [24]. In addition, linalool exerts antifungal activity by disrupting the membrane integrity and interrupting the cell cycle of planktonic C. albicans, inhibits germ tube formation and exhibits antifungal activity against C. albicans cells in biofilms. The inhibition of hyphae induction by limonene at low concentrations may be responsible for the inhibition of biofilm formation. However, ρ-cymene, a thymol precursor, is generally defined as inactive [25,26].
Nevertheless, it is complicated to point the antifungal activity of a complex mixture (EOs) to specific constituents. In fact, it is believed that the antifungal activity most probably results from the combined effect of different compounds on several cellular targets [27]. In this study, the composition of the tested EOs suggests that each oil is featured by components common to the four EOs (27–35% of the total composition) and specific components (19–33%). Thus, it is reasonable to speculate that the antifungal activity of these four EOs can also be related to the presence of specific compounds, such as terpinen-4-ol, carvacrol, α-terpineol, linalool and limonene. Studies report that terpinen-4-ol, a major component of tea tree oil (42%), has a lipophilic characteristic, probably presenting a direct action on the cell membrane structure and associated enzymes [28]. Carvacrol, present in white thyme oil (9%), and linalool, present in the four EOs under study in a range between 0.2–3%, are associated with the modification of membrane permeability and inhibition of hyphae and biofilm formation [8]. The antifungal activity of α-terpineol, present in the four EOs (3–16%), also occurs through the disruption of cell walls and cytoplasm, resulting in abnormal hyphae [29]. The limonene constituent of tea tree, niaouli and white thyme oils act on the genetic material of yeast, leading to damage to the cell wall and intracellular structures, including nuclear alterations (condensation of genetic material and specific changes in mitochondria). This compound also induces dramatic structural changes in organelles, accompanied by cell wall disruption [30]. However, to better understand the antifungal effect of these EOs, the synergistic or antagonistic effect between the most abundant compounds and also those present in a smaller percentage in the mixture should be investigated [31]. Thus, more studies are needed in order to understand the interactions between components and, consequently, the mechanisms of action of EOs.
4. Materials and Methods
4.1. Essential Oils
This study evaluated the antifungal activity of fours EOs, namely cajeput (Melaleuca cajaputi (leaf); florame® (Lot 801736), Portugal); niaouli (Melaleuca quinquenervia (leaf); florame® (Lot 100378), Portugal); tea tree (Melaleuca alternifolia (leaf); florame® (Lot 903025), Portugal) and white thyme (Thymus satureiodes (flowering tops); florame® (Lot 800180), Portugal) (all with 100% purity). All EO samples were stored wrapped in aluminium foil in order to protect from light at room temperature.
Analysis of the EOs was carried out by florame® (Saint-Rémy-de-Provence, France) (Supplementary Materials), using Gas Chromatography (GC) with Flame-Ionisation Detection (FID) under the following conditions: the hydrogen carrier gas; the polar Elite-WAX column (100% polyethylene glycol) (60 m/0.25 mm/0.25 μm) and the non-polar Elite-5 columns (5% diphenyl, 95% dimethylpolysiloxane) (60 m/0.25 mm/0.25 μm) for tea tree, white thyme and cajeput EOs. Analysis of the niaouli EO was carried out using a GC 6890 MS 5975 instrument at the following conditions: the injected sample volume was 1 μL; the helium carrier gas flow rate was 1 mL min−1; an HP5 ms capillary column (30 m/0.25 mm) was used, with a film thickness of 0.25 μm; the column temperature was 60 °C increasing at 2 °C min−1 to 250 °C; and the mass range was 40–450 m z−1.
4.2. Microorganisms and Culture Conditions in This Study
Candida auris NCPF 8971 was stored at −80 ± 2 °C in Sabouraud Dextrose Broth (SDB; Liofilchem, Roseto degli Abruzzi, Italy) with 20% (v/v) glycerol. Before each assay, C. auris was subcultured onto Sabouraud Dextrose Agar (SDA; Liofilchem) and incubated aerobically at 37 °C for 18–24 h. SDA plates were prepared from SDB supplemented with 2% (w/v) agar (Liofilchem).
4.3. Antifungal Susceptibility Testing
4.3.1. Fluconazole
MICs and MFCs of FLC were determined using the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI M27-A4), with some modifications [32]. Briefly, the initial cell concentration for C. auris was adjusted for 2 × 105 Colony-Forming Units (CFU)/mL in RPMI 1640 and dispensed into 96-well round bottom microtiter plates with the FLC solution, both in a proportion of 1:2. Positive (C. auris suspension) and negative (broth medium RPMI 1640) controls were included. All microtiter plates were incubated for 24–48 h at 37 °C. MIC was obtained by visual observation of the turbidity gradient and by the determination of the optical density at 570 nm. MIC50 endpoints were established as the lowest concentration of FLC that resulted in a decrease of growth by ≥ 50% relative to the positive control (C. auris suspension). To determine MFC, each well was sub-cultured onto SDA plates and incubated for 24 h at 37 °C. MFC endpoints were defined as the concentration in the first well with no growth on the SDA plate. The experiment was performed in three independent assays in triplicate.
4.3.2. Essential Oils
Planktonic Antimicrobial Susceptibilities
MICs and MFCs of cajeput, niaouli, tea tree and white thyme EOs were determined as described above, with some modifications due to the use of volatile compounds. After the concentration was adjusted, the cell suspension was dispensed into glass wells into glass Petri dishes (1 mL/well) with the EOs diluted (12.5% (v/v)–0.02% (v/v)) in vegetable oil and almond oil (Prunus amygdalus, AB—Agriculture Biologique, Paris, France), with both at a ratio of 1:2. Positive (C. auris suspension with almond oil) and negative (RPMI broth medium) controls were included. The set glass wells into glass Petri dishes were incubated for 48 h at 37 °C. MIC50 and MFC were determined as described above [17].
The agar disc diffusion method described by Tran et al. and Touati et al. [11,33], with some modifications, was used for the determination of the antifungal activities of cajeput, niaouli, tea tree and white thyme oils. Briefly, a swab dipped in cell suspensions (pre-inocula) adjusted to 1 × 108 cells/mL was spread onto SDA plates. Then, sterile 6 mm blank disks (Liofilchem) impregnated with 25 µL of each oil (100% (v/v)) were placed over the agar surface, and plates were incubated at 37 °C for 24 h. Plates with disks loaded with ethanol 70% (v/v) and almond oil were also included as positive and negative controls, respectively. The inhibition zones induced by different EOs were measured (mm).
Both experiments were performed in triplicate and in three independent assays.
Biofilm Antimicrobial Susceptibilities
C. auris biofilms were developed as described by Stepanovi’c et al. and Ribeiro et al. [17,34], with some modifications due to the use of volatile compounds. Briefly, pre-inocula (pure liquid cultures) of C. auris were maintained in SDB for 18 h at 37 °C under agitation (120 rev/min). Then, the initial cell concentration was adjusted for 1 × 105 cells/mL. The cellular suspension was transferred, under aseptic conditions, to glass wells inside glass Petri plates (1 mL/well). The C. auris biofilm culture was incubated aerobically for 24 h on a horizontal shaker at 120 rpm and 37 °C. The effect of four EOs (100%) (tea tree, niaouli, white thyme and cajeput) was evaluated on biofilm formation and on 24 h old biofilms. For this, two drops (25 µL with a final concentration of 2.4% (v/v)) of each oil were added to the wells containing a cell suspension (biofilm formation) or pre-formed biofilms (24 h old biofilms). Positive (C. auris suspension) and negative (SDB broth medium) controls were included. All plates were then incubated in the same conditions. After 24 h, the wells were washed with saline solution to discard the planktonic fraction, and the microdrop technique was used. Biofilm cell suspensions were serially diluted in saline and plated on SDA plates (incubated aerobically for 24 h at 37 °C), and the cultivable cells were enumerated. Values of cultivable sessile cells were expressed as Log CFU per ml (Log10 (CFU/mL)). The biofilm experiment was performed in triplicate with three independent assays.
4.4. Statistical Analysis
Biofilm antimicrobial susceptibility results were analysed using the Prism software package (GraphPad Software version 6.01 for Macintosh). Two-way ANOVA tests were performed, and means were compared by applying Tukey’s multiple comparison test. Statistically significant differences were considered significant when p < 0.05. At least three independent experiments were performed (in triplicate).
5. Conclusions
C. auris is recognised as a notorious nosocomial pathogen that requires urgent efforts to develop more efficient and safer alternative treatments due to high transmissibility, incorrect use of antifungal drugs, identification challenges and consequent treatment failures. In this sense, EOs are an alternative therapy for this important pathogen. The results of this study demonstrate a high efficacy of the four EOs tested to inhibit the planktonic growth and completely inhibit C. auris biofilm formation. Altogether, the data suggest that the application of these four EOs, particularly white thyme and cajeput, is an efficient alternative for preventing and treating infections caused by C. auris biofilms and also reduces dependence on existing conventional antimicrobials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11070977/s1, File S1: Essential Oils Analysis Bulletins (florame® (Saint-Rémy-de-Provence, France)).
Author Contributions
Conceptualisation, L.F., M.E.R. and M.H.; methodology, L.F. and R.R.; validation, M.E.R. and M.H.; formal analysis, L.F., R.R., M.E.R. and M.H.; investigation, L.F.; resources, R.C.; data curation, L.F.; writing—original draft preparation, L.F., M.E.R. and M.H.; writing—review and editing, R.C., M.E.R. and M.H.; visualisation, L.F., M.E.R. and M.H.; supervision, M.E.R. and M.H.; project administration, M.H.; funding acquisition, L.F. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit and grant ref 2020.05720.BD for Liliana Fernandes. Also, this study was supported by LABBELS—Associate Laboratory in Biotechnology, Bioengineering and Microelectromechnaical Systems, LA/P/0029/2020 and M. Elisa Rodrigues thanks FCT for funding through program DL 57/2016—Norma transitória.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-Resistant Candida Auris: ‘New Kid on the Block’ in Hospital-Associated Infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple Introductions and Subsequent Transmission of Multidrug-Resistant Candida Auris in the USA: A Molecular Epidemiological Survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Aldejohann, A.M.; Wiese-Posselt, M.; Gastmeier, P.; Kurzai, O. Expert Recommendations for Prevention and Management of Candida Auris Transmission. Mycoses 2022, 65, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Browna, C.S. Candida Auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef] [Green Version]
- Shaban, S.; Patel, M.; Ahmad, A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida Auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida Auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [Green Version]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczynska, A. Effect of Clove and Thyme Essential Oils on Candida Biofilm Formation and the Oil Distribution in Yeast Cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [Green Version]
- Palmeira-de-Oliveira, A.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Pina-Vaz, C.; Queiroz, J.; Rodrigues, A. Anti-Candida Activity of Essential Oils. Mini-Rev. Med. Chem. 2009, 9, 1292–1305. [Google Scholar] [CrossRef]
- Di Vito, M.; Mattarelli, P.; Modesto, M.; Girolamo, A.; Ballardini, M.; Tamburro, A.; Meledandri, M.; Mondello, F. In Vitro Activity of Tea Tree Oil Vaginal Suppositories against Candida Spp. and Probiotic Vaginal Microbiota. Phytother. Res. 2015, 29, 1628–1633. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complementary Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In Vitro Antifungal Activity of Cinnamomum Zeylanicum Bark and Leaf Essential Oils against Candida Albicans and Candida Auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Cosmétiques et Huiles Essentielles Bio Florame. Available online: https://fr.florame.com/?fbclid=IwAR34JDp1K64QMDTNYyauialnpoCUJlF3LBN9w85ZM8dYb4XCoL3OAnggVDQ (accessed on 2 November 2021).
- Antifungal Susceptibility Testing and Interpretation|Candida Auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 2 November 2021).
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft Genome of a Commonly Misdiagnosed Multidrug Resistant Pathogen Candida Auris. BMC Genom. 2015, 16, 686. [Google Scholar] [CrossRef] [Green Version]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [Green Version]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In Vivo Activity of Terpinen-4-Ol, the Main Bioactive Component of Melaleuca Alternifolia Cheel (Tea Tree) Oil against Azole-Susceptible and -Resistant Human Pathogenic Candida Species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.; Fernandes, L.; Costa, R.; Cavaleiro, C.; Salgueiro, L.; Henriques, M.; Rodrigues, M.E. Comparing the Effect of Thymus Spp. Essential Oils on Candida Auris. Ind. Crops Prod. 2022, 178, 114667. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida Auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere 2020, 5, e00910-19. [Google Scholar] [CrossRef] [Green Version]
- Asdadi, A.; Alilou, H.; Akssira, M.; Mina, L.; Hassani, I.; Chebli, B.; Moutaj, R.; Gonzặlez-Mas, C.; Amparo Blặzquez, M. Chemical Composition and Anticandidal Effect of Three Thymus Species Essential Oils from Southwest of Morocco against the Emerging Nosocomial Fluconazole-Resistant Strains. J. Biol. Agric. Healthc. 2014, 4, 16–26. [Google Scholar]
- Salgueiro, L.R.; Cavaleiro, C.; Gonçalves, M.J.; Proença Da Cunha, A. Antimicrobial Activity and Chemical Composition of the Essential Oil of Lippia Graveolens from Guatemala. Planta Med. 2003, 69, 80–83. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial Activity of Essential Oils from Australian Native Plants. Phytother. Res. 2005, 19, 643–646. [Google Scholar] [CrossRef]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal Activity of Thai Cajuput Oil and Its Effect on Efflux-Pump Gene Expression in Fluconazole-Resistant Candida Albicans Clinical Isolates. Int. J. Microbiol. 2020, 2020, 5989206. [Google Scholar] [CrossRef]
- Oliva, B.; Piccirilli, E.; Ceddia, T.; Pontieri, E.; Aureli, P.; Ferrini, A.M. Antimycotic Activity of Melaleuca Alternifolia Essential Oil and Its Major Components. Lett. Appl. Microbiol. 2003, 37, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids Inhibit Candida Albicans Growth by Affecting Membrane Integrity and Arrest of Cell Cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Tampieri, M.P.; Galuppi, R.; MacChioni, F.; Carelle, M.S.; Falcioni, L.; Cioni, P.L.; Morelli, I. The Inhibition of Candida Albicans by Selected Essential Oils and Their Major Components. Mycopathologia 2005, 159, 339–345. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between Components of the Essential Oil of Melaleuca Alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Caetano, É.P.; de Lima, R.A.C.; Marques, F.J.D.F.; Castelo-Branco, D.D.S.C.M.; Melo, C.V.S.D.; Guedes, G.M.D.M.; Oliveira, J.S.D.; Camargo, Z.P.D.; Moreira, J.L.B.; et al. Terpinen-4-Ol, Tyrosol, and β-Lapachone as Potential Antifungals against Dimorphic Fungi. Braz. J. Microbiol. 2016, 47, 917. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Zhang, L.; An, P.; Qi, J.; Yu, X.; Lu, J.; Ren, X. Antifungal Mechanisms of α-Terpineol and Terpene-4-Alcohol as the Critical Components of Melaleuca alternifolia Oil in the Inhibition of Rot Disease Caused by Aspergillus ochraceus in Postharvest Grapes. J. Appl. Microbiol. 2019, 126, 1161–1174. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In Vitro and In Vivo Inhibitory Activity of Limonene against Different Isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. Essential Oil to Inhibit the Growth of Food Spoiling Yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017. [Google Scholar]
- Touati, I.; Ruiz, N.; Thomas, O.; Druzhinina, I.S.; Atanasova, L.; Tabbene, O.; Elkahoui, S.; Benzekri, R.; Bouslama, L.; Pouchus, Y.F.; et al. Hyporientalin A, an Anti-Candida Peptaibol from a Marine Trichoderma Orientale. World J. Microbiol. Biotechnol. 2018, 34, 98. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).