The Impact of Point-of-Care Blood C-Reactive Protein Testing on Prescribing Antibiotics in Out-of-Hours Primary Care: A Mixed Methods Evaluation
Abstract
:1. Introduction
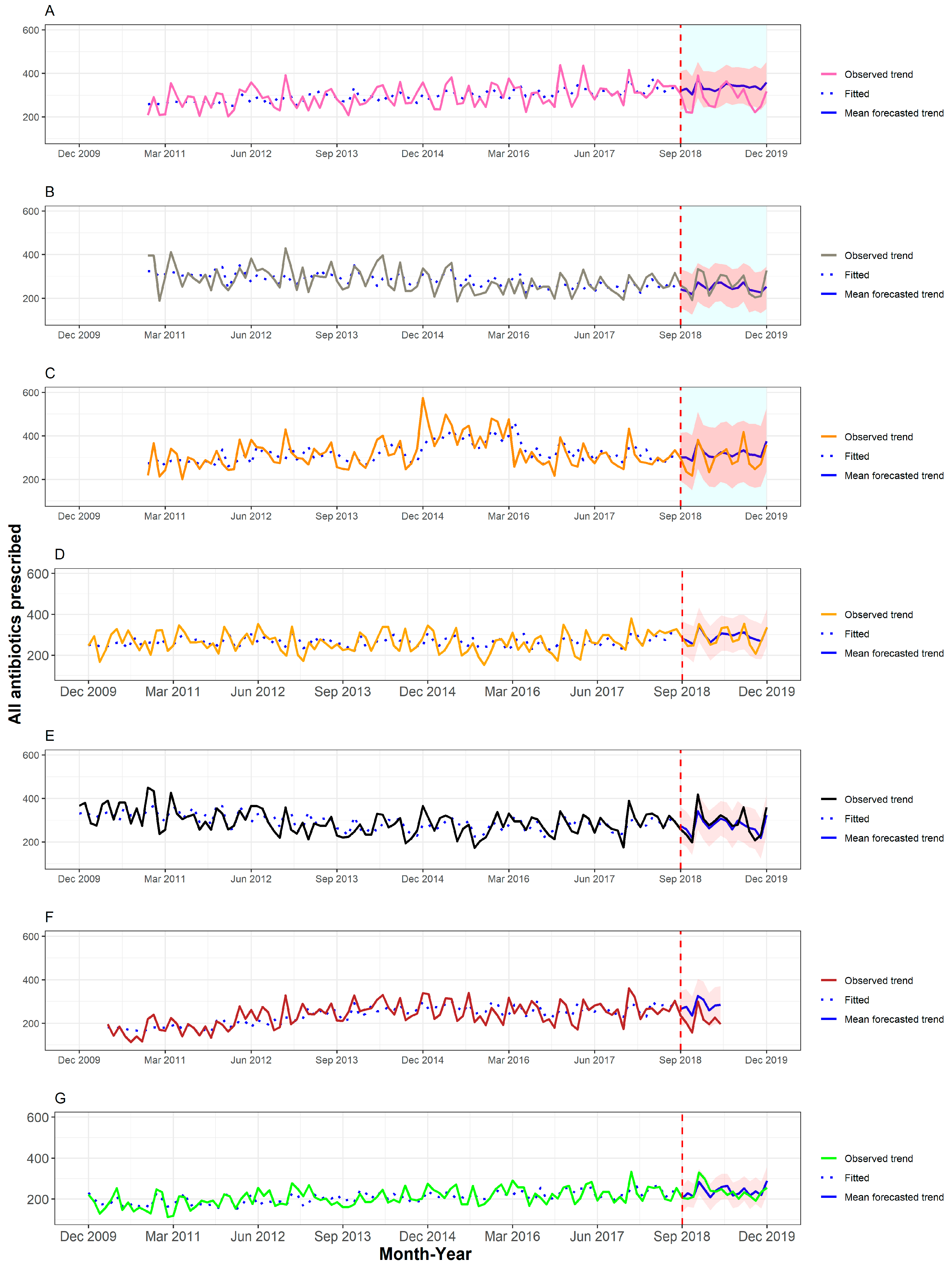
2. Results
2.1. Antibiotic Prescribing
2.2. CRP Test Use
2.3. Qualitative Findings
2.4. The Potential Role(s) for CRP POC Testing in OOH Care
[I]t has made a difference to who I prescribe for, and you know plenty of people who I’d before have said, “Oh go away, you’re absolutely fine,” CRP’s of 75 got standby scripts to take home with them just in case things were getting worse in 48 h And some people I would have prescribed for before just didn’t get any antibiotics...your CRP is less than 5, I know it is viral. Clinician 2 (GP).
The patients seem quite happy with that, once they’ve got something there to look at. I think having the CRP machine is something to back up what you’re saying to the patient. It just helps me to know that I’ve made the right decision as well. Clinician 8 (AHP)
2.5. Considerations about Test Usage
I mean given the time constraints, cos you know you can be up to your neck in patients, and you’ve got a waiting room full of patients, and if I’m just faffing around taking blood and taking it there.. I know it doesn’t take very long, but even so it’s still an extra… Clinician 5 (GP)
You have to leave the patient in the room and [] come out and do the analysis and then I’ve left a patient on their own in my room with my bag there and everything, so practically it’s quite difficult. Maybe if it was actually on the desk it would be more practical than having to leave the patient and go through the waiting room. Clinician 5 (GP)
So, because it’s there [in my room] I tend to use it. If it wasn’t there, I probably wouldn’t walk all the way to the office… when you’ve only got 15 min, it’s two or three minutes more out of your time. Clinician 8 (AHP)
It’s just accuracy of things isn’t it, that you worry about sort of these machines and things….. And you’ve based your decision round that. Clinician 15 (AHP)
[Y]ou did do a CRP and someone comes back and it’s a high CRP, and then goes on to deteriorate, you didn’t send them in, Where do you stand there when you’ve got this, are you more likely to send people in then? I mean you kind of, you’ve got to know what you’re going to do with the results as well, if you start using it as a tool. [] it could go to increasing your uncertainty. Clinician 17 (GP)
2.6. Training Considerations
[F]ormal training is difficult because lots of people do one session every two weeks in the evening in addition to their day jobs, so training in out-of-hours is tricky. You know to catch everybody you need to run five sessions on something almost. Your chances of finding a time where the ten people who work most regularly are free – not that easy.. Clinician 2 (GP)
It’s always good to have something in black and white, and I’ll always follow it. It’s good to have guidelines, isn’t it? Clinician 12 (GP)
3. Discussion
3.1. Mixed Methods Integration
3.2. Strengths and Limitations
3.3. Comparison with Existing Literature
3.4. Implications for Research and Practice
4. Conclusions
5. Materials and Methods
6. Outcomes
6.1. Quantitative
6.2. Quantitative Data Collection and Analysis
6.3. Qualitative Data Collection and Analysis
6.4. Mixed Method Integration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Dregan, A.; Moore, M.V.; Ashworth, M.; Van Staa, T.; McCann, G.; Charlton, J.; Yardley, L.; Little, P.; McDermott, L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: Survey of 568 UK general practices. BMJ Open 2014, 4, e006245. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J. Antimicrob. Chemother. 2018, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, M.; Agbebiyi, A.; Ashiru-Oredope, D.; Hopkins, S. Trends and patterns in antibiotic prescribing among out-of-hours primary care providers in England, 2010–2014. J. Antimicrob. Chemother. 2017, 72, 3490–3495. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Bartholomeeusen, S.; Ryckebosch, P.; Coenen, S. Quality of antibiotic prescription during office hours and out-of-hours in Flemish primary care, using European quality indicators. Eur. J. Gen. Pract. 2014, 20, 114–120. [Google Scholar] [CrossRef]
- Cals, J.W.L.; Butler, C.C.; Hopstaken, R.M.; Hood, K.; Dinant, G.J. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: Cluster randomised trial. BMJ 2009, 338, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Holm, A.; Pedersen, S.S.; Nexoe, J.; Obel, N.; Nielsen, L.P.; Koldkjaer, O. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br. J. Gen. Pract. 2007, 57, 555–560. [Google Scholar]
- Van Der Meer, V.; Neven, A.K.; Van Den Broek, P.J.; Assendelft, W.J.J. Diagnostic value of C reactive protein in infections of the lower respiratory tract: Systematic review. BMJ 2005, 331, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, E.; Melbye, H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: An open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam. Pract. 2014, 15, 80. [Google Scholar] [CrossRef] [Green Version]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Stevens, F.; Zeijen, C.G.; Dinant, G.J.; van Vugt, C.; Cals, J.W. Access to diagnostic tests during GP out-of-hours care: A cross-sectional study of all GP out-of-hours services in the Netherlands. Eur. J. Gen. Pract. 2016, 22, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schols, A.; Dinant, G.J.; Cals, J.W.L. Point-of-care testing in general practice: Just what the doctor ordered? Br. J. Gen. Pract. 2018, 68, 362–363. [Google Scholar] [CrossRef] [Green Version]
- Hayward, G.; Dixon, S.; Garland, S.; Glogowska, M.; Hunt, H.; Lasserson, D. Point-of-care blood tests during home visits by out-of-hours primary care clinicians; A mixed methods evaluation of a service improvement. BMJ Open 2020, 10, e033428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schot, M.J.; Van den Bruel, A.; Broekhuizen, B.D.; Cals, J.W.; Noteboom, E.A.; Balemans, W. Point-of-care C-reactive protein to assist in primary care management of children with suspected non-serious lower respiratory tract infection: A randomised controlled trial. BJGP Open 2018, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C. Point-of-care C-reactive protein testing to optimise antibiotic use in a primary care urgent care centre setting. BMJ Open Qual. 2018, 7, e000391. [Google Scholar] [CrossRef] [Green Version]
- Practice Plus Group. Out of Hours Services. Available online: https://practiceplusgroup.com/our-services/integrated-urgent-care/out-of-hours-services/ (accessed on 17 July 2022).
- Abbott. Afinion CRP. 2022. Available online: https://www.globalpointofcare.abbott/en/product-details/afinion-crp.html (accessed on 17 July 2022).
- Tonkin-Crine, S.K.; San Tan, P.; van Hecke, O.; Wang, K.; Roberts, N.W.; McCullough, A.; Hansen, M.P.; Butler, C.C.; Del Mar, C.B. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: An overview of systematic reviews. Cochrane Database Syst. Rev. 2017, CD012252. [Google Scholar] [CrossRef] [Green Version]
- Aabenhus, R.; Jensen, J.U.S.; Jørgensen, K.J.; Hróbjartsson, A.; Bjerrum, L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2014, CD010130. [Google Scholar] [CrossRef]
- Martínez-González, N.A.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.Y.J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-care c-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: Systematic review and meta-analysis of randomised controlled trials. Antibiotics 2020, 9, 610. [Google Scholar] [CrossRef]
- Cooke, J.; Butler, C.; Hopstaken, R.; Dryden, M.S.; McNulty, C.; Hurding, S.; Moore, M.; Livermore, D.M. Narrative review of primary care point-of- care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir. Res. 2015, 2, e000086. [Google Scholar] [CrossRef]
- Eley, C.V.; Sharma, A.; Lee, H.; Charlett, A.; Owens, R.; McNulty, C.A.M. Effects of primary care C-reactive protein point-of-care testing on antibiotic prescribing by general practice staff: Pragmatic randomised controlled trial, England, 2016 and 2017. Eurosurveillance 2020, 25, e1900408. [Google Scholar] [CrossRef] [PubMed]
- Debets, V.E.; Verheij, T.J.; van der Velden, A.W. Antibiotic prescribing during office hours and out-of-hours: A comparison of quality and quantity in primary care in the Netherlands. Br. J. Gen. Pract. 2017, 67, e178–e186. [Google Scholar] [CrossRef] [PubMed]
- Hayward, G.N.; Fisher, R.F.; Spence, G.T.; Lasserson, D.S. Increase in antibiotic prescriptions in out-of-hours primary care in contrast to in-hours primary care prescriptions: Service evaluation in a population of 600,000 patients. J. Antimicrob. Chemother. 2016, 71, 2612–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balla, J.; Heneghan, C.; Thompson, M.; Balla, M. Clinical decision making in a high-risk primary care environment: A qualitative study in the UK. BMJ Open 2012, 2, e000414. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.J.; Halls, A.V.; Tonkin-Crine, S.; Moore, M.V.; Latter, S.E.; Little, P.; Eyles, C.; Postle, K.; Leydon, G.M. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J. Antimicrob. Chemother. 2018, 73, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Howick, J.; Cals, J.W.; Jones, C.; Price, C.P.; Plüddemann, A.; Heneghan, C.; Berg, M.Y.; Buntinx, F.; Hickner, J.; Pace WBadrick, T. Current and future use of point-of-care tests in primary care: An international survey in Australia, Belgium, The Netherlands, the UK and the USA. BMJ Open 2014, 4, e005611. [Google Scholar] [CrossRef]
- Dixon, S.; Glogowska, M.; Garland, S.; Hunt, H.; Lasserson, D.; Hayward, G. Clinician perspectives on having point of care tests made available to them during out of hours home visiting. BMC Fam. Pract. 2021, 22, 246. [Google Scholar] [CrossRef]
- Butler, C.C.; Simpson, S.; Wood, F. General practitioners’ perceptions of introducing near-patient testing for common infections into routine primary care: A qualitative study. Scand. J. Prim. Health Care 2008, 26, 17–21. [Google Scholar] [CrossRef]
- Jones, C.H.; Howick, J.; Roberts, N.W.; Price, C.P.; Heneghan, C.; Plüddemann, A.; Thompson, M. Primary care clinicians’ attitudes towards point-of-care blood testing: A systematic review of qualitative studies. BMC Fam. Pract. 2013, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Di Gennaro, F.; Marotta, C.; Amicone, M.; Bavaro, D.F.; Bernaudo, F.; Frisicale, E.M.; Kurotschka, P.K.; Mazzari, A.; Veronese, N.; Murri, R.; et al. Italian young doctors’ knowledge, attitudes and practices on antibiotic use and resistance: A national cross-sectional survey. J. Glob. Antimicrob. Resist. 2020, 23, 167–173. [Google Scholar] [CrossRef]
- Alves, P.G.; Hayward, G.; Leydon, G.; Barnes, R.; Woods, C.; Webb, J.; Booker, M.; Ireton, H.; Latter, S.; Little, P.; et al. Antibiotic prescribing in UK out-of-hours primary care services: A realist-informed scoping review of training and guidelines for healthcare professionals. BJGP Open 2021, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Fanshawe, T.R.; Turner, P.J.; Gillespie, M.M.; Hayward, G.N. The comparative interrupted time series design for assessment of diagnostic impact: Methodological considerations and an example using point-of-care C-reactive protein testing. Diagnostic Progn. Res. 2022, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 17 July 2022).
- Hyndman, R.; Athanasopoulos, G.; Bergmeir, C.; Caceres, G.; Chhay, L.; O’Hara-Wild, M.; Petropoulos, F.; Razbash, S. Forecast: Forecasting Functions for Time Series and Linear Models. R Package Version 8.16. 2022. Available online: https://pkg.robjhyndman.com/forecast/ (accessed on 17 July 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 17 July 2022).
- Pope, C.; Ziebland, S.; Mays, N. Qualitative research in health care: Analysing qualitative data. BMJ Br. Med. J. 2000, 320, 114. [Google Scholar] [CrossRef] [PubMed]
- Ziebland, S.; McPherson, A. Making sense of qualitative data analysis: An introduction with illustrations from DIPEx (personal experiences of health and illness). Med. Educ. 2006, 40, 405–414. [Google Scholar] [CrossRef]

| Reason | N | % |
|---|---|---|
| Lower respiratory tract infection | 108 | 71% |
| Not reported | 10 | 7% |
| Cough | 5 | 3% |
| Abdominal symptoms | 5 | 3% |
| Upper respiratory tract infection | 4 | 3% |
| Reassurance or advice | 4 | 3% |
| Sinusitis | 2 | 1% |
| Temporal arteritis | 2 | 1% |
| Tonsillitis | 2 | 1% |
| Confusion | 1 | 1% |
| Cystic Fibrosis | 1 | 1% |
| Diverticulitis | 1 | 1% |
| Knee pain post-operation | 1 | 1% |
| Meningitis | 1 | 1% |
| Recurrent ear pain/headache | 1 | 1% |
| Sepsis | 1 | 1% |
| Urinary tract infection | 1 | 1% |
| Uvulitis | 1 | 1% |
| Vasculitis | 1 | 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, S.; Fanshawe, T.R.; Mwandigha, L.; Edwards, G.; Turner, P.J.; Glogowska, M.; Gillespie, M.M.; Blair, D.; Hayward, G.N. The Impact of Point-of-Care Blood C-Reactive Protein Testing on Prescribing Antibiotics in Out-of-Hours Primary Care: A Mixed Methods Evaluation. Antibiotics 2022, 11, 1008. https://doi.org/10.3390/antibiotics11081008
Dixon S, Fanshawe TR, Mwandigha L, Edwards G, Turner PJ, Glogowska M, Gillespie MM, Blair D, Hayward GN. The Impact of Point-of-Care Blood C-Reactive Protein Testing on Prescribing Antibiotics in Out-of-Hours Primary Care: A Mixed Methods Evaluation. Antibiotics. 2022; 11(8):1008. https://doi.org/10.3390/antibiotics11081008
Chicago/Turabian StyleDixon, Sharon, Thomas R. Fanshawe, Lazaro Mwandigha, George Edwards, Philip J. Turner, Margaret Glogowska, Marjorie M. Gillespie, Duncan Blair, and Gail N. Hayward. 2022. "The Impact of Point-of-Care Blood C-Reactive Protein Testing on Prescribing Antibiotics in Out-of-Hours Primary Care: A Mixed Methods Evaluation" Antibiotics 11, no. 8: 1008. https://doi.org/10.3390/antibiotics11081008
APA StyleDixon, S., Fanshawe, T. R., Mwandigha, L., Edwards, G., Turner, P. J., Glogowska, M., Gillespie, M. M., Blair, D., & Hayward, G. N. (2022). The Impact of Point-of-Care Blood C-Reactive Protein Testing on Prescribing Antibiotics in Out-of-Hours Primary Care: A Mixed Methods Evaluation. Antibiotics, 11(8), 1008. https://doi.org/10.3390/antibiotics11081008






