Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Antibiotic Preparation and Exposure
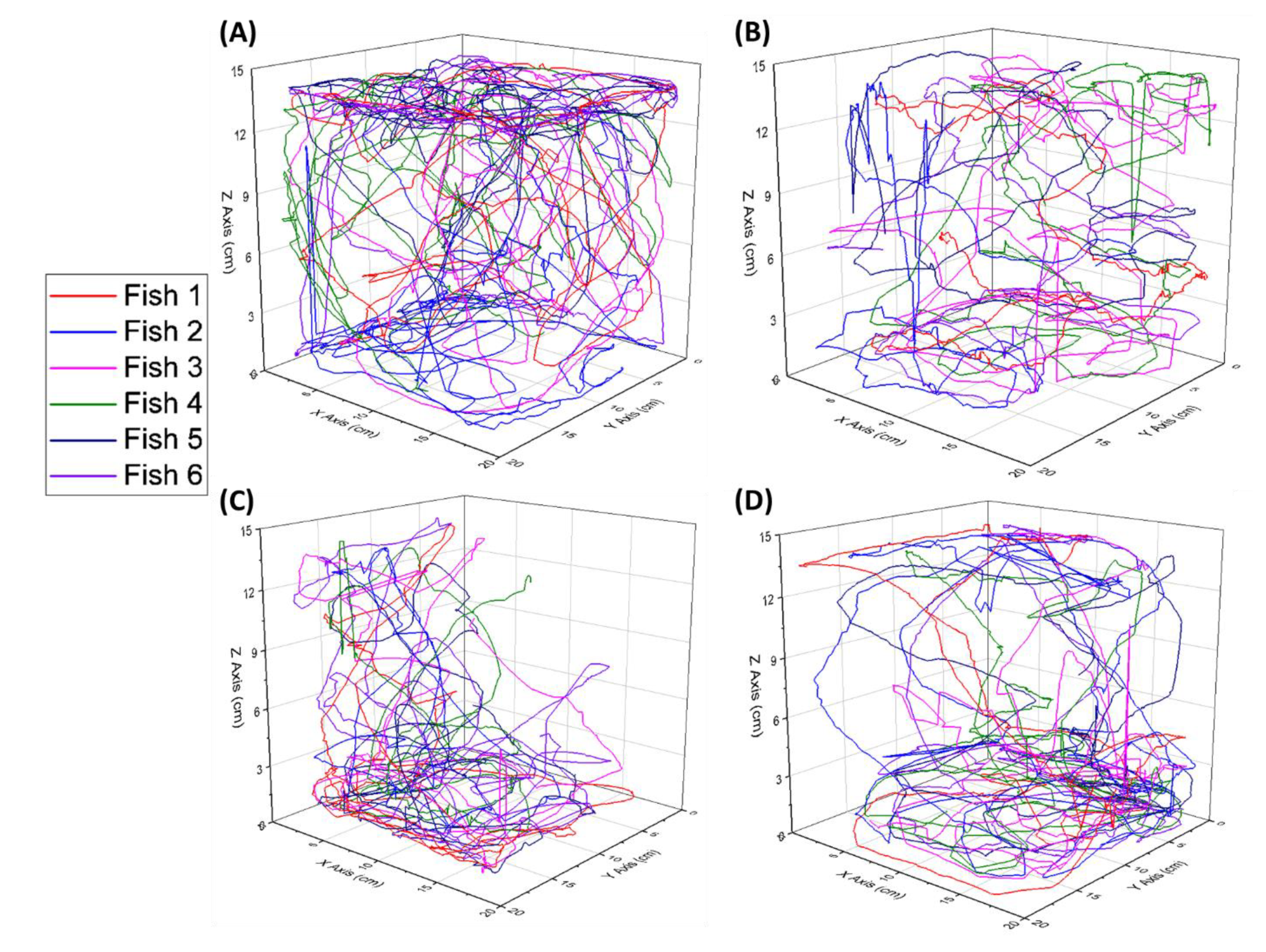
2.3. Three-Dimensional (3D) Locomotion Tracking
2.4. Locomotion Trajectory Analysis by Using VBA Macro
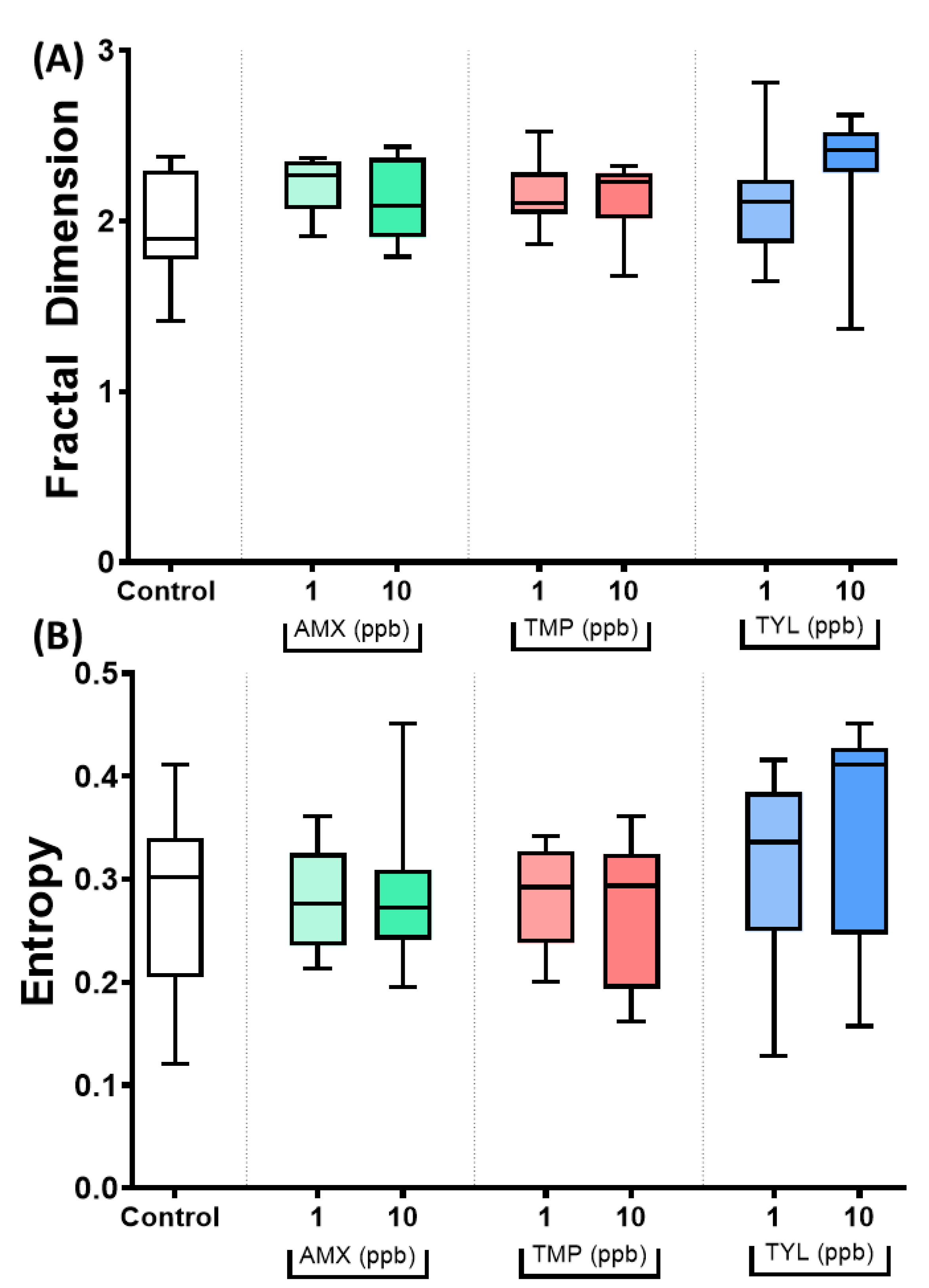
2.5. Fractal Dimension and Entropy Analysis
2.6. Principal Component Analysis (PCA) and Hierarchy Clustering
2.7. Statistics
3. Results
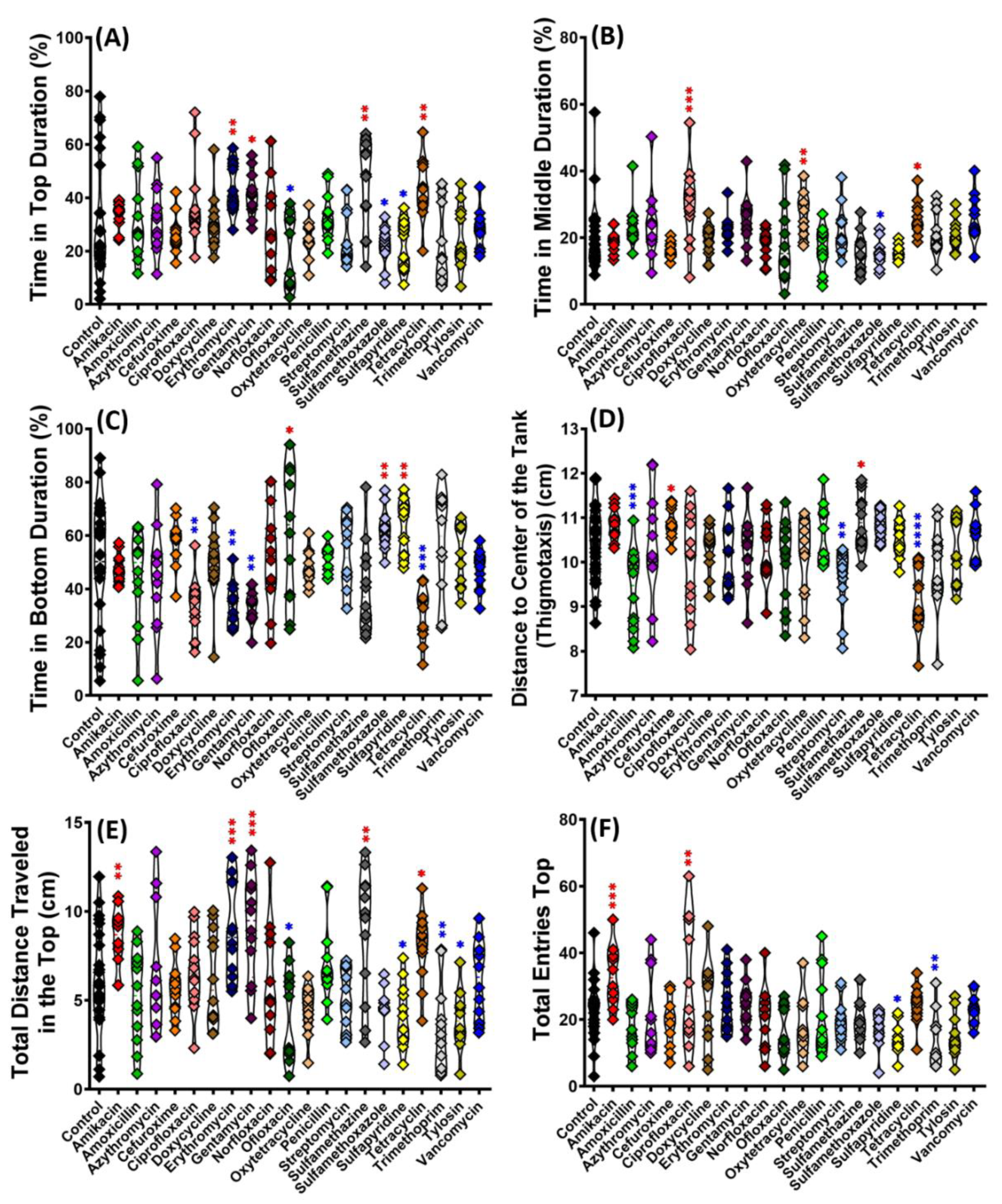
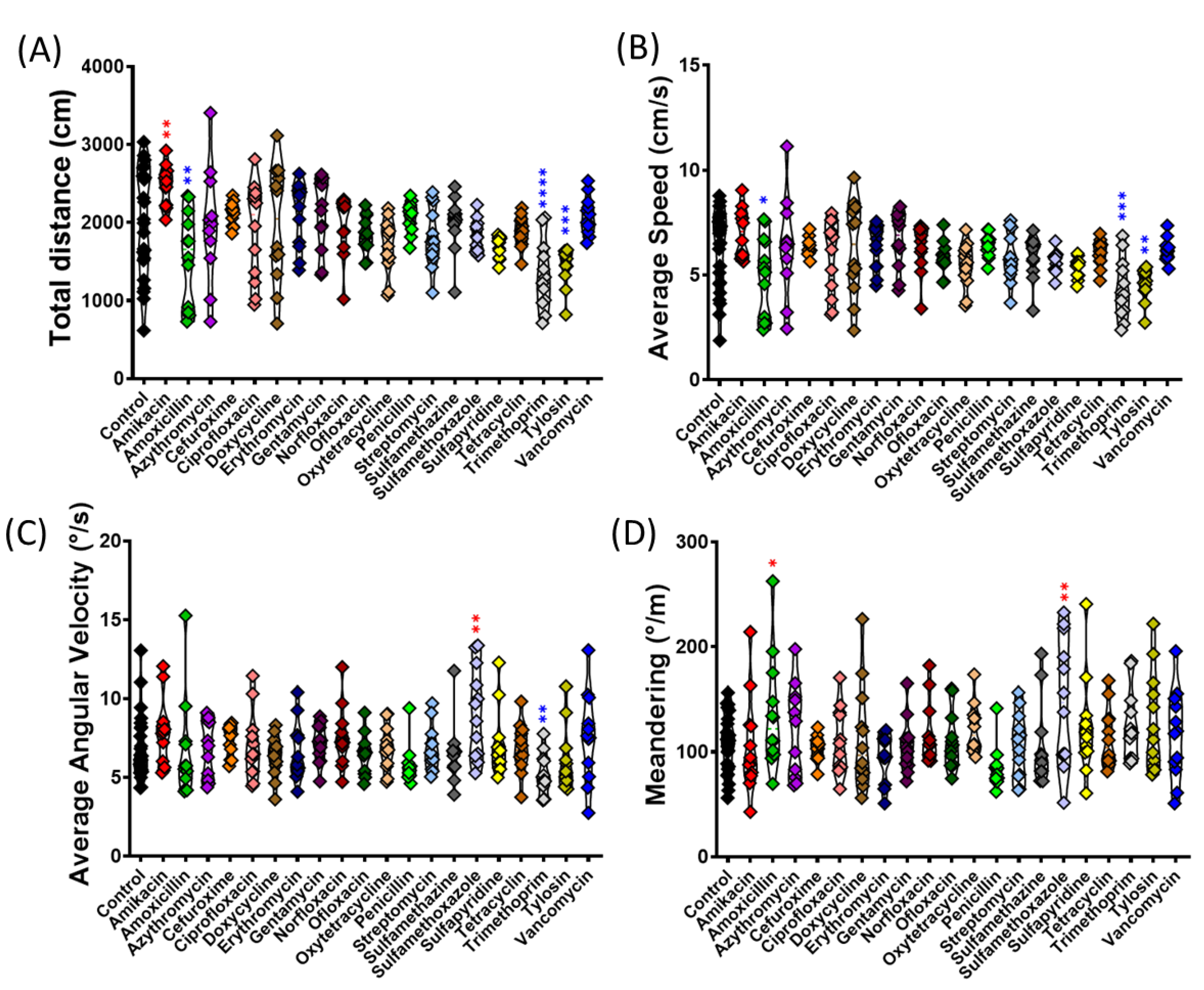
3.1. Swimming Movement Activity Assessment by 3D Locomotion Test
3.2. Exploratory Behavior Assessment by 3D Locomotion Test
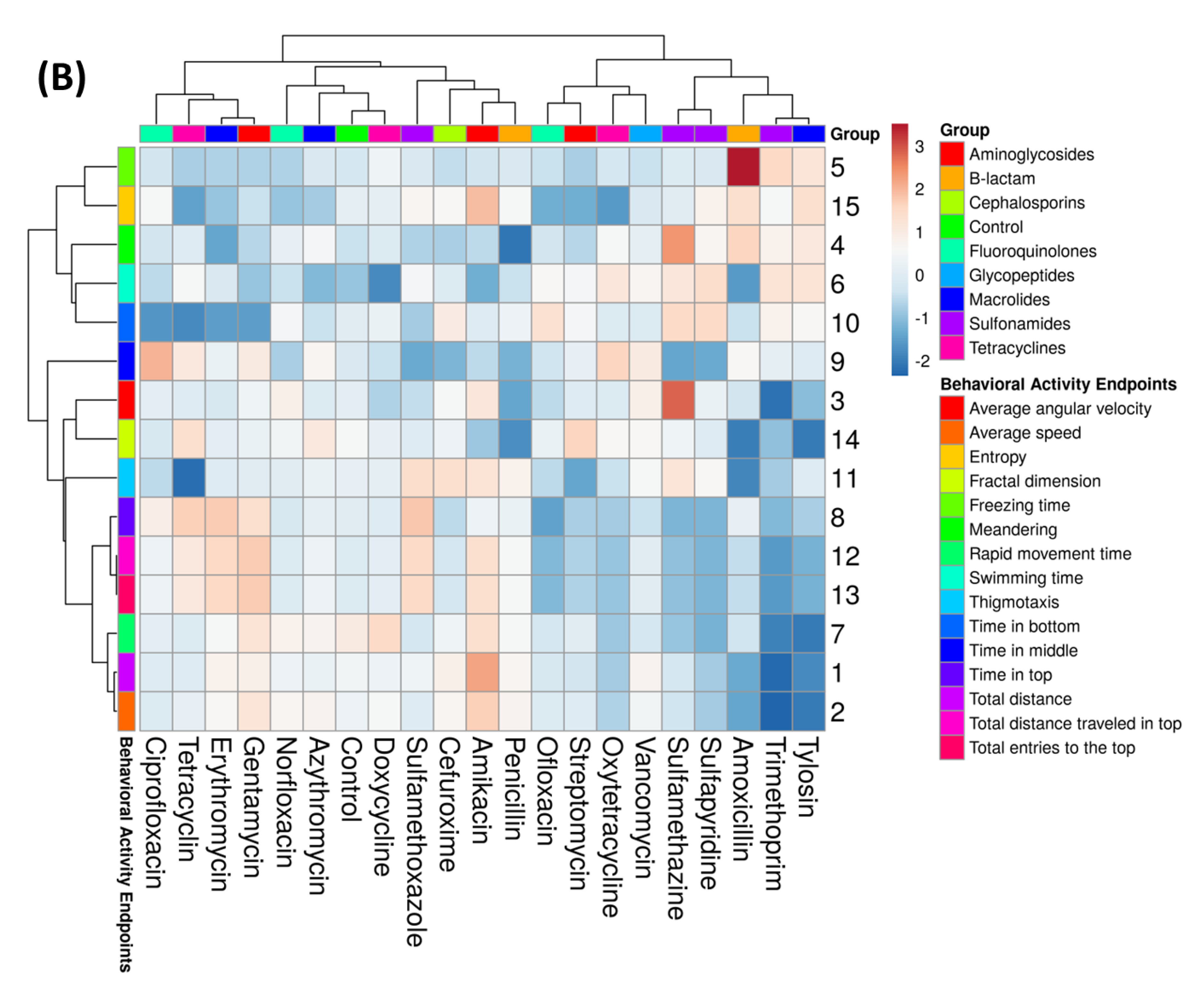
3.3. Dimensional Reduction Assessment of Behavioral Alterations after Antibiotic Exposure
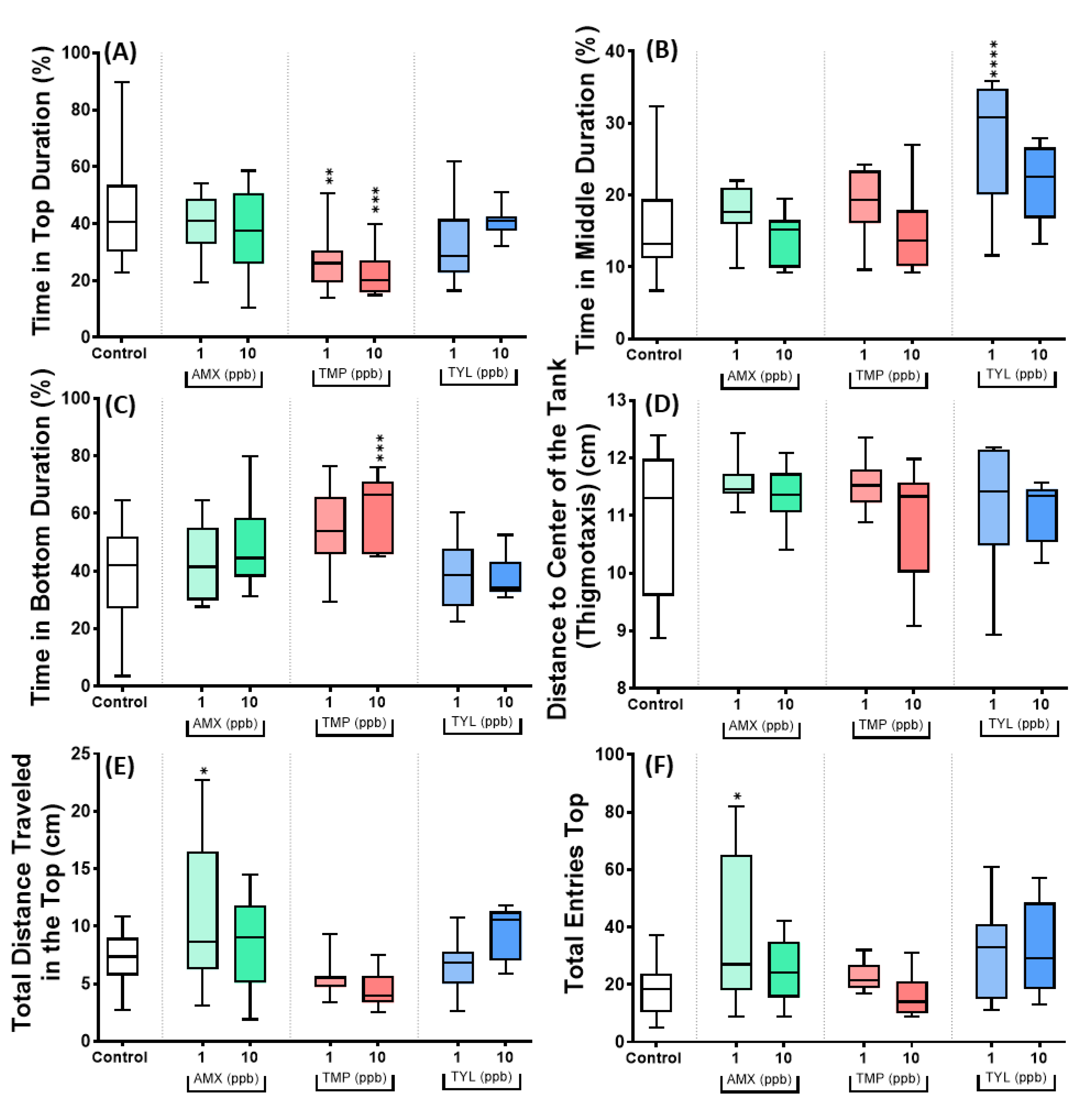
3.4. Data Validation for Three Selected Antibiotics of Amoxicillin, Trimethoprim and Tylosin
4. Discussion
4.1. Several Antibiotics Were Not Induced Toxicity Behavior Alterations
4.2. Most of the Antibiotic-Induced Behavioral Changes in Locomotor Activity as Their Toxicity Was Also Reported in Previous Studies
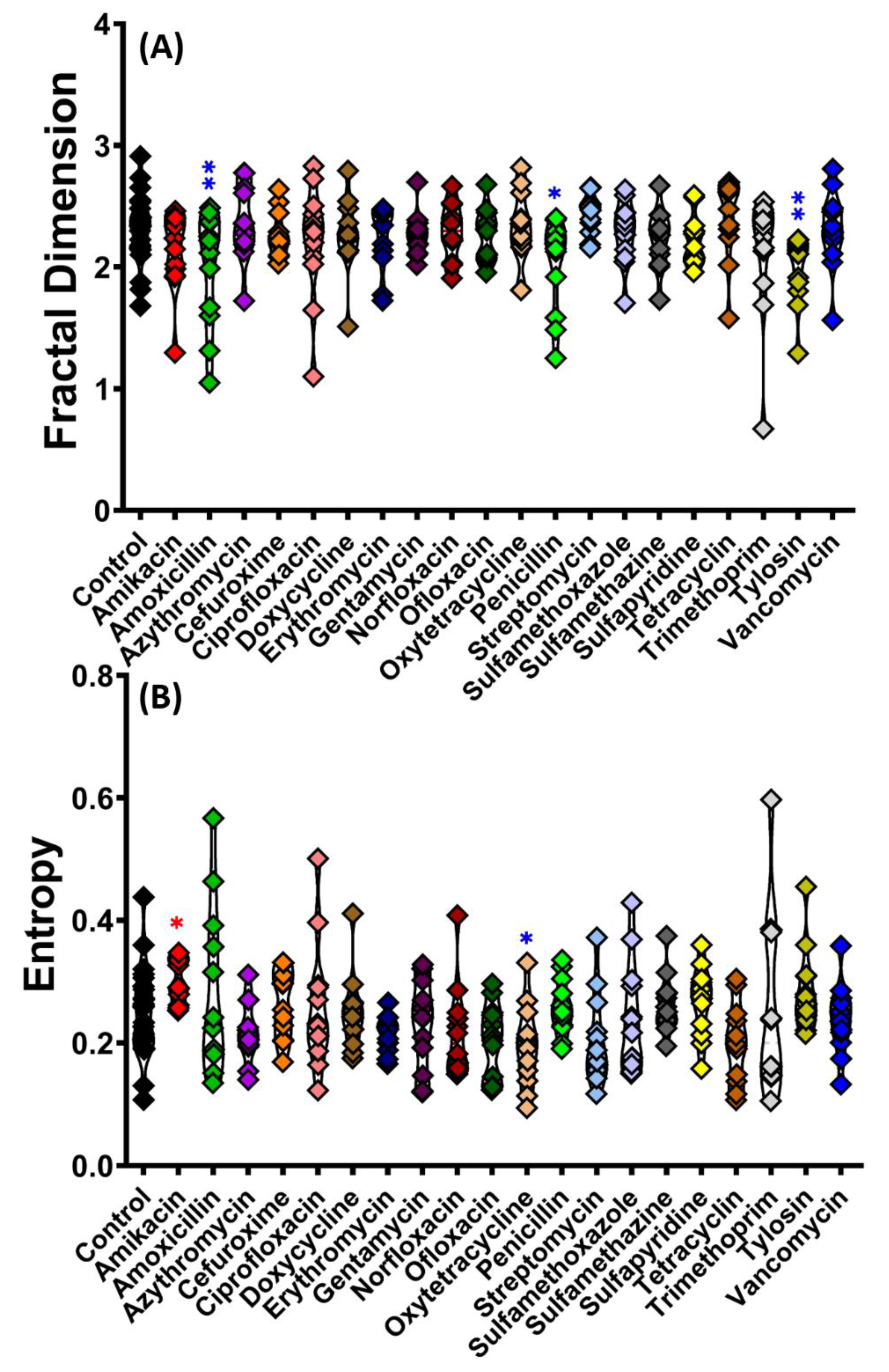
4.3. Fractal Dimension and Entropy Value Served as Algorithms to Identify the Differences in Fish Responses Exposed to Antibiotic
4.4. Amoxicillin, Trimethoprim, and Tylosin Caused Contrast Behavioral Effects Compared to Other Antibiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| No. | Class | Antibiotics | CAS No. | Purity (%) | Half-Life in Water (days) | Defined Daily Dose (DDD) * (g) | Concentration (s) Used in This Study (ppb) |
|---|---|---|---|---|---|---|---|
| 1 | Aminoglycosides | Amikacin | 37517-28-5 | 98 | 2–3 | 1 | 100 |
| Gentamycin | 1405-41-0 | 98 | 1–4 | 0.24 | 100 | ||
| Streptomycin | 3810-74-0 | 95 | 30.9 | 1 | 100 | ||
| 2 | Cephalosporins | Cefuroxime | 55268-75-2 | 99 | 2.8–3.3 | 0.5 | 100 |
| 3 | Fluoroquinolones | Ciprofloxacin | 86393-32-0 | 98 | 0.5–1 | 1 | 100 |
| Norfloxacin | 68077-27-0 | 98 | 5.3-5.9 | 0.8 | 100 | ||
| Ofloxacin | 82419-36-1 | 99 | 0.83–1 | 0.4 | 100 | ||
| 4 | Glycopeptides | Vancomycin | 1404-93-9 | 99 | 9–10 | 2 | 100 |
| 5 | Macrolides | Azithromycin | 83905-01-5 | 98 | 0.05–0.83 | 0.3 | 100 |
| Erythromycin | 114-07-8 | 95 | 5–365 | 1 | 100 | ||
| Tylosin | 1401-69-0 | 99 | 1.2–4.7 | 1.2 | 1, 10, 100 | ||
| 6 | Sulfonamides | Sulfamethazine | 1981-58-4 | 98 | 2.7–4.2 | 0.5 | 100 |
| Sulfamethoxazole | 723-46-6 | 98 | 0.42–2.4 | 2 | 100 | ||
| Sulfapyridine | 144-83-2 | 99 | 0.25–0.58 | 1 | 100 | ||
| Trimethoprim | 60834-30-2 | 98 | 20–100 | 0.4 | 1, 10, 100 | ||
| 7 | Tetracyclines | Doxycycline | 564-25-0 | 98 | 7–14 | 0.1 | 100 |
| Oxytetracycline | 2058-46-0 | 95 | 0.26–9 | 1 | 100 | ||
| Tetracycline | 64-75-5 | 99 | 20.6–106.6 | 1 | 100 | ||
| 8 | β-lactam | Amoxicillin | 26787-78-0 | 99 | 0.9–3.3 | 3 | 1, 10, 100 |
| Penicillin G | 113-98-4 | 96 | 4.1–11 | 3.6 | 100 |
| No. | Behavior Endpoints | Definition | Interpretation |
|---|---|---|---|
| 1 | Total distance | The total distance traveled by zebrafish in the novel tank | This phenotype is representative of general motor and neurological characteristics. In general, zebrafish are very sensitive to non-specific motor disorders and the sedative effects of drugs |
| 2 | Average speed | Magnitude and direction of zebrafish speed | Based on the nature of the behavioral test, this value may be increased or decreased to reflect motor aspects of the zebrafish swimming |
| 3 | Average angular velocity | Angle of angular speed of zebrafish measured in magnitude and direction | Based on the nature of the behavioral test, this value may be increased or decreased to reflect motor aspects of the zebrafish swimming |
| 4 | Meandering | Turning degree (compared to straight locomotion) | Based on the nature of the behavioral test, this value may be increased or decreased to reflect motor aspects of the zebrafish swimming |
| 5 | Freezing time | The total duration of all freezing episodes (immobility) | Generally higher among stressed zebrafish and indicates increased anxiety |
| 6 | Swimming time | Total duration of all normal swimming bouts | Indicates a normal behavior and commonly observed, which may be maintained for minutes or hours |
| 7 | Rapid movement time | Total duration of all high swimming movement above the average speed | May be part of dashing or erratic movement as part of alarm reaction, typically triggered by acute stressors or characterized by baseline anxiety/fear |
| 8 | Time in top | The total amount of time spent in the upper portion of the novel tank | Low anxiety levels are reflected in a longer duration in the top of the tank |
| 9 | Time in middle | The total amount of time spent in the middle portion of the novel tank | Zebrafish dive to the bottom of novel tank as soon as they are introduced to a novel environment and start exploring as they become accustomed to it |
| 10 | Time in bottom | Total time spent in the lower portion of the novel tank | Zebrafish dive to the bottom of novel tank as soon as they are introduced to a novel environment and start exploring as they become accustomed to it |
| 11 | Thigmotaxis | A tendency to stay near the edge or side of an area (and avoid the center) | Zebrafish anxiety is measured by this parameter |
| 12 | Total distance traveled in top | The total distance traveled within the top portion of the defined area | Zebrafish with significant anxiety would move further in the tank’s bottom area |
| 13 | Total entries to the top | The number of crosses that run from the bottom of the novel tank to the top area. | Lower anxiety levels are associated with more top entries. |
| 14 | Fractal dimension | The variations or patterns in spatial complexity of zebrafish movement | Lower value indicates a decrease in complexity as a result of stressful or painful therapy, with arbitrary points reflecting the consequences of stress and mild, moderate, and severe pain |
| 15 | Entropy | Detecting behavioral changes in response to stressors by elucidating the time-space organized characteristic in behavioral data | A valuable tool for assessing behavioral complexity and tracking the influence of environmental stresses. The entropy decreases with increasing perturbation of the fish |
References
- Chen, H.; Liu, S.; Xu, X.-R.; Diao, Z.-H.; Sun, K.-F.; Hao, Q.-W.; Liu, S.-S.; Ying, G.-G. Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J. Hazard. Mater. 2018, 343, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.D.; Pereira, T.C.B.; Altenhofen, S.; Nabinger, D.D.; de Abreu Ferreira, P.M.; Bogo, M.R.; Bonan, C.D. Antibiotic drugs alter zebrafish behavior. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Steele, J.C.; Meng, X.-Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.; Henriksson, P.J.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; Van Den Brink, P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Pei, J.; Zhang, R.; Wang, S.; Zeng, W.; Huang, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yu, K. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018, 154, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhang, R.-Q.; Lai, H.-J.; Chen, Z.-F.; Pan, C.-G. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013, 444, 183–195. [Google Scholar] [CrossRef]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Liu, C.-Q.; Li, L.; Xiang, M. The occurrence of chloramphenicol and tetracyclines in municipal sewage and the Nanming River, Guiyang City, China. J. Environ. Monit. 2009, 11, 1199–1205. [Google Scholar] [CrossRef]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. Int. J. 2005, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Liu, J.; Yan, Z.; Ma, B.; Zhang, Z.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Antibiotics and antimicrobials phylogeny. Cap. Med. 2004, 2, 14–16. [Google Scholar]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Chung, J.K. Agriculture, Forestry. In The Research of the Antibiotics Reduction in Aquarium and the Environmental Influence of Parasiticide; Ministry of Food, Agriculture, Forestry Fisheries Pukyong National University Press: Busan, Korea, 2008. [Google Scholar]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; World Health Organization Advisory Group; Agerso, Y.; Andremont, A.; Collignon, P.; Conly, J.; et al. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance from Food Animal Production. In Clinical Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 63, pp. 1087–1093. [Google Scholar]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.-B. Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef]
- Legradi, J.; Di Paolo, C.; Kraak, M.; Van der Geest, H.; Schymanski, E.; Williams, A.; Dingemans, M.; Massei, R.; Brack, W.; Cousin, X. An ecotoxicological view on neurotoxicity assessment. Environ. Sci. Eur. 2018, 30, 1–34. [Google Scholar] [CrossRef]
- Michelotti, P.; Quadros, V.A.; Pereira, M.E.; Rosemberg, D.B. Ketamine modulates aggressive behavior in adult zebrafish. Neurosci. Lett. 2018, 684, 164–168. [Google Scholar] [CrossRef]
- Jijie, R.; Mihalache, G.; Balmus, I.-M.; Strungaru, S.-A.; Baltag, E.S.; Ciobica, A.; Nicoara, M.; Faggio, C. Zebrafish as a Screening Model to Study the Single and Joint Effects of Antibiotics. Pharmaceuticals 2021, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; von Keyserlingk, M.A.; Franks, B. Zebrafish welfare: Natural history, social motivation and behaviour. Appl. Anim. Behav. Sci. 2018, 200, 13–22. [Google Scholar] [CrossRef]
- Suriyampola, P.S.; Shelton, D.S.; Shukla, R.; Roy, T.; Bhat, A.; Martins, E.P. Zebrafish social behavior in the wild. Zebrafish 2016, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zheng, L.; Zhou, J.L. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio). Chemosphere 2017, 182, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Schnörr, S.; Steenbergen, P.; Richardson, M.; Champagne, D. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- da Silva Santos, N.; Oliveira, R.; Lisboa, C.A.; e Pinto, J.M.; Sousa-Moura, D.; Camargo, N.S.; Perillo, V.; Oliveira, M.; Grisolia, C.K.; Domingues, I. Chronic effects of carbamazepine on zebrafish: Behavioral, reproductive and biochemical endpoints. Ecotoxicol. Environ. Saf. 2018, 164, 297–304. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Audira, G.; Lai, Y.H.; Huang, J.C.; Chen, K.H.C.; Hsiao, C.D. Phenomics Approach to Investigate Behavioral Toxicity of Environmental or Occupational Toxicants in Adult Zebrafish (Danio rerio). Curr. Protoc. 2021, 1, e223. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Kalueff, A.V. Of mice and zebrafish: The impact of the experimenter identity on animal behavior. Lab Anim. 2021, 50, 7. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. JoVE 2012, 69, e4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.Y.-C.; Yu, T.-H.; Lin, C.-F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- aan het Rot, M.; Mathew, S.J.; Charney, D.S. Neurobiological mechanisms in major depressive disorder. Cmaj 2009, 180, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Amao, A.M. Introduction to Computing and VBA. Available online: http://fac.ksu.edu.sa/sites/default/files/1-introductiontocomputingvba_0.pdf (accessed on 23 March 2021).
- Jacobson, R. Microsoft Office Excel 2007 Visual Basic for Applications Step by Step; Pearson Education: London, UK, 2007. [Google Scholar]
- Audira, G.; Suryanto, M.E.; Chen, K.H.-C.; Vasquez, R.D.; Roldan, M.J.M.; Yang, C.-C.; Hsiao, C.-D.; Huang, J.-C. Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration. Biology 2022, 11, 969. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Shiogiri, N.S.; Ikefuti, C.V.; Carraschi, S.P.; da Cruz, C.; Fernandes, M.N. Effects of azithromycin on tilapia (Oreochromis niloticus): Health status evaluation using biochemical, physiological and morphological biomarkers. Aquac. Res. 2017, 48, 3669–3683. [Google Scholar] [CrossRef]
- Fairgrieve, W.T.; Masada, C.L.; McAuley, W.C.; Peterson, M.E.; Myers, M.S.; Strom, M.S. Accumulation and clearance of orally administered erythromycin and its derivative, azithromycin, in juvenile fall Chinook salmonOncorhynchus tshawytscha. Dis. Aquat. Org. 2005, 64, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Delyfer, M.-N.; Rougier, M.-B.; Leoni, S.; Zhang, Q.; Dalbon, F.; Colin, J.; Korobelnik, J.-F. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J. Cataract Refract. Surg. 2011, 37, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.S.; Hyldig, N.; Krych, Ł.; Greisen, G.; Krogfelt, K.A.; Zachariassen, G.; Nielsen, D.S. Impact of early exposure to cefuroxime on the composition of the gut microbiota in infants following cesarean delivery. J. Pediatrics 2019, 210, 99–105.e2. [Google Scholar] [CrossRef] [PubMed]
- Vutukuru, S. Doxycycline Induced Alterations in the Glycogen and Protein Content of the Zebra Fish, Danio rerio. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 1, 271220031. [Google Scholar]
- Wang, D.D.; Englot, D.J.; Garcia, P.A.; Lawton, M.T.; Young, W.L. Minocycline-and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav. 2012, 24, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Wang, F.; Li, K.; Nie, X.; Fang, H. Effects of norfloxacin nicotinate on the early life stage of zebrafish (Danio rerio): Developmental toxicity, oxidative stress and immunotoxicity. Fish Shellfish Immunol. 2020, 96, 262–269. [Google Scholar] [CrossRef]
- Rosenberg, C.R.; Fang, X.; Allison, K.R. Potentiating aminoglycoside antibiotics to reduce their toxic side effects. PLoS ONE 2020, 15, e0237948. [Google Scholar] [CrossRef]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, D.; Wu, D.; Xia, J.; Zhou, W.; Cui, C. Residual β-lactam antibiotics and ecotoxicity to Vibrio fischeri, Daphnia magna of pharmaceutical wastewater in the treatment process. J. Hazard. Mater. 2022, 425, 127840. [Google Scholar] [CrossRef]
- Bownik, A.; Ślaska, B.; Bochra, J.; Gumieniak, K.; Gałek, K. Procaine penicillin alters swimming behaviour and physiological parameters of Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 18662–18673. [Google Scholar] [CrossRef] [Green Version]
- Magdaleno, A.; Saenz, M.; Juárez, A.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histopathological effects in gills and liver of Sparus aurata following acute and chronic exposures to erythromycin and oxytetracycline. Environ. Sci. Pollut. Res. 2019, 26, 15481–15495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J. The effect of acute erythromycin exposure on the swimming ability of Zebrafish (Danio rerio) and medaka (Oryzias latipes). Int. J. Environ. Res. Public Health 2020, 17, 3389. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Hashmi, I. Assessment of immunohematological, hematological and biochemical responses in cultivable fish Cyprinus carpio exposed to an antibiotic sulfamethoxazole (SMX). J. Water Health 2021, 19, 108–119. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.; Gomes, R.; Campos, J.; Braga, M.; Ramos, A.; Correia, A. Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Keerthisinghe, T.P.; Wang, F.; Wang, M.; Yang, Q.; Li, J.; Yang, J.; Xi, L.; Dong, W.; Fang, M. Long-term exposure to TET increases body weight of juvenile zebrafish as indicated in host metabolism and gut microbiome. Environ. Int. 2020, 139, 105705. [Google Scholar] [CrossRef] [PubMed]
- Mandelbrot, B. How long is the coast of Britain? Statistical self-similarity and fractional dimension. Science 1967, 156, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Deakin, A.G.; Spencer, J.W.; Cossins, A.R.; Young, I.S.; Sneddon, L.U. Welfare challenges influence the complexity of movement: Fractal analysis of behaviour in zebrafish. Fishes 2019, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Eguiraun, H.; López-de-Ipiña, K.; Martinez, I. Application of entropy and fractal dimension analyses to the pattern recognition of contaminated fish responses in aquaculture. Entropy 2014, 16, 6133–6151. [Google Scholar] [CrossRef] [Green Version]
- Gisiger, T. Scale invariance in biology: Coincidence or footprint of a universal mechanism? Biol. Rev. 2001, 76, 161–209. [Google Scholar] [CrossRef] [Green Version]
- Reebs, S.G. Sleep in Fishes. 2008. Available online: http://www.howfishbehave.ca/pdf/sleep%20in%20fishes.pdf (accessed on 6 July 2021).
- Sigurgeirsson, B.; Þorsteinsson, H.; Sigmundsdóttir, S.; Lieder, R.; Sveinsdóttir, H.S.; Sigurjónsson, Ó.E.; Halldórsson, B.; Karlsson, K. Sleep–wake dynamics under extended light and extended dark conditions in adult zebrafish. Behav. Brain Res. 2013, 256, 377–390. [Google Scholar] [CrossRef]
- Sneddon, L.U. Evolution of nociception and pain: Evidence from fish models. Philos. Trans. R. Soc. B 2019, 374, 20190290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, J.-M.; Chon, T.-S.; Liu, Y.; Kim, H.; Bae, M.-J.; Park, Y.-S. Analysis of movement behavior of zebrafish (Danio rerio) under chemical stress using hidden Markov model. Mod. Phys. Lett. B 2013, 27, 1350014. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.; Tien, D.H. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses. In Zebrafish Neurobehavioral Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–14. [Google Scholar]
- Brinn, R.; Marcon, J.; McComb, D.; Gomes, L.; Abreu, J.; Baldisseroto, B. Stress responses of the endemic freshwater cururu stingray (Potamotrygon cf. histrix) during transportation in the Amazon region of the Rio Negro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D.; Wilson, S.P. The utility of behavioral studies for aquatic toxicology testing: A meta-analysis. Chemosphere 2013, 93, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C., Jr.; Day, N.P. Global antibiotic consumption and usage in humans, 2000–18: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Gonçalves, C.L.; Vasconcelos, F.F.; Wessler, L.B.; Lemos, I.S.; Candiotto, G.; Lin, J.; Matias, M.B.; Rico, E.P.; Streck, E.L. Exposure to a high dose of amoxicillin causes behavioral changes and oxidative stress in young zebrafish. Metab. Brain Dis. 2020, 35, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; McDonough, S.; Ladewig, J.C.; Soares, A.M.; Nogueira, A.J.; Domingues, I. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2013, 36, 903–912. [Google Scholar] [CrossRef]
- Deprey, K.L.; Uno, J.K. Amoxicillin decreases intestinal microbial diversity and increases stress-associated behaviors in zebrafish. FASEB J. 2016, 30, 1027.7. [Google Scholar]
- Rao, R.; Manu, B.; Thalla, A.K. Behavioral, Physical and Biochemical Responses Induced by Amoxicillin Exposure from Cyprinus carpio. Int. J. Earth Sci. Eng. 2017, 10, 673–676. [Google Scholar]
- Papis, E.; Davies, S.J.; Jha, A.N. Relative sensitivity of fish and mammalian cells to the antibiotic, trimethoprim: Cytotoxic and genotoxic responses as determined by neutral red retention, Comet and micronucleus assays. Ecotoxicology 2011, 20, 208–217. [Google Scholar] [CrossRef]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; Plaza, J.J.G.; Milaković, M.; Udiković-Kolić, N. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kolar, B.; Arnuš, L.; Jeretin, B.; Gutmaher, A.; Drobne, D.; Durjava, M.K. The toxic effect of oxytetracycline and trimethoprim in the aquatic environment. Chemosphere 2014, 115, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Lützhøft, H.-C.H.; Andersen, H.R.; Ingerslev, F. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 46, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, A.; Pinckney, J.L. Size-selective toxicity effects of the antimicrobial tylosin on estuarine phytoplankton communities. Environ. Pollut. 2016, 216, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, D.; Sun, H.; Guo, J.; Mo, J. Tylosin toxicity in the alga Raphidocelis subcapitata revealed by integrated analyses of transcriptome and metabolome: Photosynthesis and DNA replication-coupled repair. Aquat. Toxicol. 2021, 239, 105964. [Google Scholar] [CrossRef]
- Hu, J.; Liang, Y.; Chen, M.; Wang, X. Assessing contamination levels of amprolium and tylosin using the embryo toxicity assay and three biomarkers. Aquat. Ecosyst. Health Manag. 2010, 13, 335–341. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, X.; Xie, Y.; Song, M.; Zhu, K.; Ding, S. Macrolides induce severe cardiotoxicity and developmental toxicity in zebrafish embryos. Sci. Total Environ. 2019, 649, 1414–1421. [Google Scholar] [CrossRef]



| Antibiotics | Concentrations | Exposure Period | Stages | Effects | Refs. |
|---|---|---|---|---|---|
| Amoxicillin | 100 ppm | 7 days | Adult zebrafish | Locomotor alteration and decreased social interaction Induced oxidative stress in brain tissue | [72] |
| 0, 75, 128, 221, 380, 654 and 1125 ppm | 96 h | Embryos and adult zebrafish | Caused premature hatching Induced oxidative stress | [73] | |
| 70 ppm | 7 days | Adult zebrafish | Decreased intestinal microbial diversity Increased stress-associated behaviors | [74] | |
| 10, 11, 13, 15, 25, 40, 70, and 80 ppm | 96 h | Fingerlings and adult common carp | Behavioral, physical, and biochemical abnormalities Toxicity in liver | [75] | |
| Trimethoprim | 0–100 ppm | 48 h | In vitro (rainbow trout gonad-2 cell) | Exhibit cytotoxic and genotoxic effects DNA damage and oxidative stress | [76] |
| 200 ppb | 72 h | Zebrafish embryos | Embryotoxicity with reduced hatching rate, body malformations, and high mortality | [77] | |
| 100 ppm | 48 h | Daphnia magna | Growth inhibition Toxic effect led to death | [78] | |
| 30–300 ppm | 5 days | Daphnia magna and green algae | Growth inhibition | [79] | |
| Tylosin | 5–400 ppb | 48 h | Phytoplankton | Growth inhibition Alteration of size structure and composition | [80] |
| 3–400 ppb | 7 days | Green algae | Growth inhibition DNA damage Impairment of molecular pathways related to photosynthesis | [81] | |
| 0, 0.05, 0.2, 1, 5, 25, 100 ppm | 96 h | Zebrafish embryos | Decreased survival rate Induced oxidative stress | [82] | |
| 12.5 and 50 ppm | 48 h | Zebrafish embryos | Promoted tachycardia and bradycardia | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryanto, M.E.; Yang, C.-C.; Audira, G.; Vasquez, R.D.; Roldan, M.J.M.; Ger, T.-R.; Hsiao, C.-D. Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis. Antibiotics 2022, 11, 1059. https://doi.org/10.3390/antibiotics11081059
Suryanto ME, Yang C-C, Audira G, Vasquez RD, Roldan MJM, Ger T-R, Hsiao C-D. Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis. Antibiotics. 2022; 11(8):1059. https://doi.org/10.3390/antibiotics11081059
Chicago/Turabian StyleSuryanto, Michael Edbert, Chun-Chuen Yang, Gilbert Audira, Ross D. Vasquez, Marri Jmelou M. Roldan, Tzong-Rong Ger, and Chung-Der Hsiao. 2022. "Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis" Antibiotics 11, no. 8: 1059. https://doi.org/10.3390/antibiotics11081059
APA StyleSuryanto, M. E., Yang, C.-C., Audira, G., Vasquez, R. D., Roldan, M. J. M., Ger, T.-R., & Hsiao, C.-D. (2022). Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis. Antibiotics, 11(8), 1059. https://doi.org/10.3390/antibiotics11081059









