Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain
Abstract
:1. Introduction
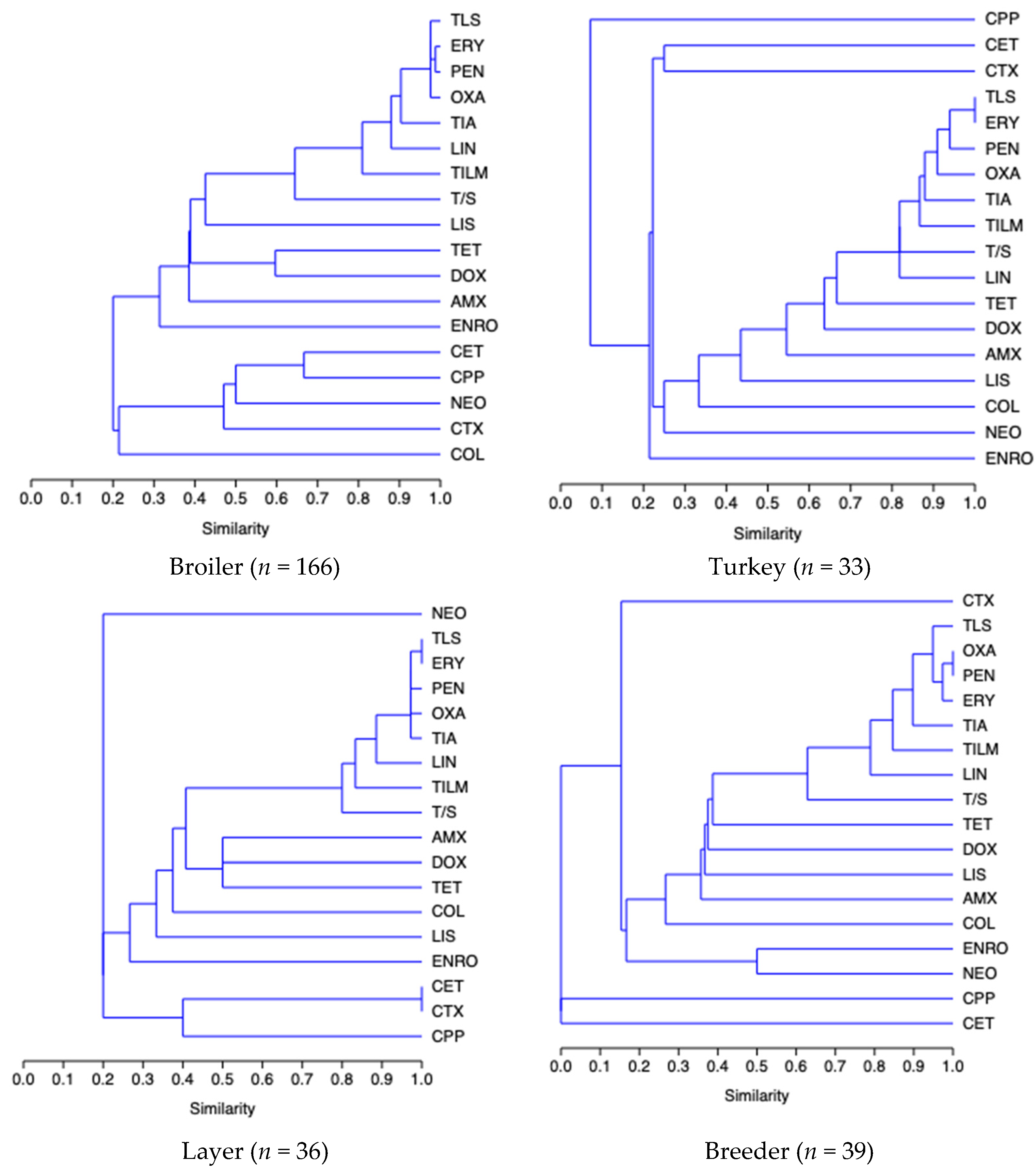
2. Results
3. Discussion
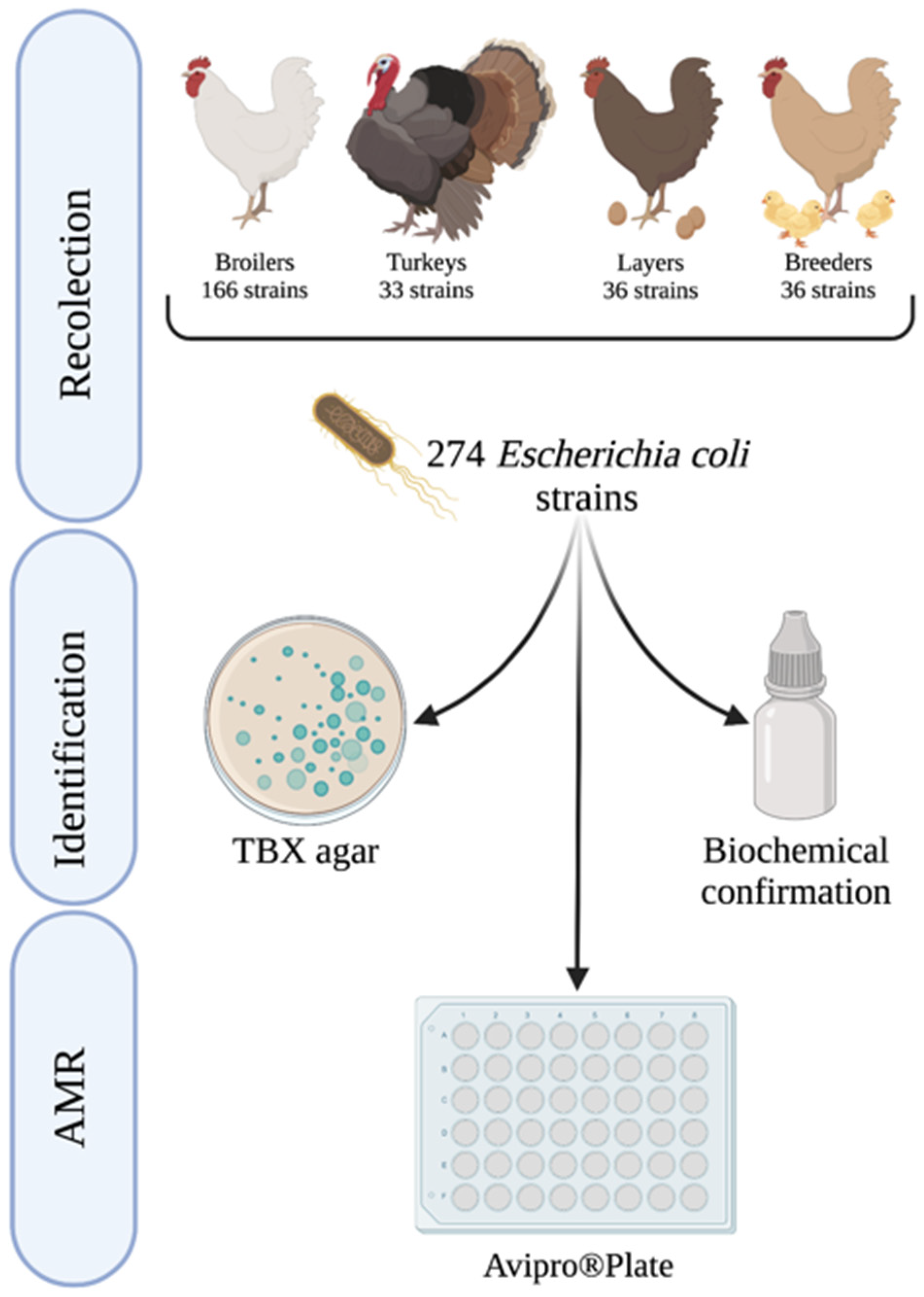
4. Materials and Methods
4.1. Study Design
4.2. Microbiological Isolation of E. coli
4.3. Antimicrobial Susceptibility Testing and Classification
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naghavi, M.; Murray, C.J.L.; Ikuta, K.S.; Mestrovic, T.; Swetschinski, L.; Sartorius, B. Global Burden of Antimicrobial Resistance: Essential Pieces of a Global Puzzle—Authors’ Reply. Lancet 2022, 399, 2349–2350. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. In Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Moffo, F.; Mouiche, M.M.M.; Djomgang, H.K.; Tombe, P.; Wade, A.; Kochivi, F.L.; Dongmo, J.B.; Mbah, C.K.; Mapiefou, N.P.; Ngogang, M.P.; et al. Poultry Litter Contamination by Escherichia Coli Resistant to Critically Important Antimicrobials for Human and Animal Use and Risk for Public Health in Cameroon. Antibiotics 2021, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 1–286. [Google Scholar] [CrossRef]
- Mathew, P.; Sivaraman, S.; Chandy, S. Communication Strategies for Improving Public Awareness on Appropriate Antibiotic Use: Bridging a Vital Gap for Action on Antibiotic Resistance. J. Family Med. Prim. Care 2019, 8, 1867. [Google Scholar] [CrossRef]
- Saraiva, M.D.M.S.; Lim, K.; Monte, D.F.M.D.; Givisiez, P.E.N.; Alves, L.B.R.; Neto, O.C.D.F.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial Resistance in the Globalized Food Chain: A One Health Perspective Applied to the Poultry Industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- World Health Organitzation (WHO). E. coli. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli (accessed on 4 July 2022).
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef]
- Apostolakos, I.; Laconi, A.; Mughini-Gras, L.; Yapicier, Ö.Ş.; Piccirillo, A. Occurrence of Colibacillosis in Broilers and Its Relationship with Avian Pathogenic Escherichia coli (APEC) Population Structure and Molecular Characteristics. Front. Vet. Sci. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Zou, M.; Ma, P.P.; Liu, W.S.; Liang, X.; Li, X.Y.; Li, Y.Z.; Liu, B.T. Prevalence and Antibiotic Resistance Characteristics of Extraintestinal Pathogenic Escherichia Coli among Healthy Chickens from Farms and Live Poultry Markets in China. Animals 2021, 11, 1112. [Google Scholar] [CrossRef]
- Bojesen, A.M.; Ahmed, U.; Skaarup, H.; Espinosa-Gongora, C. Recurring Outbreaks by the Same Escherichia coli ST10 Clone in a Broiler Unit during 18 Months. Vet. Res. 2022, 53, 2. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman. Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; Elmougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; et al. Poultry Hatcheries as Potential Reservoirs for Antimicrobial-Resistant Escherichia coli: A Risk to Public Health and Food Safety. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA; ECDC. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020. EFSA J. 2022, 20, 68–88. [Google Scholar] [CrossRef]
- López Navas, A.; Muñoz Madero, C.; Carapeto García, R.; García Caballero, A.; Aguilera Moyano, C.; Isabelteixeira Justo, C.; Cubillo Dapena, G.; Trillo Contreras, J.L.; Alonso Irujo, L.; Villar Gómara, L.; et al. Informe Anual PRAN. 2022. Available online: https://www.resistenciaantibioticos.es/en/publicaciones (accessed on 5 July 2022).
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. 2021. Available online: https://op.europa.eu/en/publication-detail/-/publication/d20a0041-83db-11ec-8c40-01aa75ed71a1/language-en (accessed on 5 July 2022).
- Racewicz, P.; Majewski, M.; Biesiada, H.; Nowaczewski, S.; Wilczyński, J.; Wystalska, D.; Kubiak, M.; Pszczoła, M.; Madeja, Z.E. Prevalence and Characterisation of Antimicrobial Resistance Genes and Class 1 and 2 Integrons in Multiresistant Escherichia coli Isolated from Poultry Production. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia coli: A Global Overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Österblad, M.; Hakanen, A.; Manninen, R.; Leistevuo, T.; Peltonen, R.; Meurman, O.; Huovinen, P.; Kotilainen, P. A between-Species Comparison of Antimicrobial Resistance in Enterobacteria in Fecal Flora. Antimicrob. Agents Chemother. 2000, 44, 1479–1484. [Google Scholar] [CrossRef] [Green Version]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.K.; Lo, D.Y.; Wei, H.W.; Kuo, H.C. Antimicrobial Resistance of Escherichia Coli Isolates from Canine Urinary Tract Infections. J. Vet. Med. Sci. 2015, 77, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- Horigan, V.; Kosmider, R.D.; Horton, R.A.; Randall, L.; Simons, R.R.L. An Assessment of Evidence Data Gaps in the Investigation of Possible Transmission Routes of Extended Spectrum β-Lactamase Producing Escherichia coli from Livestock to Humans in the UK. Prev. Vet. Med. 2016, 124, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Sharma, M.; Millner, P.D.; Hashem, F.; Camp, M.; Whyte, C.; Graham, L.; Cotton, C.P. Survival and Persistence of Nonpathogenic Escherichia coli and Attenuated Escherichia coli O157:H7 in Soils Amended with Animal Manure in a Greenhouse Environment. J. Food Prot. 2016, 79, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Hussain, T.; Das, C.R.; Andleeb, S. Isolation and Characterization of a Myoviridae MJ1 Bacteriophage against Multi-Drug Resistant Escherichia coli 3. Jundishapur J. Microbiol. 2015, 8, e25917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, A.A.; Palma, M.S.A. Tetracycline: Production, Waste Treatment and environmental Impact Assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Rivera-Gomis, J.; Marín, P.; Martínez-Conesa, C.; Otal, J.; Jordán, M.J.; Escudero, E.; Cubero, M.J. Antimicrobial Resistance of Campylobacter jejuni, Escherichia coli and Enterococcus faecalis Commensal Isolates from Laying Hen Farms in Spain. Animals 2021, 11, 1284. [Google Scholar] [CrossRef]
- Dawadi, P.; Bista, S.; Bista, S. Prevalence of Colistin-Resistant Escherichia coli from Poultry in South Asian Developing Countries. Vet. Med. Int. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Schreier, J.; Karasova, D.; Crhanova, M.; Rychlik, I.; Rautenschlein, S.; Jung, A. Influence of Lincomycin-Spectinomycin Treatment on the Outcome of Enterococcus cecorum Infection and on the Cecal Microbiota in Broilers. Gut Pathog. 2022, 14, 1–13. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable Sources of Community-Acquired Carriage of Escherichia coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [Green Version]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [Green Version]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial Resistance in Fecal Escherichia coli and Salmonella enterica Isolates: A Two-Year Prospective Study of Small Poultry Flocks in Ontario, Canada. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia Coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Martínez-álvarez, S.; Sanz, S.; Olarte, C.; Hidalgo-Sanz, R.; Carvalho, I.; Fernández-Fernández, R.; Campaña-Burguet, A.; Latorre-Fernández, J.; Zarazaga, M.; Torres, C. Antimicrobial Resistance in Escherichia coli from the Broiler Farm Environment, with Detection of SHV-12-Producing Isolates. Antibiotics 2022, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Leekitcharoenphon, P.; Johansson, M.H.K.; Munk, P.; Malorny, B.; Skarżyńska, M.; Wadepohl, K.; Moyano, G.; Hesp, A.; Veldman, K.T.; Bossers, A.; et al. Genomic Evolution of Antimicrobial Resistance in Escherichia coli. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Thanki, A.M.; Clavijo, V.; Healy, K.; Wilkinson, R.C.; Sicheritz-Pontén, T.; Millard, A.D.; Clokie, M.R.J. Development of a Phage Cocktail to Target Salmonella Strains Associated with Swine. Pharmaceuticals 2022, 15, 58. [Google Scholar] [CrossRef]
- Shousha, A.; Awaiwanont, N.; Sofka, D.; Smulders, F.J.M.; Paulsen, P.; Szostak, M.P.; Humphrey, T.; Hilbert, F. Bacteriophages Isolated from Chicken Meat and the Horizontal Transfer of Antimicrobial Resistance Genes. Appl. Environ. Microbiol. 2015, 81, 4600–4606. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]

| Antimicrobial Class | Antimicrobial | Year (%; CI) | |||
|---|---|---|---|---|---|
| 2019 n = 92 | 2020 n = 93 | 2021 n = 89 | |||
| β-Lactams | PEN | 97 (0.91–0.99) | 99 (0.94–1) | 97 (0.91–0.99) | 97 G (0.95–0.99) |
| AMX | 35 ab (0.05–0.45) | 45 b (0.35–0.55) | 26 a (0.18–0.36) | 35 A (0.3–0.41) | |
| OXA | 99 (0.94–1) | 95 (0.88–0.98) | 96 (0.89–0.98) | 96 G (0.94–0.98) | |
| Fluoroquinolones | ENRO | 17 (0.11–0.26) | 12 (0.06–0.20) | 10 (0.05–0.18) | 13 C (0.1–0.18) |
| Cephalosporin | CET | 4 (0.02–0.10) | 6 (0.03–0.13) | 3 (0.01–0.09) | 5 B (0.03–0.08) |
| CPP | 2 (0.00–0.07) | 6 (0.03–0.13) | 4 (0.02–0.11) | 4 B (0.02–0.07) | |
| CTX | 11 (0.06–0.19) | 11 (0.06–0.18) | 7 (0.03–0.14) | 9 C (0.06–0.13) | |
| Macrolides | ERY | 100 (0–1) | 99 (0.94–1) | 100 (0–1) | 100 C (0.98–1) |
| TILM | 97 b (0.91–0.99) | 77 a (0.68–0.85) | 72 a (0.62–0.80) | 82 D (0.77–8.86) | |
| TLS | 97 (0.91–0.99) | 100 (0–1) | 98 (0.92–1.00) | 98 EG (0.96–0.99) | |
| Tetracyclines | DOX | 43 b (0.33–0.53) | 18 a (0.11–0.27) | 20 a (0.13–0.30) | 27 D (0.22–0.33) |
| TET | 49 b (0.39-0.59) | 34 a (0.25–0.44) | 31 a (0.23–0.42) | 38 A (0.33–0.44) | |
| Aminoglycosides | NEO | 9 (0.04–0.15) | 6 (0.03–0.13) | 1 (0.00–0.06) | 5 B (0.03–0.08) |
| Lincosamides | LIN | 66 a (0.45–0.65) | 92 b (0.16–0.33) | 98 b (0.23–0.42) | 85 A (0.31–0.43) |
| LIS | 55 b (0.56–0.75) | 24 a (0.86–0.97) | 31 a (0.92–1.00) | 37 B (0.81–0.89) | |
| Folate inhibitors | T/S | 78 a (0.69–0.86) | 62 a (0.52–0.72) | 57 b (0.47–0.67) | 66 H (0.6–0.71) |
| Polymyxins | COL | 17 b (0.09–0.24) | 11 ab (0.06–0.18) | 4 a (0.02–0.11) | 11 C (0.07–0.14) |
| Pleuromutilins | TIA | 95 (0.88–0.98) | 90 (0.83–0.95) | 89 (0.81–0.94) | 91 I (0.87–0.94) |
| BROILER n = 166 | TURKEY n = 33 | LAYER n = 36 | BREEDER n = 39 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |||||||||||||
| 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | |||||
| PEN | 100 | 100 | 97 | 99 | 83 | 100 | 100 | 94 | 100 | 100 | 88 | 96 | 95 | 93 | 100 | 96 |
| AMX | 38 b | 56 b | 18 a | 37 | 25 a | 25 a | 69 b | 40 | 55 | 41 | 13 | 36 | 21 | 21 | 33 | 25 |
| OXA | 100 | 98 | 95 | 98 | 100 | 75 | 92 | 89 | 100 | 94 | 100 | 98 | 95 | 93 | 100 | 96 |
| ENRO | 24 | 15 | 10 | 16 | 17 | 0 | 8 | 8 | 9 | 12 | 25 | 15 | 5 | 7 | 0 | 4 |
| CET | 4 | 6 | 3 | 4 | 8 | 0 | 8 | 5 | 9 | 18 | 0 | 9 | 0 | 0 | 0 | 0 |
| CPP | 4 | 7 | 3 | 5 | 0 | 0 | 8 | 3 | 0 | 12 | 13 | 8 | 0 | 0 | 0 | 0 |
| CTX | 12 | 11 | 8 | 10 | 25 b | 0 ab | 0 a | 8 | 9 | 18 | 0 | 9 | 0 | 7 | 17 | 8 |
| ERY | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 93 | 100 | 98 |
| TILM | 96 b | 80 a | 69 a | 82 | 100 b | 63 a | 77 a | 80 | 100 | 71 | 88 | 86 | 95 | 86 | 100 | 82 |
| TLS | 96 | 100 | 97 | 98 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 100 | 100 | 98 |
| DOX | 44 b | 19 a | 16 a | 26 A | 67 | 25 | 46 | 46 B | 45 b | 12 a | 0 a | 19 A | 26 | 21 | 33 | 27 A |
| TET | 46 | 33 | 27 | 36 | 75 | 50 | 54 | 60 | 55 | 29 | 38 | 40 | 37 | 36 | 17 | 30 |
| NEO | 10 | 9 | 2 | 7 | 17 | 0 | 0 | 6 | 0 | 6 | 0 | 2 | 5 | 0 | 0 | 2 |
| LIN | 72 a | 91 b | 98 b | 87 | 50 a | 100 b | 100 b | 83 | 76 | 94 | 88 | 85 | 58 a | 93 b | 100 b | 84 |
| LIS | 66 b | 28 a | 31 a | 41 | 75 b | 25 a | 15 a | 38 | 36 | 18 | 38 | 31 | 26 ab | 14 a | 67 b | 36 |
| T/S | 78 b | 59 a | 58 a | 65 | 83 | 88 | 77 | 83 | 100 b | 53 a | 50 a | 68 | 63 | 71 | 17 | 50 |
| COL | 10 | 7 | 5 | 7 A | 42 | 25 | 8 | 25 B | 18 | 12 | 0 | 10 AB | 21 | 14 | 0 | 12 AB |
| Antimicrobial nº | Antimicrobial Resistance Pattern | n | % |
|---|---|---|---|
| 15 | PEN-AMX-OXA-ENRO-CET-CPP-CTX-TLS-TILM-ERY-DOX-NEI-COL-LIS-TIA | 2 | 1.1 |
| 13 | PEN-AMX-OXA-ENRO-TLS-TILM-ERY-TET-DOX-LIS-LIN-T/S -TIA | 2 | 1.1 |
| PEN-AMX-OXA-TLS-TILM-ERY-TET-DOX-COL-LIS-LIN-T/S-TIA | 3 | 1.7 | |
| 12 | PEN-AMX-OXA-ENRO-TLS-TILM-ERY-TET-DOX-LIS-T/S-TIA | 2 | 1.1 |
| OXA-TLS-TILM-ERY-TET-DOX-NEO-COL-LIS-LIN-T/S-TIA | 2 | 1.1 | |
| PEN-OXA-ENRO-TLS-TILM-ERY-TET-DOX-LIS-LIN-T/S-TIA | 3 | 1.7 | |
| PEN-AMX-OXA-TLS-TILM-ERY-TET-DOX-LIS-LIN-T/S-TIA | 7 | 4.0 | |
| 11 | PEN-AMX-OXA-TLS-TILM-ERY-TET-DOX-LIS-T/S-TIA | 4 | 2.3 |
| PEN-OXA-TLS-TILM-ERY-TET-DOX-LIS-LIN-T/S-TIA | 5 | 2.9 | |
| PEN-AMX-OXA-TLS-TILM-ERY-DOX-LIS-LIN-T/S-TIA | 2 | 1.1 | |
| 10 | PEN-AMX-OXA-TLS-TILM-ERY-TET-LIS-T/S-TIA | 2 | 1.1 |
| PEN-OXA-TLS-TILM-ERY-DOX-COL-LIS-T/S-TIA | 2 | 1.1 | |
| PEN-AMX-OXA-TLS-TILM-ERY-TET-LIS-LIN-TIA | 4 | 2.3 | |
| PEN-OXA-TLS-TILM-ERY-TET-DOX-LIS-T/S-TIA | 4 | 2.3 | |
| PEN-AMX-OXA-TLS-TILM-ERY-LIS-LIN-T/S-TIA | 5 | 2.9 | |
| 9 | PEN-OXA-TLS-TILM-ERY-TET-COL-LIS-TIA | 2 | 1.1 |
| PEN-OXA-TLS-TILM-ERY-TET-LIS -T/S-TIA | 4 | 2.3 | |
| PEN-OXA-TLS-TILM-ERY-LIN-LIN-T/S-TIA | 9 | 5.1 | |
| PEN-AMX-OXA-TLS-TILM-ERY-LIS-T/S-TIA | 11 | 6.3 | |
| 8 | PEN-AMX-OXA-TLS-ERY-TET-LIS-TIA | 2 | 1.1 |
| PEN-OXA-TLS-ERY-LIS-LIN-T/S-TIA | 2 | 1.1 | |
| PEN-AMX-OXA-TLS-TILM-ERY-T/S-TIA | 3 | 1.7 | |
| PEN-AMX-OXA-TLS-TILM-ERY-LIS-TIA | 4 | 2.3 | |
| PEN-OXA-TLS-TILM-ERY-LIN-T/S-TIA | 5 | 2.9 | |
| PEN-OXA-TLS-TILM-ERY-LIS-LIN-TIA | 5 | 2.9 | |
| PEN-OXA-TLS-TILM-ERY-LIS-T/S-TIA | 26 | 14.9 | |
| 7 | PEN-OXA-TLS-TILM-ERY-T/S-TIA | 5 | 2.9 |
| PEN-OXA-TLS-ERY-LIS-T/S-TIA | 7 | 4.0 | |
| PEN-OXA-TLS-TILM-ERY-LIS-TIA | 16 | 9.1 | |
| 6 | PEN-TLS-ERY-LIS-T/S-TIA | 2 | 1.1 |
| PEN-OXA-TLS-ERY-LIS-T/S | 3 | 1.7 | |
| PEN-OXA-TLS-TILM-ERY-T/S | 3 | 1.7 | |
| PEN-OXA-TLS-TILM-ERY-TIA | 4 | 2.3 | |
| PEN-OXA-TLS-ERY-LIS-TIA | 7 | 4.0 | |
| 5 | PEN-OXA-TLS-ERY-LIS | 4 | 2.3 |
| PEN-OXA-TLS-ERY-TIA | 2 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevilla-Navarro, S.; Catalá-Gregori, P.; Torres-Boncompte, J.; Orenga, M.T.; Garcia-Llorens, J.; Cortés, V. Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain. Antibiotics 2022, 11, 1064. https://doi.org/10.3390/antibiotics11081064
Sevilla-Navarro S, Catalá-Gregori P, Torres-Boncompte J, Orenga MT, Garcia-Llorens J, Cortés V. Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain. Antibiotics. 2022; 11(8):1064. https://doi.org/10.3390/antibiotics11081064
Chicago/Turabian StyleSevilla-Navarro, Sandra, Pablo Catalá-Gregori, Jan Torres-Boncompte, Maria Teresa Orenga, Josep Garcia-Llorens, and Verónica Cortés. 2022. "Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain" Antibiotics 11, no. 8: 1064. https://doi.org/10.3390/antibiotics11081064
APA StyleSevilla-Navarro, S., Catalá-Gregori, P., Torres-Boncompte, J., Orenga, M. T., Garcia-Llorens, J., & Cortés, V. (2022). Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain. Antibiotics, 11(8), 1064. https://doi.org/10.3390/antibiotics11081064








