Abstract
Although carbon materials are widely used in surface engineering, particularly graphene (GP) and carbon nanotubes (CNTs), the application of these nanocomposites for the development of antibiofilm marine surfaces is still poorly documented. The aim of this study was, thus, to gather and discuss the relevant literature concerning the antifouling performance of carbon-based coatings against marine micro- and macrofoulers. For this purpose, a PRISMA-oriented systematic review was conducted based on predefined criteria, which resulted in the selection of thirty studies for a qualitative synthesis. In addition, the retrieved publications were subjected to a quality assessment process based on an adapted Methodological Index for Non-Randomized Studies (MINORS) scale. In general, this review demonstrated the promising antifouling performance of these carbon nanomaterials in marine environments. Further, results from the revised studies suggested that functionalized GP- and CNTs-based marine coatings exhibited improved antifouling performance compared to these materials in pristine forms. Thanks to their high self-cleaning and enhanced antimicrobial properties, as well as durability, these functionalized composites showed outstanding results in protecting submerged surfaces from the settlement of fouling organisms in marine settings. Overall, these findings can pave the way for the development of new carbon-engineered surfaces capable of preventing marine biofouling.
1. Introduction
Carbon nanomaterials, such as graphene (GP), carbon nanotubes (CNTs), fullerenes, and diamond-like carbon, are recognized for their proven antimicrobial and antiadhesive properties [1]. Graphene consists of a single-layer sheet of sp2-hybridized carbon with a two-dimensional honeycomb structure [2]. GP materials show a high specific surface area, electron conductivity, and thermal stability, making them attractive for applications like photocatalysis, energy production, and storage [3,4]. These nanocomposites stand out as some of the strongest and thinnest materials available and have significantly better electrochemical properties than CNTs, which are formed by rolling up either a single graphene sheet, single-walled carbon nanotubes (SWCNTs), or a series of concentric graphene sheets, multi-walled carbon nanotubes (MWCNTs) [5]. CNTs, therefore, exhibit a concentric cylindrical structure with a diameter in the order of nanometers (depending on the number of walls) and a length of several microns (100 µm) extendable to up to a few millimeters (about 4 mm) [6]. Due to their unique properties, such as a remarkable mechanical strength, high thermal conductivity, and structural stability, CNTs are promising nanomaterials for several applications, namely in the industrial, environmental, and medical fields [7,8,9].
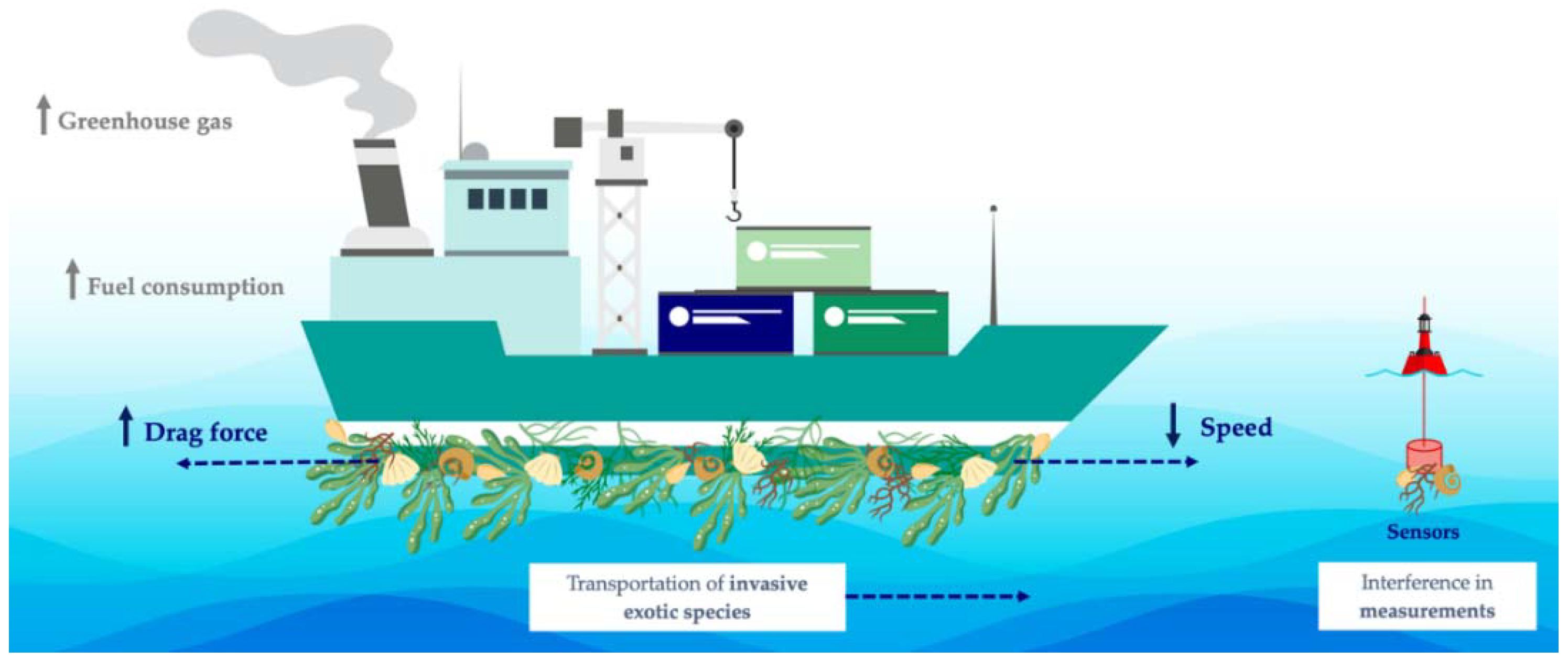
The antimicrobial and antifouling (AF) performance of carbon nanomaterials, as well as their outstanding mechanical properties, have led to their application in coatings for marine vessels and underwater structures and devices [10,11,12]. Biofouling is caused by the adhesion of microorganisms to surfaces, which rapidly grow and form a biofilm, building the basis for the further settlement of macroorganisms [13,14]. This biological process has a high impact on several industries, compromising, for instance, the performance of membrane separation processes and water filtration devices and deteriorating marine surfaces [15,16,17,18]. In cooling water circuits from power plants, biofouling leads to efficiency losses and accelerates the deterioration due to biocorrosion by about 20% [19]. In turn, in marine environments, submerged surfaces are rapidly colonized by different types of micro- and macroorganisms. Marine biofouling presents several economic, industrial, environmental, and health-related consequences (Figure 1) [20]. The gradual attachment of fouling organisms to watercraft promotes the increase of the vessel’s drag. It has been shown that the increased roughness caused by a heavily fouled ship hull can result in a power penalty of 86% at cruising speed and even light fouling can generate up to 16% penalty [21]. Consequently, more frequent ship maintenance and higher fuel consumption are required to sustain a given navigation speed, therefore increasing the costs related to that vessel and its circulation [22,23]. In fact, it is estimated that transport delays, hull repairs, and maintenance operations lead to losses of about 150 billion USD per year [24]. Additionally, the requirement for high fuel consumption leads to higher greenhouse gas emissions, negatively impacting air quality and causing severe environmental issues, such as global warming and climate change [25]. Fouled hull vessels also play a crucial role in the introduction of invasive exotic species into non-native environments since attached organisms can be transported over greater distances, impacting the local ecosystem and, ultimately, harming global biodiversity [26]. Furthermore, marine biofouling on marine facilities, such as underwater support structures, sensors, and housings, also poses an important concern [27]. Besides contributing to the deterioration and corrosion of these surfaces, the attachment of fouling species to marine devices used for monitoring can interfere with the measurements performed [28,29].
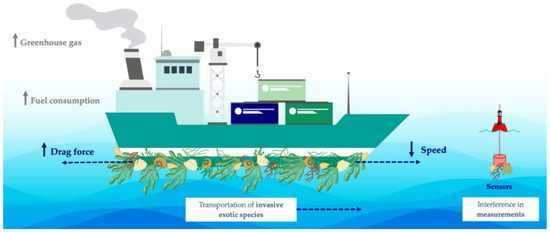
Figure 1.
Schematic representation of the negative impact of marine biofouling.
Several approaches have been used to tackle biofouling. These strategies can prevent, and delay biofilm formation or control already developed biofilms [24,30,31]. While preventive measures comprise both antimicrobial and AF surfaces, control procedures can involve the use of bacteriophages, QS inhibitors, the disruption of the biofilm matrix, as well as the use of matrices with self-cleaning properties [32,33,34,35,36].
During the twentieth century, the development of AF coatings emerged as a new approach to protecting ship hulls from biofouling. Among all the coatings developed and tested, tributyltin (TBT) stands out as one of the most effective in biofouling mitigation [37]. However, TBT was exclusively used in the 1970s, in combination with several copper compounds, since it was discovered that TBT-based compounds were highly toxic for nontarget organisms. Moreover, they were also able to persist in the marine environment for a longer period than initially estimated [38,39].
The prohibition of organotin compounds such as TBT strongly motivated AF coating manufacturers to find additional solutions that allow a similar biofouling mitigation performance obtained with TBT-based compounds but without adverse environmental impacts. The current challenge is to develop environmentally friendly marine AF coatings that are effective toward a broad range of marine organisms with no negative impact on nontarget organisms. Furthermore, leached coating compounds should have a high degradation rate in the marine environment, as well as a low bioaccumulation rate [38].
Modifications of surface charge potential, hydrophobicity, and surface topography can constitute promising strategies [40]. In this context, the use of carbon nanomaterials as fillers for polymer composites is becoming increasingly relevant [41].
The application of these nanomaterials can increase the mechanical strength of the final composite [42] and its ability to prevent or delay biofouling [43].
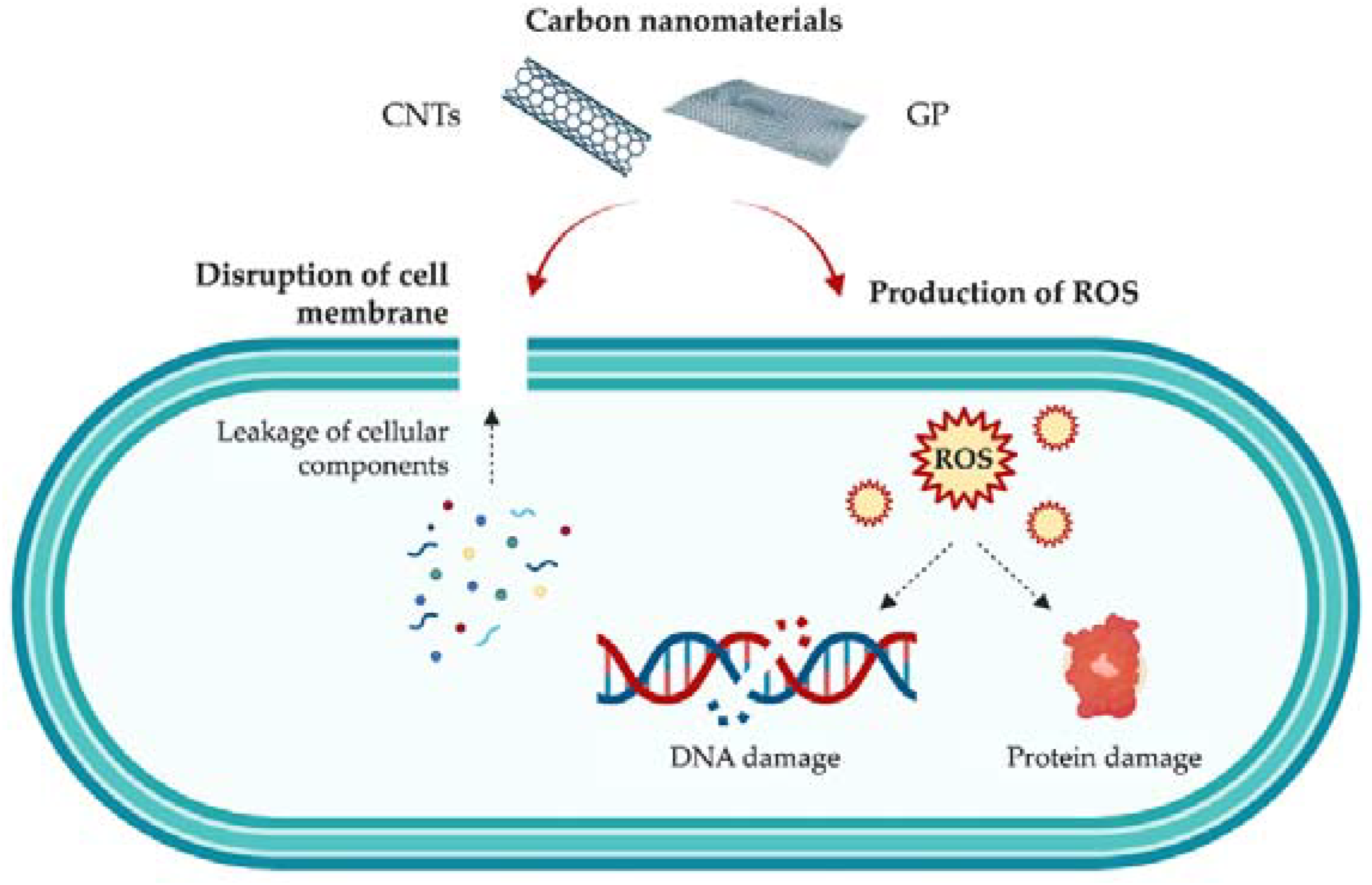
Both GP and CNTs have shown good antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as bacterial spores [1,5]. Regarding CNTs specifically, SWCNTs have exhibited significantly higher antibacterial activity than MWCNTs against Gram-positive bacteria [44]. However, the mechanisms of action behind these carbon-based nanomaterials are still not fully understood, due to their complexity and the wide array of factors that may influence their antibacterial activity, including their composition and geometry, as well as the type, morphology, and growth state of bacteria (planktonic or sessile) [7,45]. It is hypothesized that the antibacterial properties of these nanomaterials rely mainly on mechanical factors: their sharp structures act as ‘‘nano-darts’’ that pierce bacterial membranes, leading to cell death [44]. Nevertheless, other authors defend that CNTs produce not only mechanical damage with consequent cell disruption and release of intracellular content (a primary killing mechanism), but also generate oxidative stress [46] (Figure 2). It has also been reported that the length, diameter, surface area, concentration, and chemical modifications of CNTs play a significant role in both the AF and antimicrobial activity of these carbon materials [46,47,48].
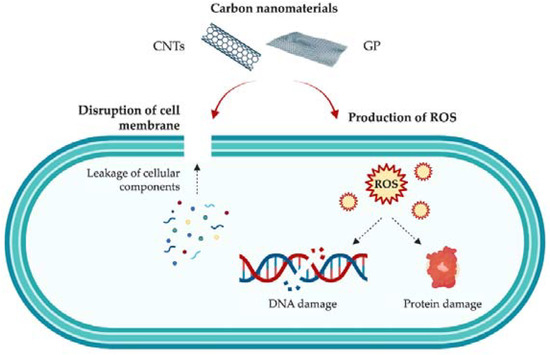
Figure 2.
Schematic representation of the mechanisms of action of carbon nanomaterials, namely GP and CNTs, against bacteria.
As for graphene-based nanomaterials, it is also assumed that both physical and chemical factors come into play. Similar to CNTs, the sharp edges of GP sheets cause membrane damage [49]. Thanks to the large surface area of graphene-based materials, they can also lead to bacterial cell entrapment [50]. Moreover, GP is assumed to be able to induce oxidative stress through the formation of reactive oxygen species (ROS), which disrupt microorganisms’ DNA and proteins (Figure 2). According to the literature, the mechanisms through which GP-based nanomaterials induce cell inhibition/death are not only dependent on their own physical and chemical properties (e.g., dimensions, number of layers, functionalization), but also on factors related to the production of the surface (e.g., graphene loading, nanoparticle dispersion, aggregation) [51,52,53].
Thanks to these appealing properties, the use of these nanomaterials for improved marine AF coatings is currently on the rise [41]. As such, assessing the effectiveness of GP- and CNTs-based coatings in preventing marine biofouling, namely on pioneer bacterial attachment and biofilm formation, can contribute to optimizing and reaching a better understanding of their AF properties. However, the currently available data regarding the potential of carbon-based coatings to prevent marine biofouling need to be critically discussed to assist researchers in the design of improved marine surfaces.
2. Results and Discussion
2.1. Study Selection and Characterization
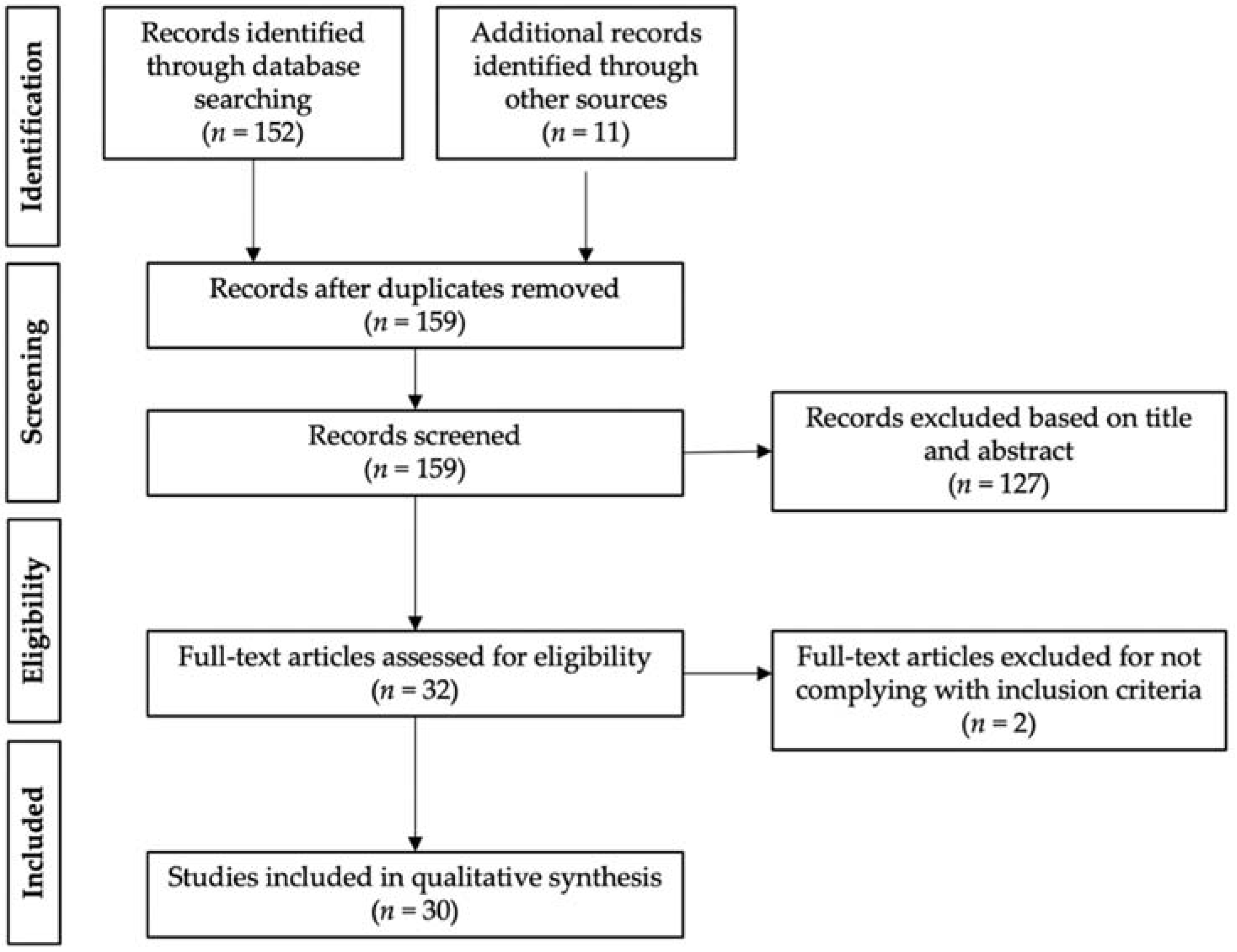
A total of 152 articles were found using the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) search methodology. This number was increased to 163 studies with the inclusion of 11 records retrieved through other sources (prior searches and references of the chosen publications). After screening out duplicates, a total of 159 articles were assessed based on title and abstract. Out of these, 127 records were disqualified for not meeting the prerequisites for inclusion. Lastly, 2 records were ruled out upon thorough analysis of the remaining 32 full-text publications since these were nonoriginal articles. Therefore, 30 studies were included in the qualitative synthesis (Figure 3).
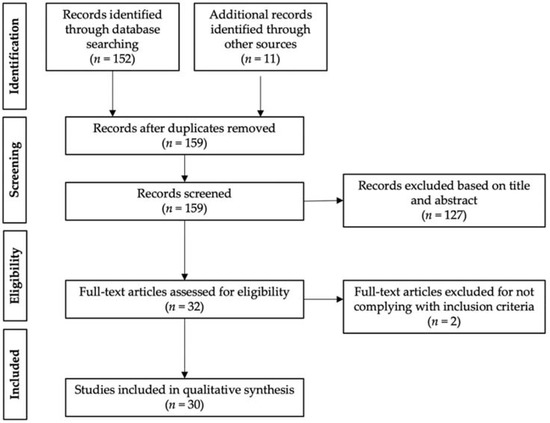
Figure 3.
Schematic summary of the PRISMA literature search.
With the increasing search for environmentally friendly AF materials, the study of carbon nanomaterials and their unique properties has been rising during the past decade. These nanomaterials are generally incorporated into commercial AF paints based on polymeric matrices, such as polydimethylsiloxane (PDMS), to assess how they influence the physicochemical properties and AF performance of coatings.
Due to the appealing physical and chemical properties it possesses, such as high hydrophobicity, low surface energy, adhesion, and endurance, PDMS stands out as one of the most widely used polymers for AF purposes [54]. However, PDMS also presents certain limitations, namely, its mechanical weakness and propensity to become damaged when immersed in a marine environment [55]. Thus, any additive capable of improving the efficacy and durability of PDMS-based AF coatings is extremely promising.
CNTs-modified PDMS composites have gained worldwide attention due to their facile fabrication, ecological stability, and remarkable AF performance [56]. Furthermore, the incorporation of GP or CNTs in polymeric coatings has been shown to improve their physical properties, namely in terms of mechanical strength [42,57], increasing their capability to delay marine biofouling.
In this systematic review, 30 full-text articles focused on carbon materials incorporated into polymeric coatings and their AF properties in the marine biofouling context were reviewed and summarized, as shown in Table 1 and Table 2. Among the selected publications, 12 focused on the incorporation of pristine or modified MWCNTs and 11 used graphene oxide (GO), either on its own or functionalized with other nanoparticles. In addition, five studies addressed the AF performance of other GP forms. PDMS stood out as the most used nanocoated polymer, with a total of 14 out of the 30 studies.

Table 1.
Studies focused on graphene-based AF coatings in marine environments.

Table 2.
Studies focused on CNT-based AF coatings in marine environments.
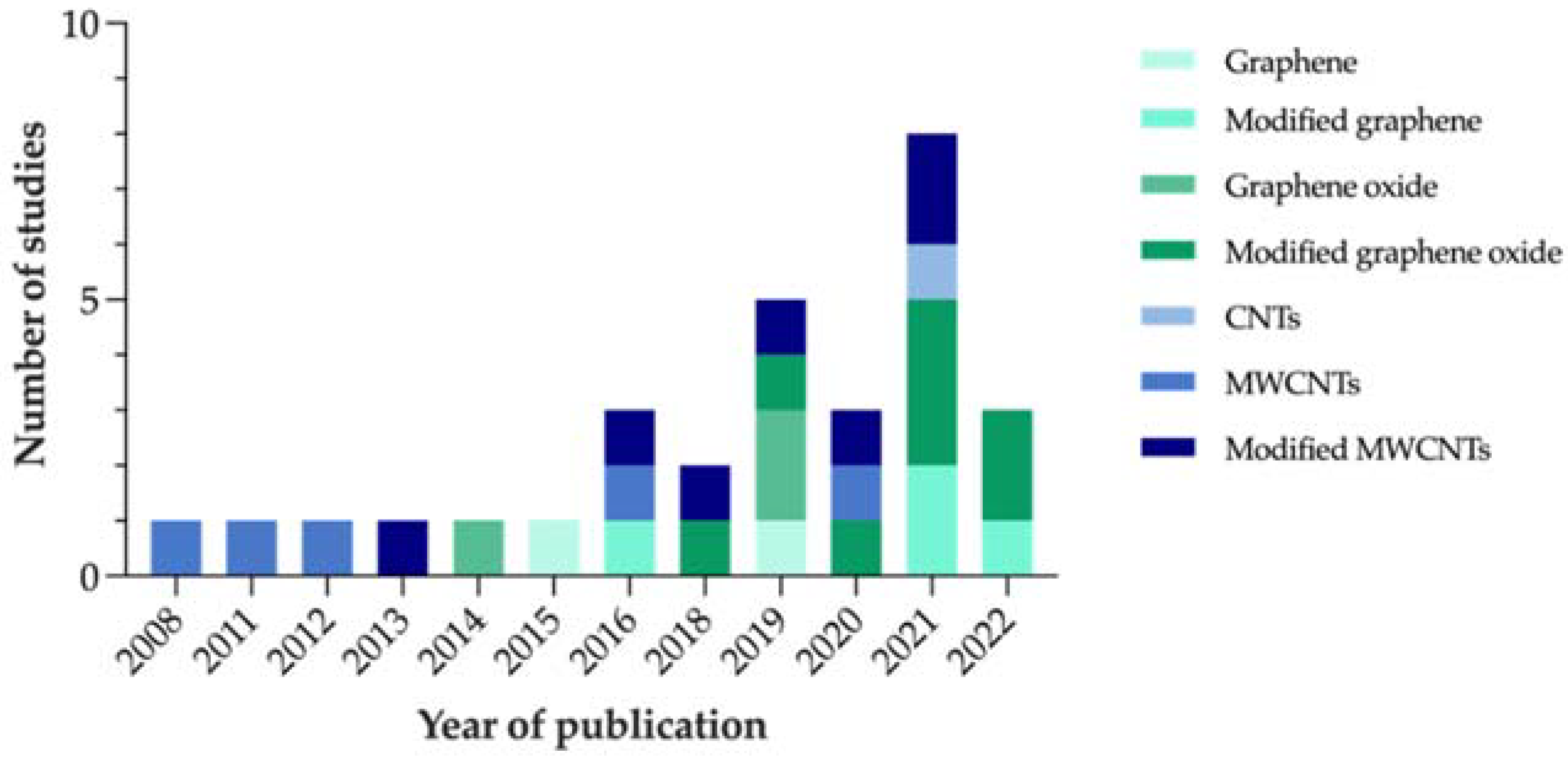
Based on the reviewed articles, Figure 4 represents the evolution that the number of studies focused on carbon-nanocoated polymeric materials for marine AF purposes has shown over the years.
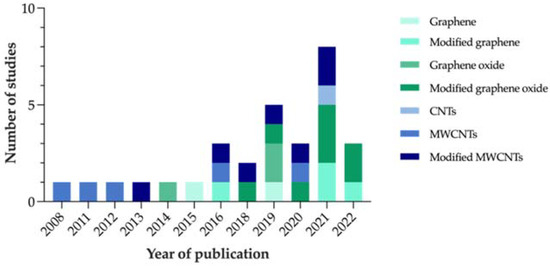
Figure 4.
Number of studies on carbon-modified AF marine coatings included in the systematic review, per year of publication.
Considering that no restrictions were applied to the search in terms of publication year, the fact that the first article identified on the subject dates to only 2008 proves that this strategy is fairly recent. In addition, the increasing number of studies published over the last few years confirms the relevance of this topic as a research matter.
In this systematic review, particular attention was given to carbon-based coatings with application in the marine field and their antifouling potential against micro- and macrofoulers.
2.2. Graphene-Based Coatings
Several authors have demonstrated the promising AF activity of GP coatings against marine bacteria [58,59] (Table 1). According to Jin, Zhang, et al., under dynamic conditions, graphene-based membranes were able to reduce bacteria adhesion by 40% [59].
However, a records analysis reveals that the current trend is to study the potential of modified/functionalized GP. The functionalization of GP with silver nanoparticles has demonstrated remarkable antibiofilm effects, with a 99.6% inhibition rate for Halomonas pacifica and over 80% for Dunaliella tertiolecta and Isochrysis sp. [60]. Recently, guanidine functionalized GP has also shown promising antibacterial and diatom antiadhesion properties, with reduction rates of up to 95% and up to 99.2%, respectively. Moreover, the field trial revealed no fouling or surface deterioration for 2 months [61].
Furthermore, laser-induced GP coatings reduced Cobetia marina surface coverage by up to 80% after 36 h of exposure [62].
These results indicated that functionalized GP coatings can be successfully applied for the development of AF marine surfaces.
2.3. Graphene Oxide-Based Coatings
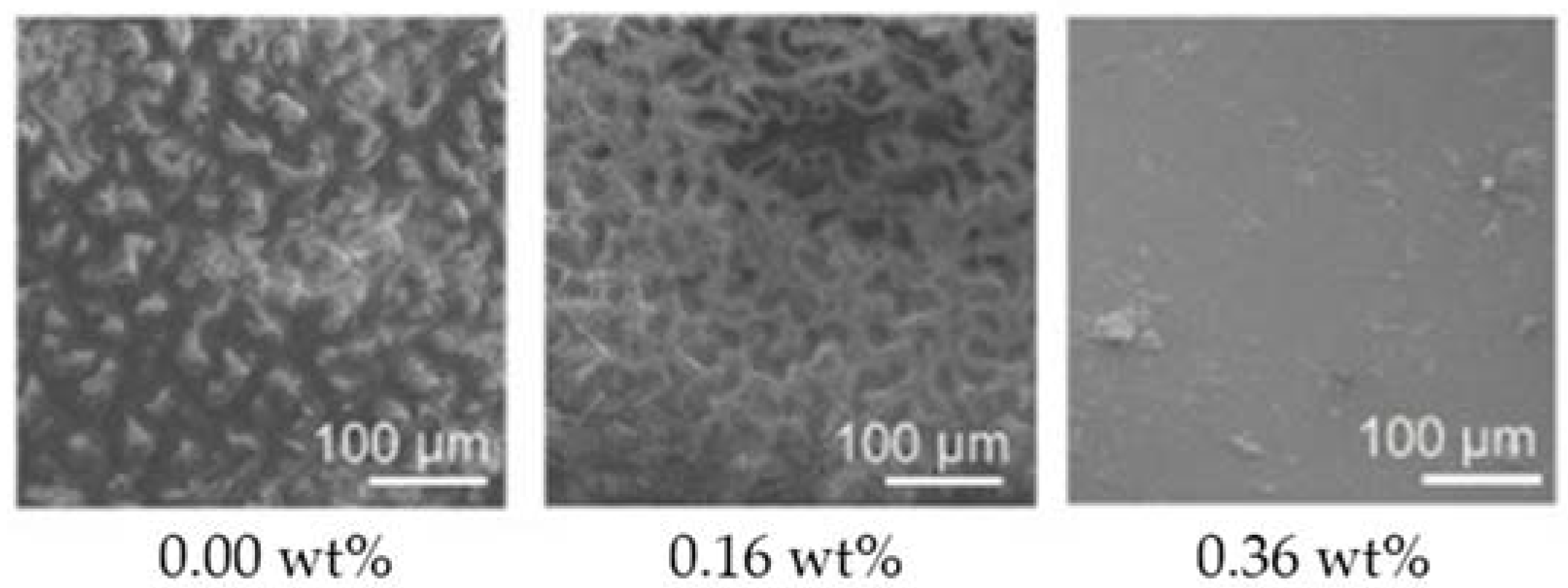
Graphene oxide, the most studied form of GP within the marine AF coatings context, has shown both in vitro and in situ high AF activity [63,64,65] (Table 1). In fact, surfaces containing GO 0.36 wt% when incubated for 10 days under dynamic conditions almost completely inhibited diatom adhesion [64] (Figure 5).
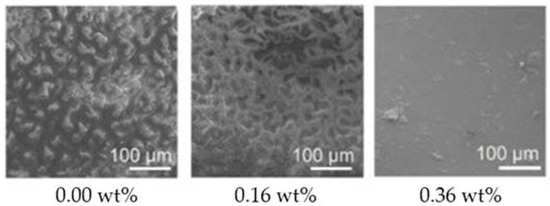
Figure 5.
Scanning electron microscopy (SEM) images of diatom adhesion on silicone surfaces containing different GO loadings, after 10 days of incubation under dynamic conditions. Reprinted with adaptations from [64], under the terms of the Creative Commons Attribution (CC BY) license.
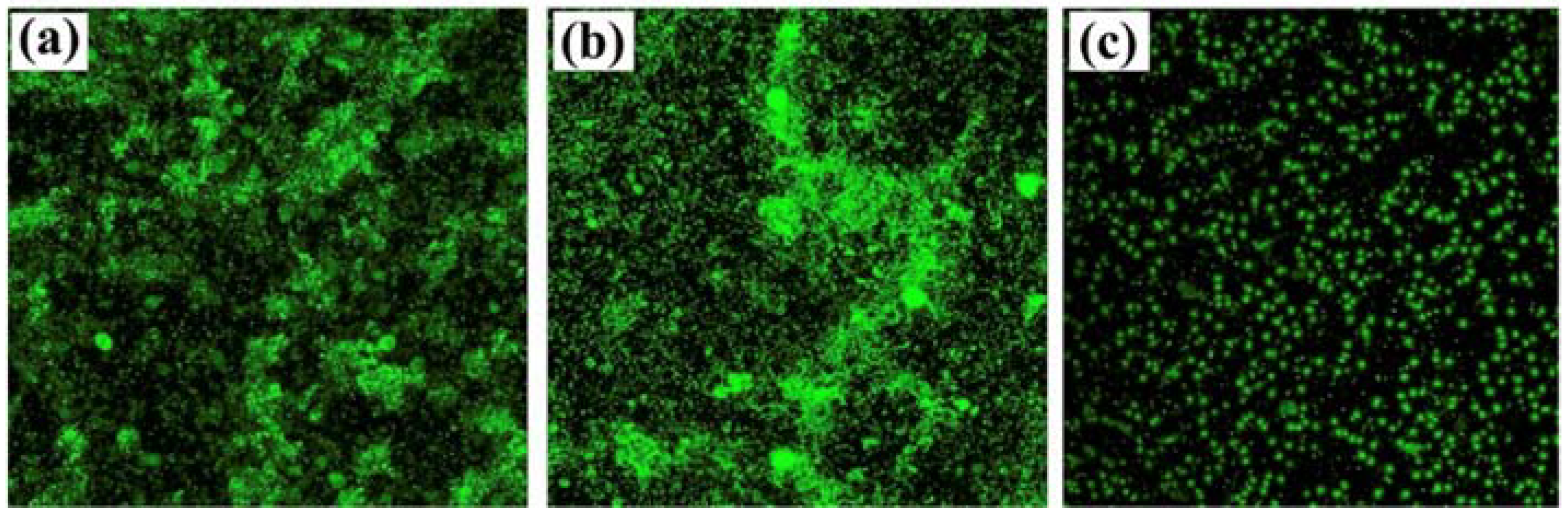
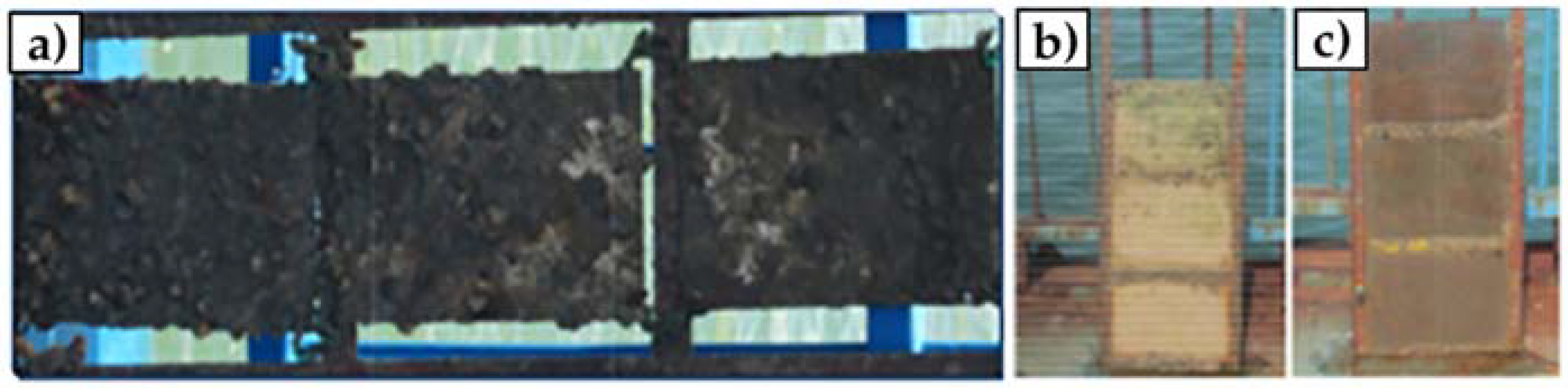
However, GO is often functionalized with metal nanoparticles, such as silver or alumina, and other compounds. These nanocomposites aim to provide GO with enhanced antimicrobial properties by improving particle dispersibility and strengthening the contact between the carbon nanomaterials and surrounding microorganisms. GO–silver nanoparticles coatings developed by Liu et al. and Zhang and Mikkelsen showed improved antibacterial and antialgal properties, and more than 80% average biofilm inhibition against H. pacifica (Figure 6), respectively [66,67]. Besides silver, alumina and silica nanoparticles have also been used in conjunction with GO, having both demonstrated excellent antimicrobial properties against a wide range of organisms [68,69]. In turn, the functionalization of GO with polyaniline/p-phenylenediamine conferred anticorrosion and AF properties to commercialized epoxy coatings [72]. Likewise, the modifications of GO materials with compounds such as cuprous oxide, acrylic acid, or boehmite nanorods, produced AF coatings with high self-cleaning performance and durability in marine environments (up to 6 months) (Figure 7) [70,71,73].
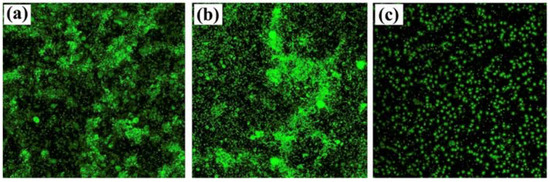
Figure 6.
Confocal laser scanning microscopy (CLSM) images of bare polypropylene (PP) (a), graphene oxide coated PP (b) and graphene oxide/silver nanoparticles coated PP (c). Reprinted with adaptations from [67], under the terms of Creative Commons CC BY license.

Figure 7.
In situ marine fouling tests over a period in a sheltered bay connected to the south China sea. Bared panels (90 days) (a); Cu2O paint-coated surfaces (365 days) (b); and GO/Cu2O paint-coated surfaces (365 days) (c). Reprinted with adaptations from [70], under the terms of the Creative Commons Attribution International License (CC BY 4.0).
In general, these results suggest that GO composites have promising AF and anticorrosion properties, which are so desirable in the marine industry.
2.4. Carbon-Nanotubes-Based Coatings
Up to date, several studies have reported the effectiveness of CNT-based coatings in the prevention and control of marine biofouling. Table 2 describes the studies demonstrating the efficacy of these coatings in marine environments, which refer essentially to MWCNTs.
Concerning the application of pristine MWCNTs (p-MWCNTs) for marine coatings, the obtained results differ. While some studies demonstrated that the incorporation of these carbon nanomaterials on PDMS improves its AF performance by reducing the abundance of pioneer eukaryotic microbes [79] and the adhesion strength of adult barnacles [75,78], other studies revealed that p-MWCNT-based coatings did not affect the settlement of micro- and macrofoulers [76,77].
In addition, recent studies have strived to assess the influence of carboxyl- and hydroxyl-modified MWCNTs on the AF performance of marine coatings. Sun and Zhang performed a thorough study of these modified surfaces by carrying out a two-month field trial focused on the addition of MWCNTs with varying hydroxyl content % (w/w), carboxyl content % (w/w), diameter, and length into PDMS coatings. Results showed that the type of MWCNTs had an impact on the coating’s AF behavior, as well as on pioneer eukaryotic communities [55]. Conversely, Ji et al. demonstrated that most carboxyl- and hydroxyl-modified coatings had weak modulating effects on pioneer biofilm communities [81], while Sun and Zhang showed that the AF behavior of these modified CNTs varied for different pioneer biofilm bacteria [55].
In turn, the production of fluorinated MWCNTs polymer-based coatings showed a promising antiadhesion effect against pseudobarnacles [84] and an impressive 98% reduction rate against E. coli [85].
Furthermore, the infusion of lubricants, such as silicone oil, into the polymeric matrices was revealed to be a promising approach to MWCNTs-based coatings [74,80,83]. This strategy aims to develop long-term superhydrophobic fouling release (FR) surfaces that leach lubricant over time, creating an isolation oil layer that protects the surface from deformation or damage caused by friction and reinforces its AF properties [83].
Altogether, these data provide important findings that should be considered in the development of new CNTs-based antifouling marine coatings.
2.5. Other Carbon-Nanomaterials-Based Coatings
Apart from GP and CNTs, the AF potential of other carbon nanomaterials has been explored. Recently, Luo et al. synthesized atomic chromium–graphitic carbon nitride coatings and demonstrated their in situ activity to control marine biofouling for approximately 2 months [86].
2.6. Qualitative Assessment
In order to assess the validity of the obtained results and their predictive value, the 30 selected articles were scored according to an adapted MINORS scale (Table 3). Out of a maximum score of 24, the studies obtained a mean score of 21.5 ± 2.0.

Table 3.
Adapted MINORS scale and mean score of the assessed studies.
All articles clearly stated the aim of the work, presented adequate methodologies, and provided enough information about the composition and fabrication method of the tested coating (criteria one, two, and four; mean score of 2.00). Additionally, 29 out of the 30 articles reported at least three replicates/independent experiments for each assay, as well as an adequate control group (criteria three and five; mean score of 1.93). Moreover, most studies provided sufficient information about the experimental setup used (criterion eight; mean score of 1.90) and implemented appropriate surface characterization methods (criterion six; mean score of 1.87).
Although these results are overall positive, some limitations were also found. It is noteworthy that 17 out of the 30 selected studies were carried out solely in vitro, most under static conditions (criterion seven; mean score of 1.73). This is quite unfortunate since experimental setups that mimic the marine environment (e.g., hydrodynamic conditions, day-to-night light variation) or in situ studies can be much more reliable for most applications. Moreover, a considerable number of studies did not report the number of organisms (e.g., cell concentration) that the surfaces were exposed to (criterion nine; mean score of 1.76), and only evaluated the performance of AF coatings for short-term adhesion (criterion 10; mean score of 1.76). Lastly, only 12 out of the 30 assessed publications clearly mentioned the implementation of statistical tests appropriate to the dataset (criterion 12; mean score of 0.87). Since a statistical analysis is a crucial part of producing trustworthy results and predictions, this is considered to be a critical flaw of most articles found.
Despite the overall high score of the selected studies, due to the wide array of methodologies used to test the AF properties of the coatings and the lack of proper statistical analysis, it is essential to highlight the importance of conducting further tests with robust, established methodologies and adequately analyzing the results, to facilitate the comparison between studies and draw more reliable conclusions about AF marine coatings. Moreover, one of the key issues in the development of new AF surfaces is the necessary screening of candidate surfaces (usually tested in vitro in a first step prior to in situ testing) before scale-up and final performance evaluation. Since it has been shown that hydrodynamics can severely affect biofilm formation [87,88,89,90] and gene expression by fouling organisms [91,92], it is recommended that these tests be performed in controlled hydrodynamic conditions that mimic the final application scenario [93].
3. Methods
3.1. Search Strategy, Study Eligibility, and Data Extraction
Previously published studies evaluating the effectiveness of carbon nanomaterials, namely GP and/or CNTs used to produce AF or FR coatings for the prevention or control of the attachment of micro- or macrofoulers in marine settings were systematically reviewed based on the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) Statement [94].
The search was conducted until June 6th, 2022, using two electronic databases, ScienceDirect and PubMed, through selective combinations of relevant keywords: “marine biofilm”, “biofouling”, “antifouling”, “coatings”, “graphene”, and “carbon nanotubes”. Published full-text articles in English were assessed for eligibility. Studies were screened based on title, abstract, and, ultimately, full content.
The reference sections of all included articles were carefully examined for additional articles that were not identified through the database search. The main inclusion criteria were: (1) in situ studies concerning the application of GP and/or CNTs composites as AF/FR coatings in the marine environment; (2) in vitro studies focused on assessing the properties of GP and/or CNTs composites, including their antiadhesion, antimicrobial, anticorrosion activities, and AF/FR performance against marine micro- and macrofoulers. As for the main exclusion criterion, any nonoriginal articles, such as reviews or reports, were discarded.
For each selected article, information concerning the materials and composition of the coatings used, identification of the tested fouling organisms, experimental setup, and relevant conclusions were extracted and analyzed by two independent reviewers.
3.2. Quality Assessment
Selected studies were subjected to a quality assessment procedure based on an adapted Methodological Index for Non-Randomized Studies (MINORS) scale [95]. MINORS is a validated instrument designed to evaluate methodological quality, as well as to detect potential biases in nonrandomized surgical studies. Although there are no methodological indices to measure the risk or the quality of nonclinical studies, the MINORS scale can be adjusted to other scientific contexts and still serve as a valuable quality assessment tool [96]. As such, the MINORS scale was adapted to the specific context of this systematic review and used to evaluate the overall quality and predictive value of each publication.
For each article, 12 parameters were evaluated: (1) clearly stated aim; (2) adequate methodology; (3) detection of bias; (4) proper identification and description of the studied coating; (5) adequate control group; (6) surface characterization; (7) type of study; (8) experimental setup; (9) organisms studied; (10) biofilm formation/fouling assay duration; (11) clarity of the results, and (12) adequate statistical analysis. Each parameter was scored according to a 3-point scale: 0 (not reported), 1 (inadequately reported), or 2 (adequately reported). Therefore, the maximum score a certain publication could achieve was 24.
4. Conclusions
Among the solutions found to control marine biofouling, the application of coatings with antifouling properties on submerged surfaces has been the most promising approach. Due to their outstanding properties, carbon nanomaterials have proven to be promising in the production of antimicrobial and antifouling surface coatings.
This systematic review demonstrated that, over the last few years, there has been an increasing interest in the synthesis and evaluation of carbon-based coatings, in particular those containing GP or CNTs, to prevent and control marine biofouling. Most of the reviewed studies investigated the efficacy of modified/functionalized GP or CNTs, which demonstrated improved AF performance compared to their pristine forms.
Although these studies provided promising results, most AF coatings were only evaluated in vitro and, therefore, in situ or case studies are missing to validate their real-world application. It is also noteworthy that 26.7% of studies only qualitatively evaluated the AF performance of developed coatings or did not disclose the extent of attachment reduction and/or foulers inactivation. Moreover, the degradation or bioaccumulation rates of carbon-based coatings in the marine environment, as well as their toxicity for nontarget organisms were not addressed in the reviewed studies.
The development of more accurate and reliable in vitro test methods that are able to mimic the hydrodynamic conditions observed for each target application is of paramount importance in obtaining reliable data. This is crucial because in situ tests should not be performed with surfaces that release compounds for which the leaching and toxicity profiles have not been determined. With an increasing environmental conscience from the public and regulatory organizations, novel coatings must be nontoxic to nontarget organisms, durable, and amenable to large-scale production in a sustainable way. This is a multidisciplinary endeavor requiring the involvement of different stakeholders who have to find a common language to address these issues. In a globalized world depending on maritime transportation, the development of more effective AF coatings is of extreme importance.
Overall, this review illustrated the antifouling potential of carbon-based surfaces for marine applications and emphasized the need for further research on this topic.
Author Contributions
Conception and design of the study: F.S.-C. and R.T.-S. Literature searches and data extraction: F.S.-C. and R.T.-S. Analysis and interpretation of data: F.S.-C. and R.T.-S. Drafting the article: F.S.-C. and R.T.-S. Critical revision of the article: F.J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by: LA/P/0045/2020 (ALiCE), UIDB/00511/2020 and UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC); project HealthyWaters (NORTE-01-0145-FEDER-000069), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); project PTDC/CTM-COM/4844/2020 (NanoCAT) supported by national funds through the FCT/MCTES (PIDDAC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The Structure of Suspended Graphene Sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, Y.; Tong, W.; Sun, L.; Huang, H.; An, Q.; Tian, N.; Chu, P.K. Graphene for Energy Storage and Conversion: Synthesis and Interdisciplinary Applications. Electrochem. Energy Rev. 2020, 3, 395–430. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, Y.-J. Recent Progress on Graphene-Based Photocatalysts: Current Status and Future Perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Lukowiak, A.; Kedziora, A.; Strek, W. Antimicrobial Graphene Family Materials: Progress, Advances, Hopes and Fears. Adv. Colloid Interface Sci. 2016, 236, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chou, T.-W. A Structural Mechanics Approach for the Analysis of Carbon Nanotubes. Int. J. Solids Struct. 2003, 40, 2487–2499. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and Anti-Adhesive Properties of Carbon Nanotube-Based Surfaces for Medical Applications: A Systematic Review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 1–47. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Gu, B.E.; Huang, C.Y.; Shen, T.H.; Lee, Y.L. Effects of Multiwall Carbon Nanotube Addition on the Corrosion Resistance and Underwater Acoustic Absorption Properties of Polyurethane Coatings. Prog. Org. Coatings 2018, 121, 226–235. [Google Scholar] [CrossRef]
- Fu, Y. Synergism of Carbon Nanotubes and Graphene Nanoplates in Improving Underwater Sound Absorption Stability under High Pressure. ChemistrySelect 2022, 7, e202103222. [Google Scholar] [CrossRef]
- Ng, K.-W.; Lam, W.-H.; Pichiah, S. A Review on Potential Applications of Carbon Nanotubes in Marine Current Turbines. Renew. Sustain. Energy Rev. 2013, 28, 331–339. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2022, 9, 1. [Google Scholar] [CrossRef]
- Novoa, A.F.; Vrouwenvelder, J.S.; Fortunato, L. Membrane Fouling in Algal Separation Processes: A Review of Influencing Factors and Mechanisms. Front. Chem. Eng. 2021, 3, 687422. [Google Scholar] [CrossRef]
- Meng, S.; Wang, R.; Zhang, K.; Meng, X.; Xue, W.; Liu, H.; Liang, D.; Zhao, Q.; Liu, Y. Transparent Exopolymer Particles (TEPs)-Associated Protobiofilm: A Neglected Contributor to Biofouling during Membrane Filtration. Front. Environ. Sci. Eng. 2020, 15, 64. [Google Scholar] [CrossRef]
- Wei, S.; Du, L.; Chen, S.; Yu, H.; Quan, X. Electro-Assisted CNTs/Ceramic Flat Sheet Ultrafiltration Membrane for Enhanced Antifouling and Separation Performance. Front. Environ. Sci. Eng. 2020, 15, 11. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental Assessment of the Performance of Two Marine Coatings to Curb Biofilm Formation of Microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Ferreira, O.; Rijo, P.; Gomes, J.; Santos, R.; Monteiro, S.; Guedes, R.; Serralheiro, M.L.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J.; et al. Antimicrobial Ceramic Filters for Water Bio-Decontamination. Coatings 2021, 11, 323. [Google Scholar] [CrossRef]
- Bott, T.R. Industrial Biofouling; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444532244. [Google Scholar]
- Callow, M.E.; Callow, J.E. Marine Biofouling: A Sticky Problem. Biologist 2002, 49, 10–14. [Google Scholar]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Edyvean, R. Consequences of Fouling on Shipping. In Biofouling; Wiley-Blackwell: Oxford, UK, 2010; pp. 217–225. ISBN 9781444315462. [Google Scholar]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-Toxic, Non-Biocide-Release Antifouling Coatings Based on Molecular Structure Design for Marine Applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Butler, A.J. Biofouling and Climate Change. In Biofouling; Wiley-Blackwell: Oxford, UK, 2010; pp. 333–347. ISBN 9781444315462. [Google Scholar]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Delauney, L.; Compare, C.; Lehaitre, M. Biofouling Protection for Marine Environmental Sensors. Ocean. Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef]
- Dang, H.; Chen, R.; Wang, L.; Shao, S.; Dai, L.; Ye, Y.; Guo, L.; Huang, G.; Klotz, M.G. Molecular Characterization of Putative Biocorroding Microbiota with a Novel Niche Detection of Epsilon- and Zetaproteobacteria in Pacific Ocean Coastal Seawaters. Environ. Microbiol. 2011, 13, 3059–3074. [Google Scholar] [CrossRef]
- Taylor, D.A. Instrumentation and Control. In Introduction to Marine Engineering; Butterworth-Heinemann: Oxford, UK, 1983; pp. 270–315. ISBN 978-0-408-00586-9. [Google Scholar]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Silva, E.R.; Ferreira, O.; Ramalho, P.A.; Azevedo, N.F.; Bayón, R.; Igartua, A.; Bordado, J.C.; Calhorda, M.J. Eco-Friendly Non-Biocide-Release Coatings for Marine Biofouling Prevention. Sci. Total Environ. 2019, 650, 2499–2511. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Meyer, R.L.; Laursen, B.S.; Shipovskov, S.; Besenbacher, F.; Poulsen, C.H. Antifouling Enzymes and the Biochemistry of Marine Settlement. Biotechnol. Adv. 2008, 26, 471–481. [Google Scholar] [CrossRef]
- Fusetani, N. Antifouling Marine Natural Products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Biofilms: New Ideas for An Old Problem. Recent Pat. Biotechnol. 2012, 6, 13–22. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef]
- Michailidis, M.; Gutner-Hoch, E.; Wengier, R.; Onderwater, R.; D’Sa, R.A.; Benayahu, Y.; Semenov, A.; Vinokurov, V.; Shchukin, D.G. Highly Effective Functionalized Coatings with Antibacterial and Antifouling Properties. ACS Sustain. Chem. Eng. 2020, 8, 8928–8937. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine Paints: The Particular Case of Antifouling Paints. Prog. Org. Coatings 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Howell, D.; Behrends, B. Consequences of Antifouling Coatings—The Chemist’s Perspective. In Biofouling; Wiley-Blackwell: Oxford, UK, 2010; pp. 226–242. ISBN 9781444315462. [Google Scholar]
- Meng, P.J.; Wang, J.T.; Liu, L.L.; Chen, M.H.; Hung, T.C. Toxicity and Bioaccumulation of Tributyltin and Triphenyltin on Oysters and Rock Shells Collected from Taiwan Maricuture Area. Sci. Total Environ. 2005, 349, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment Point Theory Revisited: The Fouling Response to a Microtextured Matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Nanocoating Is a New Way for Biofouling Prevention. Front. Nanotechnol. 2021, 3, 771098. [Google Scholar] [CrossRef]
- Dustebek, J.; Kandemir-Cavas, C.; Nitodas, S.F.; Cavas, L. Effects of Carbon Nanotubes on the Mechanical Strength of Self-Polishing Antifouling Paints. Prog. Org. Coatings 2016, 98, 18–27. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the Role of Carbon Nanotube Composites in Preventing Biofouling and Promoting Biofilms on Material Surfaces in Environmental Engineering: A Review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial Activity of Graphene-Based Materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Callow, J.A. Biological Adhesives; Springer: Berlin/Heidelberg, Germany, 2006; Volume 23, ISBN 978-3-540-31048-8. [Google Scholar]
- Gomes, M.; Gomes, L.C.; Teixeira-Santos, R.; Pereira, M.F.R.; Soares, O.S.G.P.; Mergulhão, F.J. Optimizing CNT Loading in Antimicrobial Composites for Urinary Tract Application. Appl. Sci. 2021, 11, 4038. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, M.; Zhang, Q.; Jiang, W. Graphene Nanosheets Damage the Lysosomal and Mitochondrial Membranes and Induce the Apoptosis of RBL-2H3 Cells. Sci. Total Environ. 2020, 734, 139229. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Radhi, A.; Mohamad, D.; Abdul Rahman, F.S.; Abdullah, A.M.; Hasan, H. Mechanism and Factors Influence of Graphene-Based Nanomaterials Antimicrobial Activities and Application in Dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; Rehan, Z.A.; Noreen, S.; Zahid, M. Recent Developments for Antimicrobial Applications of Graphene-Based Polymeric Composites: A Review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon Nanomaterials against Pathogens; the Antimicrobial Activity of Carbon Nanotubes, Graphene/Graphene Oxide, Fullerenes, and Their Nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Sokolova, A.; Cilz, N.; Daniels, J.; Stafslien, S.J.; Brewer, L.H.; Wendt, D.E.; Bright, F.V.; Detty, M.R. A Comparison of the Antifouling/Foul-Release Characteristics of Non-Biocidal Xerogel and Commercial Coatings toward Micro- and Macrofouling Organisms. Biofouling 2012, 28, 511–523. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. New Anti-Biofouling Carbon Nanotubes-Filled Polydimethylsiloxane Composites against Colonization by Pioneer Eukaryotic Microbes. Int. Biodeterior. Biodegrad. 2016, 110, 147–154. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, Y.; Zhang, Y.; Ren, Y.; Bai, S.-L. Three-Dimensional Graphene Foam-Filled Elastomer Composites with High Thermal and Mechanical Properties. ACS Appl. Mater. Interfaces 2017, 9, 26447–26459. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A Nanomolecular Approach to Decrease Adhesion of Biofouling-Producing Bacteria to Graphene-Coated Material. J. Nanobiotechnology 2015, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, T.; Bing, W.; Dong, S.; Tian, L. Antifouling Performance and Mechanism of Elastic Graphene-Silicone Rubber Composite Membranes. J. Mater. Chem. B 2019, 7, 488–497. [Google Scholar] [CrossRef]
- Yee, M.S.L.; Khiew, P.S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.Y.; Leong, C.O. Green Synthesis of Graphene-Silver Nanocomposites and Its Application as a Potent Marine Antifouling Agent. Colloids Surfaces B Biointerfaces 2016, 148, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, R.; Song, D.; Yu, J.; Sun, G.; Liu, Q.; Han, S.; Liu, J.; Zhang, H.; Wang, J. Guanidine-Functionalized Graphene to Improve the Antifouling Performance of Boron Acrylate Polymer. Prog. Org. Coatings 2021, 159, 106396. [Google Scholar] [CrossRef]
- Manderfeld, E.; Kleinberg, M.N.; Thamaraiselvan, C.; Koschitzki, F.; Gnutt, P.; Plumere, N.; Arnusch, C.J.; Rosenhahn, A. Electrochemically Activated Laser-Induced Graphene Coatings against Marine Biofouling. Appl. Surf. Sci. 2021, 569, 150853. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.J. Graphene Oxide Nanopaint. Carbon 2014, 72, 328–337. [Google Scholar] [CrossRef]
- Jin, H.; Bing, W.; Tian, L.; Wang, P.; Zhao, J. Combined Effects of Color and Elastic Modulus on Antifouling Performance: A Study of Graphene Oxide/Silicone Rubber Composite Membranes. Materials 2019, 12, 2608. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Qin, W. Improving the Environmental Compatibility of Marine Sensors by Surface Functionalization with Graphene Oxide. Anal. Chem. 2019, 91, 13268–13274. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, S.; Li, Q.; Wang, J.; Pu, J.; Wang, G.; Zhao, W.; Feng, F.; Qin, J.; Ren, L. Integrated Dual-Functional ORMOSIL Coatings with AgNPs@rGO Nanocomposite for Corrosion Resistance and Antifouling Applications. ACS Sustain. Chem. Eng. 2020, 8, 6786–6797. [Google Scholar] [CrossRef]
- Zhang, X.; Mikkelsen, Ø. Graphene Oxide/Silver Nanocomposites as Antifouling Coating on Sensor Housing Materials. J. Clust. Sci. 2021, 33, 627–635. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/Graphene Oxide Sheet-Alumina Nanorod Ternary Composite for Superhydrophobic Antifouling Coating. Prog. Org. Coatings 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Jena, G.; Pongachira George, R.; Philip, J. Polydimethylsiloxane–Graphene Oxide Nanocomposite Coatings with Improved Anti-Corrosion and Anti-Biofouling Properties. Environ. Sci. Pollut. Res. 2021, 28, 7404–7422. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, L.; Huang, D.; Jiang, L.; Liu, L.; Li, F.; Pang, A.; Guo, X.; Tao, B. In Situ Synthesis of Graphene@cuprous Oxide Nanocomposite Incorporated Marine Antifouling Coating with Elevated Antifouling Performance. Open J. Org. Polym. Mater. 2019, 9, 47–62. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wang, F.; Liang, W.; Yang, H.; Wu, D. Fabrication of Acrylic Acid Modified Graphene Oxide (AGO)/Acrylate Composites and Their Synergistic Mechanisms of Anticorrosion and Antifouling Properties. Prog. Org. Coatings 2022, 168, 106910. [Google Scholar] [CrossRef]
- Fazli-Shokouhi, S.; Nasirpouri, F.; Khatamian, M. Epoxy-Matrix Polyaniline/p-Phenylenediamine-Functionalised Graphene Oxide Coatings with Dual Anti-Corrosion and Anti-Fouling Performance. RSC Adv. 2021, 11, 11627–11641. [Google Scholar] [CrossRef]
- Selim, M.S.; Fatthallah, N.A.; Higazy, S.A.; Hao, Z.; Jing Mo, P. A Comparative Study between Two Novel Silicone/Graphene-Based Nanostructured Surfaces for Maritime Antifouling. J. Colloid Interface Sci. 2022, 606, 367–383. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, W.; Wu, Y. Preventing Algae Biofilm Formation via Designing Long-Term Oil Storage Surfaces for Excellent Antifouling Performance. Appl. Surf. Sci. 2021, 554, 149612. [Google Scholar] [CrossRef]
- Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubois, P. Preparation and Characterisation of Silicone-Based Coatings Filled with Carbon Nanotubes and Natural Sepiolite and Their Application as Marine Fouling-Release Coatings. Biofouling 2008, 24, 291–302. [Google Scholar] [CrossRef]
- Martinelli, E.; Suffredini, M.; Galli, G.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Williams, D.; Lyall, G. Amphiphilic Block Copolymer/Poly(Dimethylsiloxane) (PDMS) Blends and Nanocomposites for Improved Fouling-Release. Biofouling 2011, 27, 529–541. [Google Scholar] [CrossRef]
- Carl, C.; Poole, A.J.; Vucko, M.J.; Williams, M.R.; Whalan, S.; de Nys, R. Enhancing the Efficacy of Fouling-Release Coatings against Fouling by Mytilus Galloprovincialis Using Nanofillers. Biofouling 2012, 28, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Li, Y.F.; Guo, X.P.; Liang, X.; Xu, Y.F.; Ding, D.W.; Bao, W.Y.; Dobretsov, S. The Effect of Carbon Nanotubes and Titanium Dioxide Incorporated in PDMS on Biofilm Community Composition and Subsequent Mussel Plantigrade Settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lang, Y.; Sun, T.; Liu, Q.; Pan, Y.; Qi, Z.; Ling, N.; Feng, Y.; Yu, M.; Ji, Y.; et al. Antifouling Potential of Multi-Walled Carbon Nanotubes-Modified Chlorinated Rubber-Based Composites on the Colonization Dynamics of Pioneer Biofilm-Forming Eukaryotic Microbes. Int. Biodeterior. Biodegrad. 2020, 149, 104921. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.; Qi, Y. hong The Influence of MWCNTs-OH on the Properties of the Fouling Release Coatings Based on Polydimethylsiloxane with the Incorporation of Phenylmethylsilicone Oil. Prog. Org. Coatings 2019, 130, 132–143. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, Y.; Lang, Y.; Wang, L.; Liu, B.; Zhang, Z. Effect of CNT/PDMS Nanocomposites on the Dynamics of Pioneer Bacterial Communities in the Natural Biofilms of Seawater. Materials 2018, 11, 902. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Yan, Z.Y.; Wang, L.; Zhang, Z. High-Throughput Sequencing Analysis of Marine Pioneer Surface-Biofilm Bacteria Communities on Different PDMS-Based Coatings. Colloids Surfaces B Biointerfaces 2020, 185, 110538. [Google Scholar] [CrossRef]
- Fan, F.-x.; Zheng, Y.-m.; Ba, M.; Wang, Y.-f.; Kong, J.-j.; Liu, J.-h.; Wu, Q. Long Time Super-Hydrophobic Fouling Release Coating with the Incorporation of Lubricant. Prog. Org. Coatings 2021, 152, 106136. [Google Scholar] [CrossRef]
- Irani, F.; Jannesari, A.; Bastani, S. Effect of Fluorination of Multiwalled Carbon Nanotubes (MWCNTs) on the Surface Properties of Fouling-Release Silicone/MWCNTs Coatings. Prog. Org. Coatings 2013, 76, 375–383. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, G.; Yang, Z.; Cui, Y.; Li, H.; Zhang, Y. Fluorinated Carbon Nanotube Superamphiphobic Coating for High-Efficiency and Long-Lasting Underwater Antibiofouling Surfaces. ACS Appl. Bio Mater. 2021, 4, 6351–6360. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.; Huo, X.; Li, L.; Song, Y.; Chen, S.; Lin, H.; Wang, N. Atomic Chromium Coordinated Graphitic Carbon Nitride for Bioinspired Antibiofouling in Seawater. Adv. Sci. 2022, 9, 2105346. [Google Scholar] [CrossRef]
- Faria, S.I.; Gomes, L.C.; Teixeira-Santos, R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Developing New Marine Antifouling Surfaces: Learning from Single-Strain Laboratory Tests. Coatings 2021, 11, 90. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J. The Association between Initial Adhesion and Cyanobacterial Biofilm Development. FEMS Microbiol. Ecol. 2021, 97, fiab52. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.I.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; de Jong, E.; Sjollema, J.; Vasconcelos, V.; Mergulhão, F.J. Unveiling the Antifouling Performance of Different Marine Surfaces and Their Effect on the Development and Structure of Cyanobacterial Biofilms. Microorganisms 2021, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.I.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J. The Relative Importance of Shear Forces and Surface Hydrophobicity on Biofilm Formation by Coccoid Cyanobacteria. Polymers 2020, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Campos, A.; Vasconcelos, V.; Mergulhão, F.J.M. Characterization of Planktonic and Biofilm Cells from Two Filamentous Cyanobacteria Using a Shotgun Proteomic Approach. Biofouling 2020, 36, 631–645. [Google Scholar] [CrossRef]
- Romeu, M.J.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Araújo, M.J.; Osório, H.; Campos, A.; Vasconcelos, V.; Mergulhão, F.J. Quantitative Proteomic Analysis of Marine Biofilms Formed by Filamentous Cyanobacterium. Environ. Res. 2021, 201, 111566. [Google Scholar] [CrossRef]
- Romeu, M.J.; Alves, P.; Morais, J.; Miranda, J.M.; Jong, E.D.; Sjollema, J.; Ramos, V.; Vasconcelos, V.; Mergulhão, F.J.M. Biofilm Formation Behaviour of Marine Filamentous Cyanobacterial Strains in Controlled Hydrodynamic Conditions. Environ. Microbiol. 2019, 21, 4411–4424. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (MINORS): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).