Phage-Related Ribosomal Proteases (Prps): Discovery, Bioinformatics, and Structural Analysis
Abstract
:1. Introduction
1.1. Discovery of Prp & Phage Relation
1.2. Ribosomal Protein L27
1.3. Prp as a Cleavage Enzyme
2. Materials and Methods
2.1. Bioinformatics
2.2. Structural Analysis
3. Results and Discussion
3.1. Bioinformatics
3.2. Prps of Bacteria without an L27 N-Terminal Extension
3.3. Mechanism
3.3.1. Proteolytic Mechanism of Prp
3.3.2. Prp as a Ribosomal Protein Chaperone
3.4. Structure
3.4.1. Case Study: SaPrp Structural Studies
3.4.2. Firmicute Prp Homology
3.4.3. Non-L27 Cleaving Prps
4. Conclusions
- Accession Codes:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Talbot, G.H.; Jezek, A.; Murray, B.E.; Jones, R.N.; Ebright, R.H.; Nau, G.J.; Rodvold, K.A.; Newland, J.G.; Boucher, H.W. The Infectious Diseases Society of America’s 10 x ‘20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 x ‘20 a Possibility? Clin. Infect. Dis. 2019, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services. CDC, Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2019.
- Kaczor, A.A.; Polski, A.; Sobótka-Polska, K.; Pachuta-Stec, A.; Makarska-Bialokoz, M.; Pitucha, M. Novel antibacterial compounds and their drug targets-successes and challenges. Curr. Med. Chem. 2017, 24, 1948–1982. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, D.E.; Clemons, W.M.; Carter, A.P.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B, on the 30S ribosomal subunit. Cell 2000, 103, 1143–1154. [Google Scholar] [CrossRef]
- Colca, J.R.; McDonald, W.G.; Waldon, D.J.; Thomasco, L.M.; Gadwood, R.C.; Lund, E.T.; Cavey, G.S.; Mathews, W.R.; Adams, L.D.; Cecil, E.T.; et al. Cross-linking in the living cell locates the site of action of oxazolidinone antibiotics. J. Biol. Chem. 2003, 278, 21972–21979. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The mechanism of action of macrolides, lincosamides and Streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Hansen, J.L.; Ippolito, J.A.; Ban, N.; Nissen, P.; Moore, P.B.; Steitz, T.A. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 2002, 10, 117–128. [Google Scholar] [CrossRef]
- Reimer, L.G.; Stratton, C.W.; Reller, A.L.B. Minimum inhibitory and bactericidal concentrations of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob. Agents Chemother. 1981, 19, 1050–1055. [Google Scholar] [CrossRef]
- Zuo, P.; Yu, P.; Alvarez, P.J.J. Aminoglycosides antagonize bacteriophage proliferation, attenuating phage suppression of bacterial growth, biofilm formation, and antibiotic resistance. Appl. Environ. Microbiol. 2021, 87, e0046821. [Google Scholar] [CrossRef]
- Wall, E.A.; Caufield, J.H.; Lyons, C.E.; Manning, K.A.; Dokland, T.; Christie, G.E. Specific N-terminal cleavage of ribosomal protein L27 in Staphylococcus aureus and related bacteria. Mol. Microbiol. 2015, 95, 258–269. [Google Scholar] [CrossRef]
- Wall, E.A.; Johnson, A.L.; Peterson, D.L.; Christie, G.E. Structural modeling and functional analysis of the essential ribosomal processing protease Prp from Staphylococcus aureus. Mol. Microbiol. 2017, 104, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Kolár, M.; Urbánek, K.; Látal, T. Antibiotic selective pressure and development of bacterial resistance. Int. J. Antimicrob. Agents 2001, 17, 357–363. [Google Scholar] [CrossRef]
- Christie, G.E.; Matthews, A.M.; King, D.G.; Lane, K.D.; Olivarez, N.P.; Tallent, S.M.; Gill, S.R.; Novick, R.P. The complete genomes of Staphylococcus aureus bacteriophages 80 and 80α—Implications for the specificity of SaPI mobilization. Virology 2010, 407, 381. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef]
- Wall, E. Elucidation of a Novel Pathway in Staphylococcus aureus: The Essential Site-Specific Processing of Ribosomal Protein L27. Ph.D. Thesis, Virginia Commonwealth University, Richmond, VA, USA, 10 April 2015. [Google Scholar]
- Caufield, H. N-Terminal Processing of Ribosomal Protein L27 in Staphylococcus aureus. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2012. [Google Scholar]
- Shajani, Z.; Sykes, M.T.; Williamson, J.R. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 2011, 80, 501–526. [Google Scholar] [CrossRef]
- De La Cruz, J.; Karbstein, K.; Woolford, J.L. Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu. Rev. Biochem. 2015, 84, 93–129. [Google Scholar] [CrossRef]
- Wilson, D.N.; Nierhaus, K.H. Ribosomal proteins in the spotlight. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 243–267. [Google Scholar] [CrossRef]
- Kressler, D.; Hurt, E.; Baßler, J. A puzzle of life: Crafting ribosomal subunits. Trends Biochem. Sci. 2017, 42, 640–654. [Google Scholar] [CrossRef]
- Trobro, S.; Åqvist, J. Role of ribosomal protein L27 in peptidyl transfer. Biochemistry 2008, 47, 4898–4906. [Google Scholar] [CrossRef]
- Wang, Y. Single molecule fluorescence energy transfer study of ribosome protein synthesis. J. Vis. Exp. 2021, 173, e62664. [Google Scholar] [CrossRef]
- Shoji, S.; Dambacher, C.M.; Shajani, Z.; Williamson, J.R.; Schultz, P.G. Systematic chromosomal deletion of bacterial ribosomal protein genes. J. Mol. Biol. 2011, 413, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; Schluenzen, F.; Harms, J.; Bashan, A.; Auerbach, T.; Baram, D.; Yonath, A. Structural insight into the role of the ribosomal tunnel in cellular regulation. Nat. Struct. Biol. 2003, 10, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Puigbò, P.; Koonin, E.V.; Wolf, Y.I. Phylogenomics of prokaryotic ribosomal proteins. PLoS ONE 2012, 7, e36972. [Google Scholar] [CrossRef] [PubMed]
- Maracci, C.; Wohlgemuth, I.; Rodnina, M.V. Activities of the peptidyl transferase center of ribosomes lacking protein L27. RNA 2015, 21, 2047–2052. [Google Scholar] [CrossRef]
- Maguire, B.A.; Beniaminov, A.D.; Ramu, H.; Mankin, A.S.; Zimmermann, R.A. A protein component at the heart of an RNA machine: The importance of protein L27 for the function of the bacterial ribosome. Mol. Cell 2005, 20, 427–435. [Google Scholar] [CrossRef]
- Mikulík, K.; Bobek, J.; Ziková, A.; Smětáková, M.; Bezoušková, S. Phosphorylation of ribosomal proteins influences subunit association and translation of poly (U) in Streptomyces coelicolor. Mol. BioSyst. 2011, 7, 817–823. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Garcia-Vallvé, S.; Simó, F.X.; Montero, M.A.; Arola, L.; Romeu, A. Simultaneous horizontal gene transfer of a gene coding for ribosomal protein L27 and operational genes in Arthrobacter sp. J. Mol. Evol. 2002, 55, 632–637. [Google Scholar] [CrossRef]
- Hotinger, J.A.; Pendergrass, H.A.; Peterson, D.; Wright, H.T.; May, A.E. Phage-related ribosomal protease (Prp) of Staphylococcus aureus: In vitro Michaelis–Menten kinetics, screening for inhibitors, and crystal structure of a covalent inhibition product complex. Biochemistry 2022, 61, 1323–1336. [Google Scholar] [CrossRef]
- Kramer, M.J.; Cleeland, R.; Grunberg, E. Mersalyl: A diuretic with antiviral properties. Antimicrob. Agents Chemother. 1975, 8, 295–299. [Google Scholar] [CrossRef]
- Lauber, M.A.; Running, W.E.; Reilly, J.P. B. subtilis ribosomal proteins: Structural homology and post-translational modifications. J. Proteome Res. 2009, 8, 4193–4206. [Google Scholar] [CrossRef] [PubMed]
- Filbeck, S.; Cerullo, F.; Paternoga, H.; Tsaprailis, G.; Joazeiro, C.A.P.; Pfeffer, S. Mimicry of canonical translation elongation underlies alanine tail synthesis in RQC. Mol. Cell 2021, 81, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.E.; Oostergetel, G.T.; Pijning, T.; Puri, P.; Arkhipova, V.; Boekema, E.J.; Poolman, B.; Guskov, A. A general mechanism of ribosome dimerization revealed by single-particle cryo-electron microscopy. Nat. Commun. 2017, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Crowe-Mcauliffe, C.; Murina, V.; Turnbull, K.J.; Kasari, M.; Mohamad, M.; Polte, C.; Takada, H.; Vaitkevicius, K.; Johansson, J.; Ignatova, Z.; et al. Structural basis of ABCF-mediated resistance to pleuromutilin, lincosamide, and streptogramin A antibiotics in Gram-positive pathogens. Nat. Commun. 2021, 12, 3577. [Google Scholar] [CrossRef]
- Golubev, A.; Fatkhullin, B.; Khusainov, I.; Jenner, L.; Gabdulkhakov, A.; Validov, S.; Yusupova, G.; Yusupov, M.; Usachev, K. Cryo-EM structure of the ribosome functional complex of the human pathogen Staphylococcus aureus at 3.2 Å resolution. FEBS Lett. 2020, 594, 3551–3567. [Google Scholar] [CrossRef]
- Watson, Z.L.; Ward, F.R.; Méheust, R.; Ad, O.; Schepartz, A.; Banfield, J.F.; Cate, J.H. Structure of the bacterial ribosome at 2 Å resolution. eLife 2020, 9, e60482. [Google Scholar] [CrossRef]
- Murphy, E.L.; Singh, K.V.; Avila, B.; Kleffmann, T.; Gregory, S.T.; Murray, B.E.; Krause, K.L.; Khayat, R.; Jogl, G. Cryo-electron microscopy structure of the 70S ribosome from Enterococcus faecalis. Sci. Rep. 2020, 10, 16301. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Swaney, S.M.; Colca, J.R.; Mcdonald, W.G.; Blinn, J.R.; Thomasco, L.M.; Gadwood, R.C.; Shinabarger, D.; Xiong, L.; Mankin, A.S.; et al. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 2007, 26, 393–402. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Chaudhuri, R.R.; Allen, A.G.; Owen, P.J.; Shalom, G.; Stone, K.; Harrison, M.; Burgis, T.A.; Lockyer, M.; Garcia-Lara, J.; Foster, S.J.; et al. Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH). BMC Genom. 2009, 10, 291. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, L.; Johnson, S.; Zeitler, A.; Bäurle, S.; Deme, J.C.; Caesar, J.J.E.; Debo, R.; Fisher, J.; Wagner, S.; Lea, S.M. The substrate specificity switch FlhB assembles onto the export gate to regulate type three secretion. Nat. Commun. 2020, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kinoshita, M.; Namba, K.; Minamino, T. Mutational analysis of the C-terminal cytoplasmic domain of FlhB, a transmembrane component of the flagellar type III protein export apparatus in Salmonella. Genes Cells 2019, 24, 408–421. [Google Scholar] [CrossRef]
- Minamino, T.; Namba, K. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 2008, 451, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef]
- Bernard, Q.; Thakur, M.; Smith, A.A.; Kitsou, C.; Yang, X.; Pal, U. Borrelia burgdorferi protein interactions critical for microbial persistence in mammals. Cell. Microbiol. 2019, 21, e12885. [Google Scholar] [CrossRef] [PubMed]
- Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. Virulence gene expression, adhesion and invasion of Campylobacter jejuni exposed to oxidative stress (H2O2). Int. J. Food Microbiol. 2016, 220, 33–38. [Google Scholar] [CrossRef]
- Grudkowska, M.; Zagdańska, B. View of multifunctional role of plant cysteine proteinases. Acta Biochim. Pol. 2004, 51, 609–624. [Google Scholar] [CrossRef]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 1997, 59, 63–88. [Google Scholar] [CrossRef]
- Prokchorchik, M.; Choi, S.; Chung, E.; Won, K.; Dangl, J.L.; Sohn, K.H. A host target of a bacterial cysteine protease virulence effector plays a key role in convergent evolution of plant innate immune system receptors. New Phytol. 2020, 225, 1327–1342. [Google Scholar] [CrossRef]
- Vernet, T.; Tessier, D.C.; Chatellier, J.; Plouffe, C.; Lee, T.S.; Thomas, D.Y.; Storer, A.C.; Ménard, R. Structural and functional roles of asparagine 175 in the cysteine protease papain. J. Biol. Chem. 1995, 270, 16645–16652. [Google Scholar] [CrossRef] [PubMed]
- Siklos, M.; BenAissa, M.; Thatcher, G.R.J. Cysteine proteases as therapeutic targets: Does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm. Sin. B 2015, 5, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, L.; Malakar, S.; Songprakhon, P.; Morchang, A.; Srisawat, C.; Noisakran, S.; Yenchitosomanus, P.T.; Limjindaporn, T. Serine protease inhibitor AEBSF reduces dengue virus infection via decreased cholesterol synthesis. Virus Res. 2019, 271, 197672. [Google Scholar] [CrossRef] [PubMed]
- Pillet, B.; Mitterer, V.; Kressler, D.; Pertschy, B. Hold on to your friends: Dedicated chaperones mediate the safe transfer of ribosomal proteins to their site of pre-ribosome incorporation. BioEssays 2017, 39, e201600153. [Google Scholar] [CrossRef] [PubMed]
- Polymenis, M. Ribosomal proteins: Mutant phenotypes by the numbers and associated gene expression changes. Open Biol. 2020, 10, 200114. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.H.; Tan, Y.Z.; Carragher, B.; Potter, C.S.; Lyumkis, D.; Williamson, J.R. Modular assembly of the bacterial large ribosomal subunit. Cell 2016, 167, 1610–1622.e15. [Google Scholar] [CrossRef]
- Kapust, R.B.; Tözsér, J.; Fox, J.D.; Anderson, D.E.; Cherry, S.; Copeland, T.D.; Waugh, D.S. Tobacco etch virus protease: Mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. Des. Sel. 2001, 14, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A. Enhanced activity and stability of papain immobilized on CNBr-activated sepharose. Int. J. Biol. Macromol. 2015, 75, 373–377. [Google Scholar] [CrossRef]
- Brömme, D.; Bescherer, K.; Kirschke, H.; Fittkau, S. Enzyme-substrate interactions in the hydrolysis of peptides by cathepsins B and H from rat liver. Biochem. J. 1987, 245, 381–385. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Varadi, M.; Tompa, P.; Pauwels, K. Affinity purification of human m-calpain through an intrinsically disordered inhibitor, calpastatin. PLoS ONE 2017, 12, e0174125. [Google Scholar] [CrossRef]
- Petsko, G.A.; Ringe, D. X-ray crystallography in the service of structure-based drug design. In Drug Design; Cambridge University Press: Cambridge, UK, 2010; pp. 17–29. [Google Scholar]
- RCSB PDB—4PEO: Crystal Structure of a Hypothetical Protein from Staphylococcus aureus. Available online: https://www.rcsb.org/structure/4PEO (accessed on 28 May 2021).
- RCSB PDB—2IDL: Crystal Structure of Conserved Protein of Unknown Function from Streptococcus pneumoniae. Available online: https://www.rcsb.org/structure/2IDL (accessed on 28 May 2021).
- Hou, H.-F.; Gao, Z.-Q.; Li, L.-F.; Liang, Y.-H.; Su, X.-D.; Dong, Y.-H. Crystal structure of SMU.848 from Streptococcus mutans. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar]
- Shin, D.H.; Lou, Y.; Jancarik, J.; Yokota, H.; Kim, R.; Kim, S.H. Crystal structure of TM1457 from Thermotoga maritima. J. Struct. Biol. 2005, 152, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Chirgadze, Y.N.; Clarke, T.E.; Romanov, V.; Kisselman, G.; Wu-Brown, J.; Soloveychik, M.; Chan, T.S.Y.; Gordon, R.D.; Battaile, K.P.; Pai, E.F.; et al. The structure of SAV1646 from Staphylococcus aureus belonging to a new ‘ribosome-associated’ subfamily of bacterial proteins. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Characterization of a Novel Protease in Staphylococcus aureus. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2015. [Google Scholar]


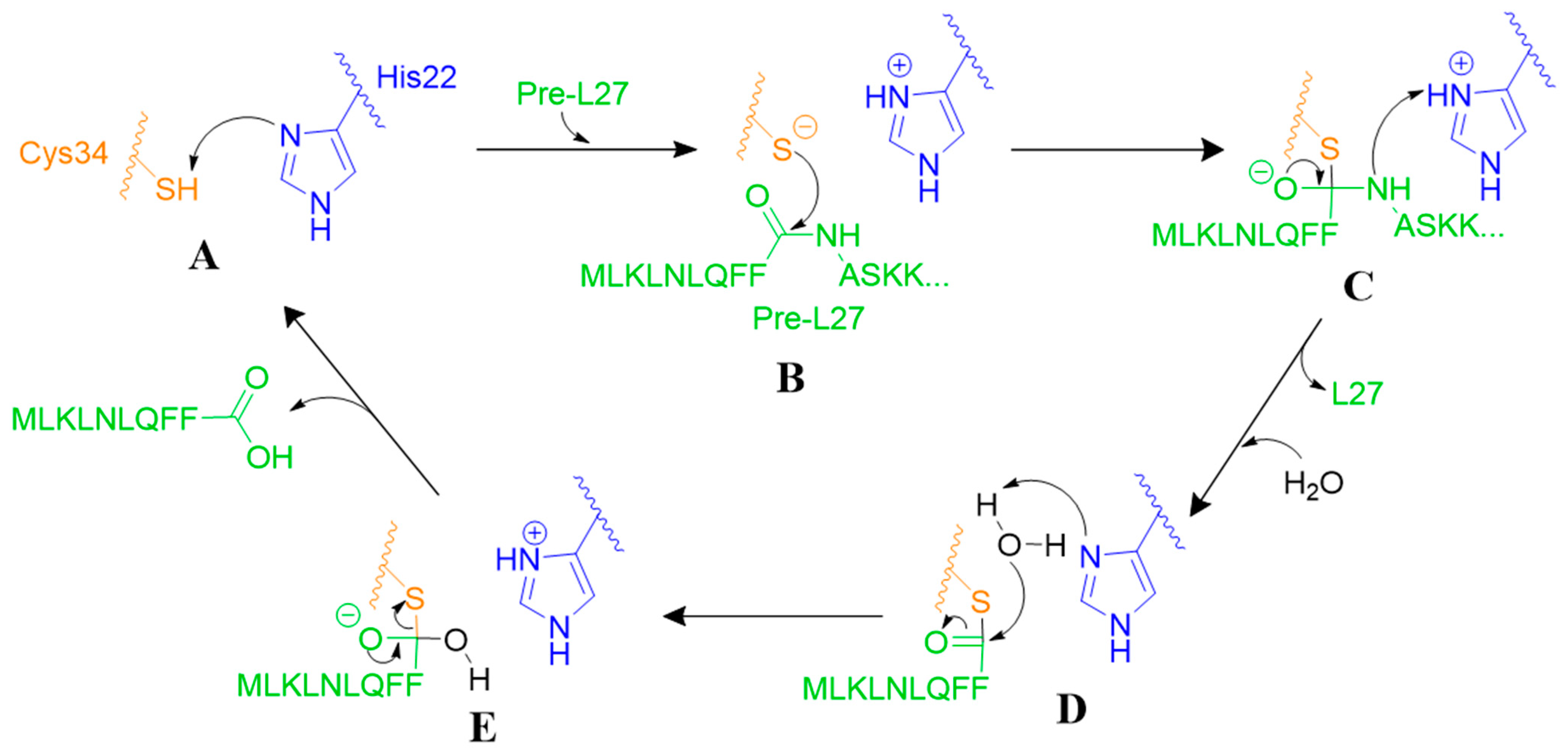


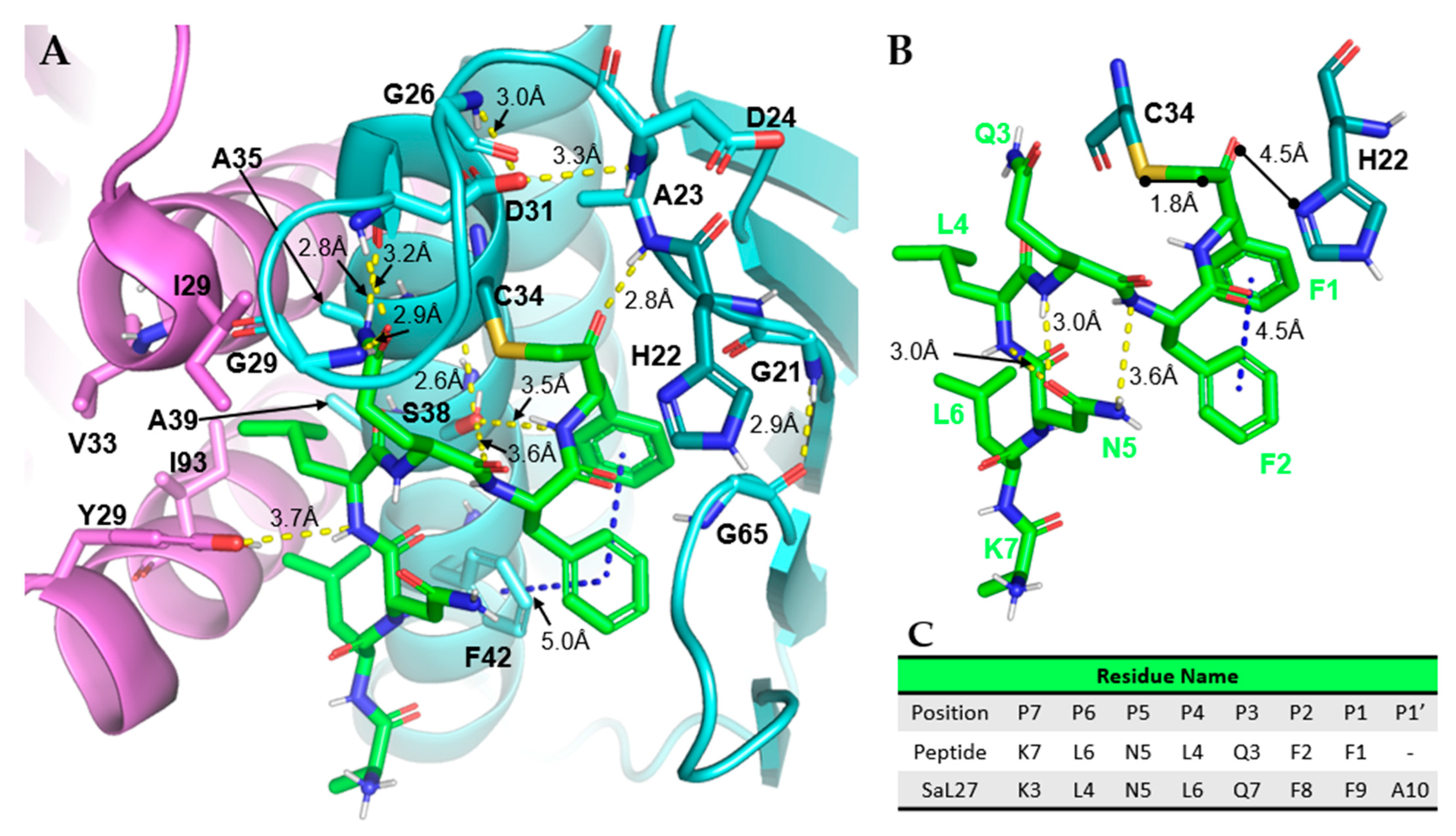
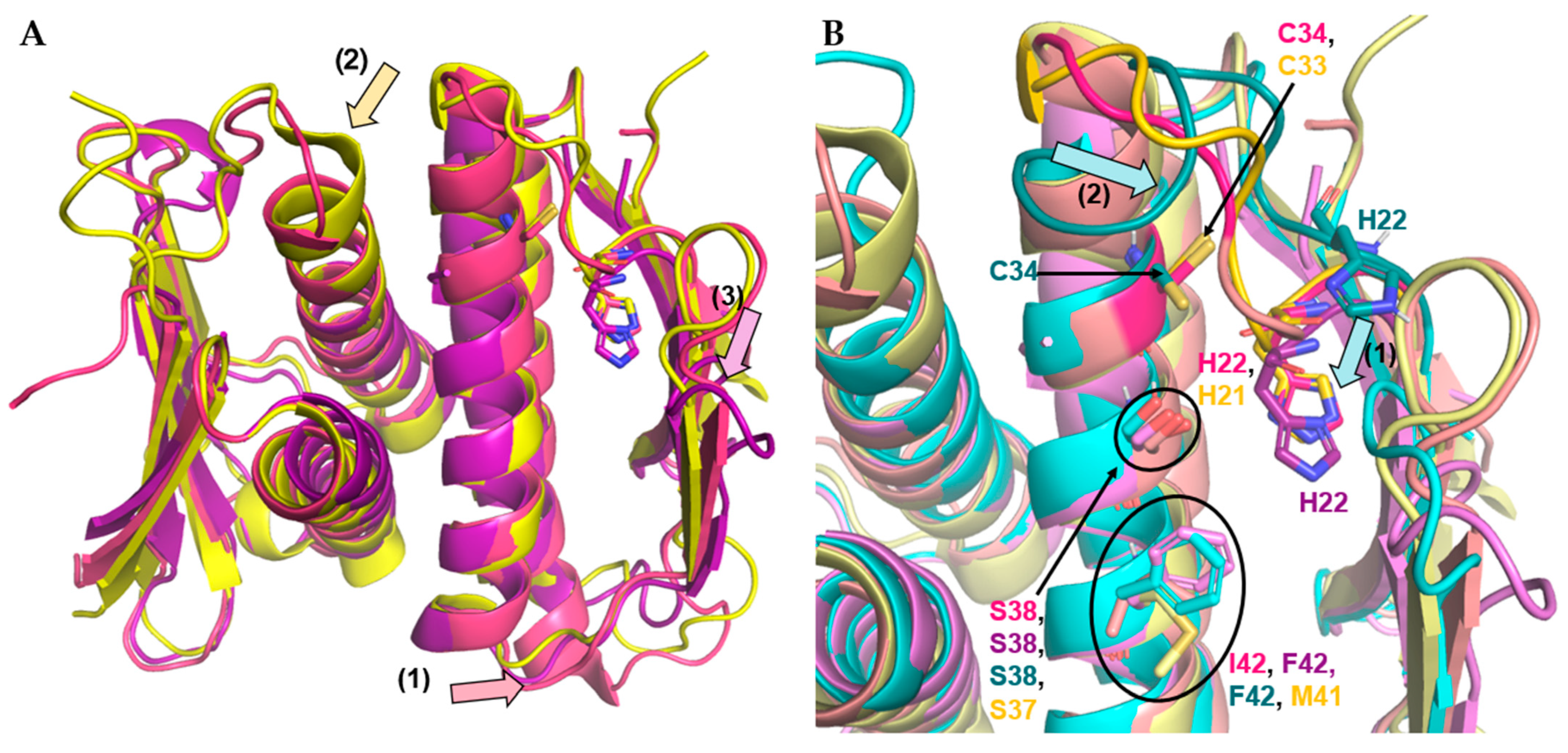
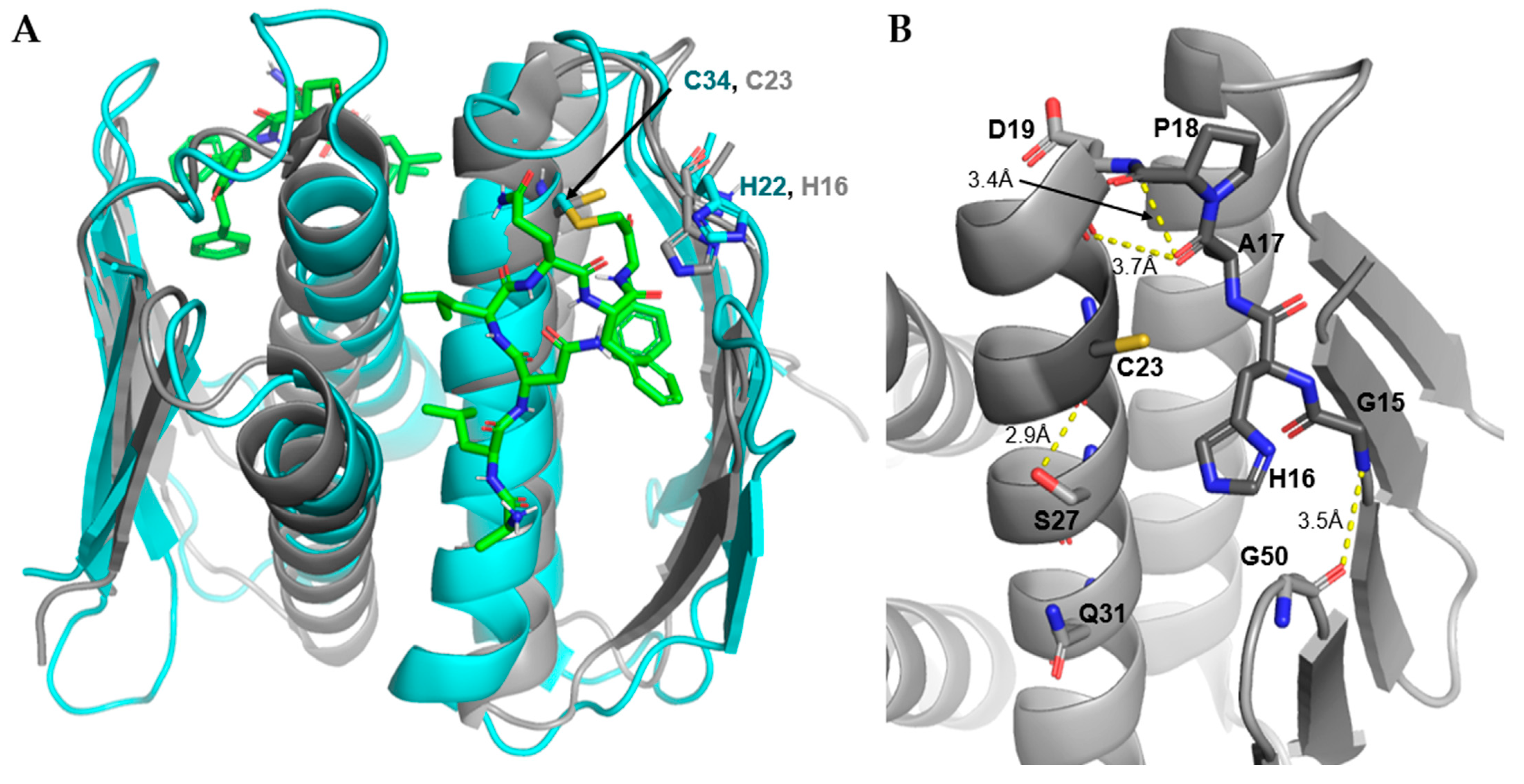
| Name | Sequence |
|---|---|
| 80α Scaffold (gp46) | ME-ENKLKFNLQFF’ADQS… |
| 80α Capsid (gp47) | MEQTQKLKLNLQHF’ASNN… |
| S. aureus L27 | M-----LKLNLQFF’ASKK… |
| Phylum | Species | L27 N-Terminus |
|---|---|---|
| Firmicutes | Bacillus subtilis | MLRL------DLQFF’ASKK… |
| Clostridioides difficile † | MLNM------NLQLL’ASKK… | |
| Clostridium tetani † | MLLM------NLQLF’ATKK… | |
| Enterococcus faecium | MLLSM-----NLQLF’AHKK… | |
| Erysipelothrix rhusiopathiae | M-KF----VLDIQLF’ASKK… | |
| Eubacterium rectale | MLNM------NLQFF’AHKK… | |
| Lactobacillus johnsonii | MM---INNLEALKLF’AHHK… | |
| Lactobacillus rhamnosus | MLKM------NLQFF’SHHK… | |
| Lactococcus lactis | MLEL------NLQLF’AHKK… | |
| Staphylococcus aureus † | MLKL------NLQFF’ASKK… | |
| Staphylococcus epidermidis | MLKL------NLQFF’ASKK… | |
| Streptococcus mutans † | MLKM---NLANLQLF’AHKK… | |
| Streptococcus oralis | MLKM---TLNNLQLF’AHKK… | |
| Streptococcus pneumoniae † | M------TLNNLQLF’AHKK… | |
| Ruminococcus torques | MMKM------NLQFF’AHKK… | |
| Fusobacteria | Fusobacterium nucleatum | M-----QFLFNIQLF’AHKK… |
| Synergistetes | Aminobacterium colombiense | M----RINFFDLQFF’AHKK… |
| Spirochete | Brachyspira pilosicoli † | M--------------’AHKK… |
| Borrelia recurrentis † | M--------------’ATSK… | |
| Borrelia burgdorferi † | M--------------’ATSK… | |
| Leptospira biflexa | M--------------’ATKK… | |
| Treponema pallidum † | M--------------’AR-K… | |
| Tenericutes | Mycoplasma genitalium † | MSKNSYCYQINLQFF’ASKK… |
| Mycoplasma pneumoniae † | M--------------’ASKK… | |
| Spiroplasma mirum † | MMKFL----LGLQLF’ASKK… | |
| Spiroplasma poulsonii | MMKFL----LGLQLF’ASKK… | |
| Ureaplasma urealyticum | MNKL--YWLTDLQLF’ASKK… | |
| Thermotogae | Thermotoga lettingae | M-------RIDIQLF’AHRK… |
| Thermotoga maritima | M--------------’AHKK… | |
| Thermotoga naphthophila | M--------------’AHKK… | |
| Proteobacteria | * Escherichia coli | M--------------’AHKK… |
| Phylum | Species | Protein | FlhB |
|---|---|---|---|
| Firmicutes | B. subtilis | L27 | M-LRLDLQFF’A-SKK… |
| FlhB | MKLRVDLQFF’AG-----EKTEKATPKKRKDTRK-KGQVAKS… | ||
| C. difficile † | L27 | M---LNMNLQLL’A-SKK… | |
| FlhB | M…FALAPMFF’MGSTD---KTEEATPKKKGEQRK-KGNIAKS… | ||
| C. tetani † | L27 | M-LLMNLQLF’A-TKK… | |
| FlhB | M…VPPILFIF’A-SED---KTEEATPHKLQEARK-KGQVAKS… | ||
| E. rectale | L27 | M-LNMNLQFF’A-HKK… | |
| FlhB | M…LCYNLQWF’AQDGEGGEKTEPATEKKLKDARE-EGKVAKS… | ||
| Spirochete | B. pilosicoli † | FlhB | M…RGFALTLF’ASAEDEG-RTELPTERKKRRAREEEGRVVNS… |
| B. recurrentis † | FlhB | M…WYIPLNFF’ASE-DEG-RTEVPTEQRKQKARR-EGQVLKS… | |
| B. burgdorferi † | FlhB | M…WYIPLDFF’SAD-DEG-RTELPTDQKKQKARE-EGRVLKS… | |
| L. biflexa | FlhB | M…YEIQLQLF’AAA-DEG-RTEPPSERRRREEKE-KGNVPKS… | |
| T. pallidum † | FlhB | M…FIIDLQWF’AAE-DEG-RSEDPTETKLRKARE-EGRVPKS… | |
| Thermotogae | T. lettingae | FlhB | M…KKLFKQLF’ADP--E--KTEKPTPRRRRKARE-EGQVATS… |
| T. maritima | FlhB | M…IE--LLLF’AEA--E--RTERATPRKRRRVRE-EGRAPVS… | |
| T. naphthophila | FlhB | M…IE--LLLF’AEA--E--RTERATPRKRRRVRE-EGRAPVS… | |
| Proteobacteria | * E. coli | FlhB | M---------’SDESDD--KTEAPTPHRLEKARE-EGQIPRS… |
| * S. Typhimurium † | FlhB | M---------’AEESDDD-KTEAPTPHRLEKARE-EGQIPRS… |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotinger, J.A.; Gallagher, A.H.; May, A.E. Phage-Related Ribosomal Proteases (Prps): Discovery, Bioinformatics, and Structural Analysis. Antibiotics 2022, 11, 1109. https://doi.org/10.3390/antibiotics11081109
Hotinger JA, Gallagher AH, May AE. Phage-Related Ribosomal Proteases (Prps): Discovery, Bioinformatics, and Structural Analysis. Antibiotics. 2022; 11(8):1109. https://doi.org/10.3390/antibiotics11081109
Chicago/Turabian StyleHotinger, Julia A., Allison Hannah Gallagher, and Aaron E. May. 2022. "Phage-Related Ribosomal Proteases (Prps): Discovery, Bioinformatics, and Structural Analysis" Antibiotics 11, no. 8: 1109. https://doi.org/10.3390/antibiotics11081109
APA StyleHotinger, J. A., Gallagher, A. H., & May, A. E. (2022). Phage-Related Ribosomal Proteases (Prps): Discovery, Bioinformatics, and Structural Analysis. Antibiotics, 11(8), 1109. https://doi.org/10.3390/antibiotics11081109







