Microbial Profiling of Potato-Associated Rhizosphere Bacteria under Bacteriophage Therapy
Abstract
:1. Introduction
2. Results
2.1. Efficacy of Bacteriophage Therapy on Potato Bacterial Diseases In Vivo
2.2. Microbial Communities: Pathogens and Phage Therapy
2.2.1. Rhizosphere Microbiome Profiling
2.2.2. Rhizosphere Microbial Communities
2.2.3. Phage Therapy-Related Microbial Communities
2.2.4. Phenotypic Prediction of Phage Treated Groups
2.2.5. Functional Prediction of Phage Treated Groups
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Culture Conditions
4.2. Phage Isolates, Amplification, and Tittering Conditions
4.3. Greenhouse Experiment Design and Treatments
4.4. Metabarcoding Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, C.; Liu, J.; Maina, A.N.; Mwaura, F.B.; Yu, J.; Yan, C.; Zhang, R.; Wei, H. Developing a bacteriophage cocktail for biocontrol of potato bacterial wilt. Virol. Sin. 2017, 32, 476–484. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.; Raffaele, S.; Vossen, J.H.; Champouret, N.; Oliva, R.; Segretin, M.E.; Rietman, H.; Cano, L.M.; Lokossou, A.; Kessel, G.; et al. Understanding and exploiting late blight resistance in the age of effectors. Annu. Rev. Phytopathol. 2011, 49, 507–531. [Google Scholar] [CrossRef]
- Spooner, D.M.; Ghislain, M.; Simon, R.; Jansky, S.H.; Gavrilenko, T. Systematics, Diversity, Genetics, and Evolution of Wild and Cultivated Potatoes. Bot. Rev. 2014, 80, 283–383. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop; Springer: Cham, Switzerland, 2020; pp. 3–35. [Google Scholar]
- Muturi, P.; Yu, J.; Maina, A.N.; Kariuki, S.; Mwaura, F.B.; Wei, H. Bacteriophages Isolated in China for the Control of Pectobacterium carotovorum Causing Potato Soft Rot in Kenya. Virol. Sin. 2019, 34, 287–294. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Charkowski, A.; Sharma, K.; Parker, M.; Secor, G.; Elphinstone, J. Bacterial Diseases of Potato. In The Potato Crop; Springer: Cham, Switzerland, 2020; pp. 351–388. [Google Scholar]
- Pérombelon, M. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar]
- Wei, Z.; Hu, J.; Gu, Y.A.; Yin, S.; Xu, Y.; Jousset, A.; Shen, Q.; Friman, V.-P. Ralstonia solanacearum pathogen disrupts bacterial rhizosphere microbiome during an invasion. Soil Biol. Biochem. 2018, 118, 8–17. [Google Scholar] [CrossRef]
- Buttimer, C.; Hendrix, H.; Lucid, A.; Neve, H.; Noben, J.P.; Franz, C.; O’Mahony, J.; Lavigne, R.; Coffey, A. Novel N4-Like Bacteriophages of Pectobacterium atrosepticum. Pharmaceuticals 2018, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Lozano-Duran, R.; Macho, A. Insights into the Root Invasion by the Plant Pathogenic Bacterium Ralstonia solanacearum. Plants 2020, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Biosca, E.G. Bacteriophage-Based Bacterial Wilt Biocontrol for an Environmentally Sustainable Agriculture. Front. Plant Sci. 2017, 8, 1218. [Google Scholar] [CrossRef] [PubMed]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Balogh, B.; Jones, J.; Iriarte, F.; Momol, M. Phage Therapy for Plant Disease Control. Curr. Pharm. Biotechnol. 2009, 11, 48–57. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, D.A. Genetics of Bactericide Resistance in Plant Pathogenic Bacteria. Annu. Rev. Phytopathol. 2003, 28, 201–219. [Google Scholar] [CrossRef]
- Behlau, F.; Canteros, B.I.; Minsavage, G.V.; Jones, J.B.; Graham, J.H. Molecular Characterization of Copper Resistance Genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfae subsp. citrumelonis. Appl. Environ. Microbiol. 2011, 77, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D. Copper- and Streptomycin-Resistant Strains and Host Differentiated Races of Xanthomonas campestris pv. vesicatoria in North Carolina. Plant Dis. 1991, 75, 733. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, T.; Friman, V.P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.P.; Gu, S.H.; Wang, X.F.; Eisenhauer, N.; Yang, T.J.; Ma, J.; Shen, Q.R.; Xu, Y.C.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function and Plant Disease Suppression. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, J. Host specificity in biological control: Insights from opportunistic pathogens. Evol. Appl. 2012, 5, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Kyselková, M.; Moënne-Loccoz, Y. Pseudomonas and other Microbes in Disease-Suppressive Soils. In Organic Fertilisation, Soil Quality and Human Health; Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–140. [Google Scholar]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Hill, C. Bacteriophages: Viruses That Infect Bacteria. Front. Young Minds 2019, 7, 146. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Yang, K.; Wang, J.; Jousset, A.; Xu, Y.; Shen, Q.; Friman, V.P. Phage combination therapies for bacterial wilt disease in tomato. Nat. Biotechnol. 2019, 37, 1513–1520. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Guo, Z.; Liu, H.; Sun, D.; Yan, G.; Hu, D.; Du, C.; Feng, X.; Han, W.; et al. A guard-killer phage cocktail effectively lyses the host and inhibits the development of phage-resistant strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wei, Z.; Wang, X.; Friman, V.-P.; Huang, J.; Wang, X.; Mei, X.; Xu, Y.; Shen, Q.; Jousset, A. Pathogen invasion indirectly changes the composition of soil microbiome via shifts in root exudation profile. Biol. Fertil. Soils 2016, 52, 997–1005. [Google Scholar] [CrossRef]
- Li, M.; Wei, Z.; Wang, J.; Jousset, A.; Friman, V.P.; Xu, Y.; Shen, Q.; Pommier, T. Facilitation promotes invasions in plant-associated microbial communities. Ecol. Lett. 2019, 22, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Carroll, T.L.; Sundin, G.W. The Role of Pigmentation, Ultraviolet Radiation Tolerance, and Leaf Colonization Strategies in the Epiphytic Survival of Phyllosphere Bacteria. Microb. Ecol. 2005, 49, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L. Chapter Two—How the Plant Growth-Promoting Bacterium Azospirillum Promotes Plant Growth—A Critical Assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Spaepen, S.; Versées, W.; Rother, D.; Pohl, M.; Steyaert, J.; Vanderleyden, J. Characterization of Phenylpyruvate Decarboxylase, Involved in Auxin Production of Azospirillum brasilense. J. Bacteriol. 2007, 189, 7626–7633. [Google Scholar] [CrossRef] [PubMed]
- Ryu, R.; Patten, C. Aromatic Amino Acid-Dependent Expression of Indole-3-Pyruvate Decarboxylase Is Regulated by TyrR in Enterobacter cloacae UW5. J. Bacteriol. 2008, 190, 7200–7208. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, W.; Hundeshagen, B.; Niederau, E. Demonstration of the indolepyruvate decarboxylase gene in different auxin-producing species of the Enterobacteriaceae. Can. J. Microbiol. 1995, 40, 1072–1076. [Google Scholar] [CrossRef]
- Suarez-Moreno, Z.; Caballero-Mellado, J.; Gonçalves Coutinho, B.; Mendonça-Previato, L.; James, E.; Venturi, V. Common Features of Environmental and Potentially Beneficial Plant-Associated Burkholderia. Microb. Ecol. 2011, 63, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Browne, P.; Prigent-Combaret, C.; Combes-Meynet, E.; Morrissey, J.; O’Gara, F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ. Microbiol. Rep. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Blaha, D.; Prigent-Combaret, C.; Mirza, M.; Moenne-Loccoz, Y. Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography: AcdS phylogeny in Proteobacteria. FEMS Microbiol. Ecol. 2006, 56, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.; Jacobson, C.; Schwarze, M.; Pasternak, J. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can. J. Microbiol. 1994, 40, 911–915. [Google Scholar] [CrossRef]
- Haas, D.; Keel, C. Regulation of Antibiotic Production in Root-Colonizing Pseudomonas spp. and Relevance for Biological Control of Plant Disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Abd Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.-M.; Pozo, M.; Azcón, R.; Azcon-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S. Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Olanrewaju, O.; Babalola, O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.-Q.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F. Genome-Resolved Metagenomics Reveals Distinct Phosphorus Acquisition Strategies between Soil Microbiomes. mSystems 2022, 7, e0110721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; You, Y.; Wang, R.; Chu, S.; Chi, Y.; Hayat, K.; Hui, N.; Liu, X.; Zhang, D.; et al. When nanoparticle and microbes meet: The effect of multi-walled carbon nanotubes on microbial community and nutrient cycling in hyperaccumulator system. J. Hazard. Mater. 2021, 423, 126947. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, J.; Liu, T.; Liu, Y.; Yan, M.; Hu, J.; Li, X.; Liu, X.; Liang, Y.; Liu, H.; et al. Effects of redox potential on soil Cadmium solubility: Insight into microbial community. J. Environ. Sci. 2018, 75, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mujakic, I.; Piwosz, K.; Koblizek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Garcia, R.; Müller, R. Simulacricoccus ruber gen. nov., sp. nov., a microaerotolerant, non-fruiting, myxospore-forming soil myxobacterium and emended description of the family Myxococcaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Kering, K.; Zhang, X.; Nyaruaba, R.; Yu, J.; Wei, H. Application of Adaptive Evolution to Improve the Stability of Bacteriophages during Storage. Viruses 2020, 12, 423. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.; Helmke, P.A.; Loeppert, R.; Soltanpour, P.N.; Tabatabai, M.; Johnston, C.; Sumner, M. Methods of Soil Analysis. Part III. Chemical Methods; John Wiley & Sons: New York, NY, USA, 1996; Volume 5. [Google Scholar]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Reyon, D.; Tsai, S.; Khayter, C.; Foden, J.; Sander, J.; Joung, J. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.; Westcott, S.; Ryabin, T.; Hall, J.; Hartmann, M.; Hollister, E.; Lesniewski, R.; Oakley, B.; Parks, D.; Robinson, C.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]






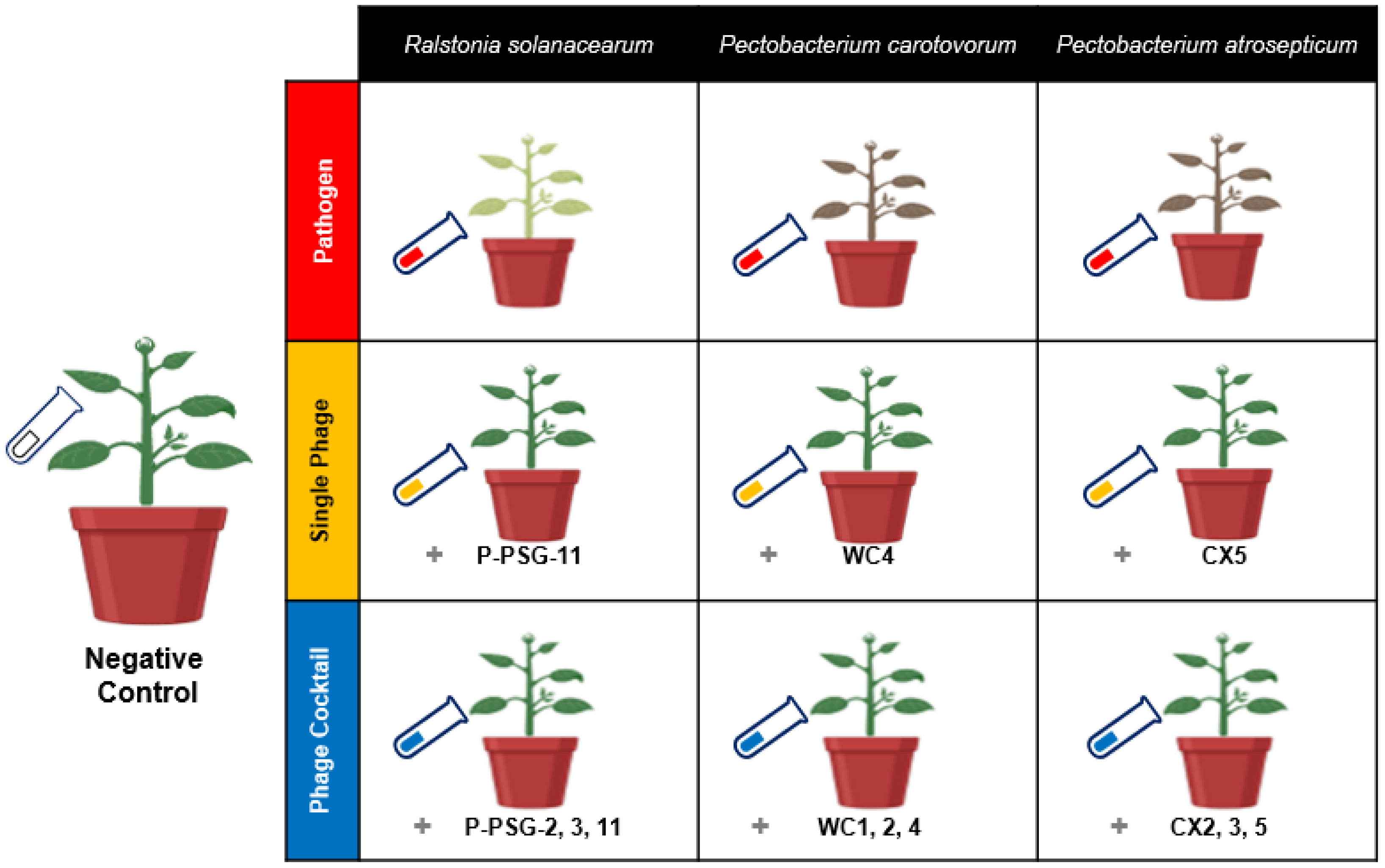
| Bacteria | Phage | ||
|---|---|---|---|
| Strain | Source | Single | Cocktail |
| Ralstonia solanacearum strain GIM1.74 (Rs) | Purchased from Guangdong Microbiology Culture Center, China | P-PSG-11 (SRs) | Rs 2, 3,11 cocktail (RsPck) |
| Pectobacterium carotovorum subsp carotovorum strain KPM17 (Pc) | Isolated from Molo, Kenya | Wc4 (SPc) | Wc1, 2, 4 cocktail (PcPck) |
| Pectobacterium atrosepticum strain WGH10001 (Pa) | Isolated from Mongolia, China | CX5 (SPa) | CX 2, 3, 5 cocktail (PaPck) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, S.; Magdy, M.; Xiong, D.; Nyaruabaa, R.; Rizk, S.M.; Yu, J.; Wei, H. Microbial Profiling of Potato-Associated Rhizosphere Bacteria under Bacteriophage Therapy. Antibiotics 2022, 11, 1117. https://doi.org/10.3390/antibiotics11081117
Mousa S, Magdy M, Xiong D, Nyaruabaa R, Rizk SM, Yu J, Wei H. Microbial Profiling of Potato-Associated Rhizosphere Bacteria under Bacteriophage Therapy. Antibiotics. 2022; 11(8):1117. https://doi.org/10.3390/antibiotics11081117
Chicago/Turabian StyleMousa, Samar, Mahmoud Magdy, Dongyan Xiong, Raphael Nyaruabaa, Samah Mohamed Rizk, Junping Yu, and Hongping Wei. 2022. "Microbial Profiling of Potato-Associated Rhizosphere Bacteria under Bacteriophage Therapy" Antibiotics 11, no. 8: 1117. https://doi.org/10.3390/antibiotics11081117
APA StyleMousa, S., Magdy, M., Xiong, D., Nyaruabaa, R., Rizk, S. M., Yu, J., & Wei, H. (2022). Microbial Profiling of Potato-Associated Rhizosphere Bacteria under Bacteriophage Therapy. Antibiotics, 11(8), 1117. https://doi.org/10.3390/antibiotics11081117









