Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults
Abstract
:1. Introduction
2. Current Epidemiology, Pathogeny and Antimicrobial Resistance of Klebsiella pneumoniae Infections in the ICU
3. Klebsiella pneumoniae in ICUs: Risk Factors and Outcome
3.1. Updated Risk Factors
3.2. Prognostic Factors
4. Clinical Entities
4.1. Pneumonia
4.1.1. Community-Acquired Pneumonia (CAP)
4.1.2. Nosocomial Pneumonia
4.1.3. Outcome
4.2. Bacteremia
4.3. Urinary Tract Infection (UTI)
4.4. Intra-Abdominal Infections
4.5. Central Nervous System (CNS) Infections
4.6. The Role of Hypervirulent Klebsiella pneumoniae
5. Clinical Management of Infections Caused by CR-Kp
5.1. General Issues: Monotherapy or Combination Therapy?
5.2. How Should CR-Kp Sensitive to Meropenem Be Treated?
5.3. How Should a CR-Kp Resistant to Meropenem Be Treated?
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epine. Available online: https://epine.es/ (accessed on 19 August 2022).
- ENVIN-HELICS. Available online: https://hws.vhebron.net/envin-helics/ (accessed on 14 May 2022).
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Jeon, J.H.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Antimicrobial Resistance of Hypervirulent Klebsiella pneumoniae: Epidemiology, Hypervirulence-Associated Determinants, and Resistance Mechanisms. Front. Cell. Infect. Microbiol. 2017, 7, 483. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Aguilar, A.C.; Caicedo, A. Carbapenem-Resistant Klebsiella pneumoniae: Microbiology Key Points for Clinical Practice. Int. J. Gen. Med. 2019, 12, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Antachopoulos, C.; Iosifidis, E.; Tsakris, A.; Roilides, E. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in Greece. Future Microbiol. 2016, 11, 809–823. [Google Scholar] [CrossRef]
- Vázquez-Ucha, J.C.; Arca-Suárez, J.; Bou, G.; Beceiro, A. New Carbapenemase Inhibitors: Clearing the Way for the β-Lactams. Int. J. Mol. Sci. 2020, 21, 9308. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Noso-comial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Aubert, D.; Naas, T.; Héritier, C.; Poirel, L.; Nordmann, P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. J. Bacteriol. 2006, 188, 6506–6514. [Google Scholar] [CrossRef] [Green Version]
- Potron, A.; Poirel, L.; Nordmann, P. Derepressed Transfer Properties Leading to the Efficient Spread of the Plasmid Encoding Carbapenemase OXA-Antimicrob. Agents Chemother. 2014, 58, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Deshpande, L.M.; Mendes, R.; Canton, R.; Sader, H.; Jones, R.N. Variations in the Occurrence of Resistance Phenotypes and Carbapenemase Genes Among Enterobacteriaceae Isolates in 20 Years of the SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6, S23–S33. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M. Multilevel population genetics in antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 705–706. [Google Scholar] [CrossRef]
- Hernández-García, M.; Pérez-Viso, B.; Carmen Turrientes, M.; Díaz-Agero, C.; López-Fresneña, N.; Bonten, M.; Malhotra-Kumar, S.; Ruiz-Garbajosa, P.; Cantón, R. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J. Antimicrob. Chemother. 2018, 73, 3039–3043. [Google Scholar] [CrossRef]
- Damjanova, I.; Tóth, A.; Pászti, J.; Hajbel-Vékony, G.; Jakab, M.; Berta, J.; Milch, H.; Füzi, M. Expansion and countrywide dissemination of ST11, ST15 and ST147 ciprofloxacin-resistant CTX-M-15-type beta-lactamase-producing Klebsiella pneumoniae epidemic clones in Hungary in 2005—The new “MRSAs”? J. Antimicrob. Chemother. 2008, 62, 978–985. [Google Scholar] [CrossRef]
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Zhang, W. Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 car-bapenem-resistant Klebsiella pneumoniae in China: A review over the last 10 years. J. Glob. Antimicrob. Resist. 2020, 23, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Turton, J.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. The Role of Epidemic Resistance Plasmids and International High-Risk Clones in the Spread of Multidrug-Resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Mammina, C.; Bonura, C.; Di Bernardo, F.; Aleo, A.; Fasciana, T.; Sodano, C.; Saporito, M.A.; Verde, M.S.; Tetamo, R.; Palma, D.M. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Eurosurveilliance 2012, 17, 20248. [Google Scholar] [CrossRef]
- Bonura, C.; Giuffrè, M.; Aleo, A.; Fasciana, T.; Di Bernardo, F.; Stampone, T.; Giammanco, A.; Palma, D.M.; Mammina, C.; The MDR-GN Working Group. An Update of the Evolving Epidemic of blaKPC Carrying Klebsiella pneumoniae in Sicily, Italy, 2014: Emergence of Multiple Non-ST258 Clones. PLoS ONE 2015, 10, e0132936. [Google Scholar] [CrossRef] [PubMed]
- Mavroidi, A.; Katsiari, M.; Likousi, S.; Palla, E.; Roussou, Z.; Nikolaou, C.; Maguina, A.; Platsouka, E.D. Characterization of ST258 Colistin-Resistant, blaKPC-Producing Klebsiella pneumoniae in a Greek Hospital. Microb. Drug Resist. 2016, 22, 392–398. [Google Scholar] [CrossRef]
- Giddins, M.J.; Macesic, N.; Annavajhala, M.K.; Stump, S.; Khan, S.; McConville, T.H.; Mehta, M.; Gomez-Simmonds, A.; Uhlemann, A.C. Successive Emergence of Ceftazidime-Avibactam Resistance through Distinct Genomic Adaptations in blaKPC-2-Harboring Klebsiella pneumoniae Sequence Type 307 Isolates. Antimicrob. Agents Chemother. 2018, 62, e02101-17. [Google Scholar] [CrossRef]
- Venditti, C.; Butera, O.; Meledandri, M.; Balice, M.P.; Cocciolillo, G.C.; Fontana, C.; D’Arezzo, S.; De Giuli, C.; Antonini, M.; Capone, A.; et al. Molecular analysis of clinical isolates of ceftazidime-avibactam-resistant Klebsiella pneumoniae. Clin. Microbiol. Infect. 2021, 27, 1040.e1–1040.e6. [Google Scholar] [CrossRef]
- Villa, L.; Feudi, C.; Fortini, D.; Brisse, S.; Passet, V.; Bonura, C.; Endimiani, A.; Mammina, C.; Ocampo, A.M.; Jiménez, J.N.; et al. Diversity, virulence, and antimicrobial resistance of the KPC-producing Klebsiella pneumoniae ST307 clone. Microb. Genom. 2017, 3, e000110. [Google Scholar] [CrossRef]
- Heiden, S.E.; Hübner, N.-O.; Bohnert, J.A.; Heidecke, C.-D.; Kramer, A.; Balau, V.; Gierer, W.; Schaefer, S.; Eckmanns, T.; Gatermann, S.; et al. A Klebsiella pneumoniae ST307 outbreak clone from Germany demonstrates features of extensive drug resistance, hypermucoviscosity, and enhanced iron acquisition. Genome Med. 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Palmieri, M.; Wyres, K.L.; Mirande, C.; Qiang, Z.; Liyan, Y.; Gang, C.; Goossens, H.; van Belkum, A.; Yan Ping, L. Genomic evolution and local epidemiology of Klebsiella pneumoniae from a major hospital in Beijing, China, over a 15 year period: Dissemination of known and novel high-risk clones. Microb. Genom. 2019, 7, 520. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.M.; Lira, A.; Lameirão, A.; Selaru, A.; Abreu, G.; Lopes, P.; Mota, M.; Novais, Â.; Peixe, L. Multiplicity of Carbapenemase-Producers Three Years after a KPC-3-Producing, K. pneumoniae ST147-K64 Hospital Outbreak. Antibiotics 2020, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Gijón, D.; Tedim, A.P.; Valverde, A.; Rodríguez, I.; Morosini, M.I.; Coque, T.M.; Manrique, M.; Pareja, E.; Tobes, R.; Ruiz-Garbajosa, P.; et al. Early OXA-48-Producing Enterobacterales Isolates Recovered in a Spanish Hospital Reveal a Complex Introduction Dominated by Sequence Type 11 (ST11) and ST405 Klebsiel-la pneumoniae Clones. mSphere 2020, 5, e00080-20. [Google Scholar] [CrossRef]
- Viale, P.; Giannella, M.; Lewis, R.; Trecarichi, E.M.; Petrosillo, N.; Tumbarello, M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev. Anti Infect. Ther. 2013, 11, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Harrington, S.; Dihmess, A.; Woo, B.; Masoud, R.; Martis, P.; Fiorenza, M.; Graffunder, E.; Evans, A.; McNutt, L.-A.; et al. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J. Antimicrob. Chemother. 2011, 66, 1600–1608. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Klarfeld-Lidji, S.; Navon-Venezia, S.; Schwartz, D.; Leavitt, A.; Carmeli, Y. Predictors of Carbapenem-Resistant Klebsiella pneumoniae Acquisition among Hospitalized Adults and Effect of Acquisition on Mortality. Antimicrob. Agents Chemother. 2008, 52, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
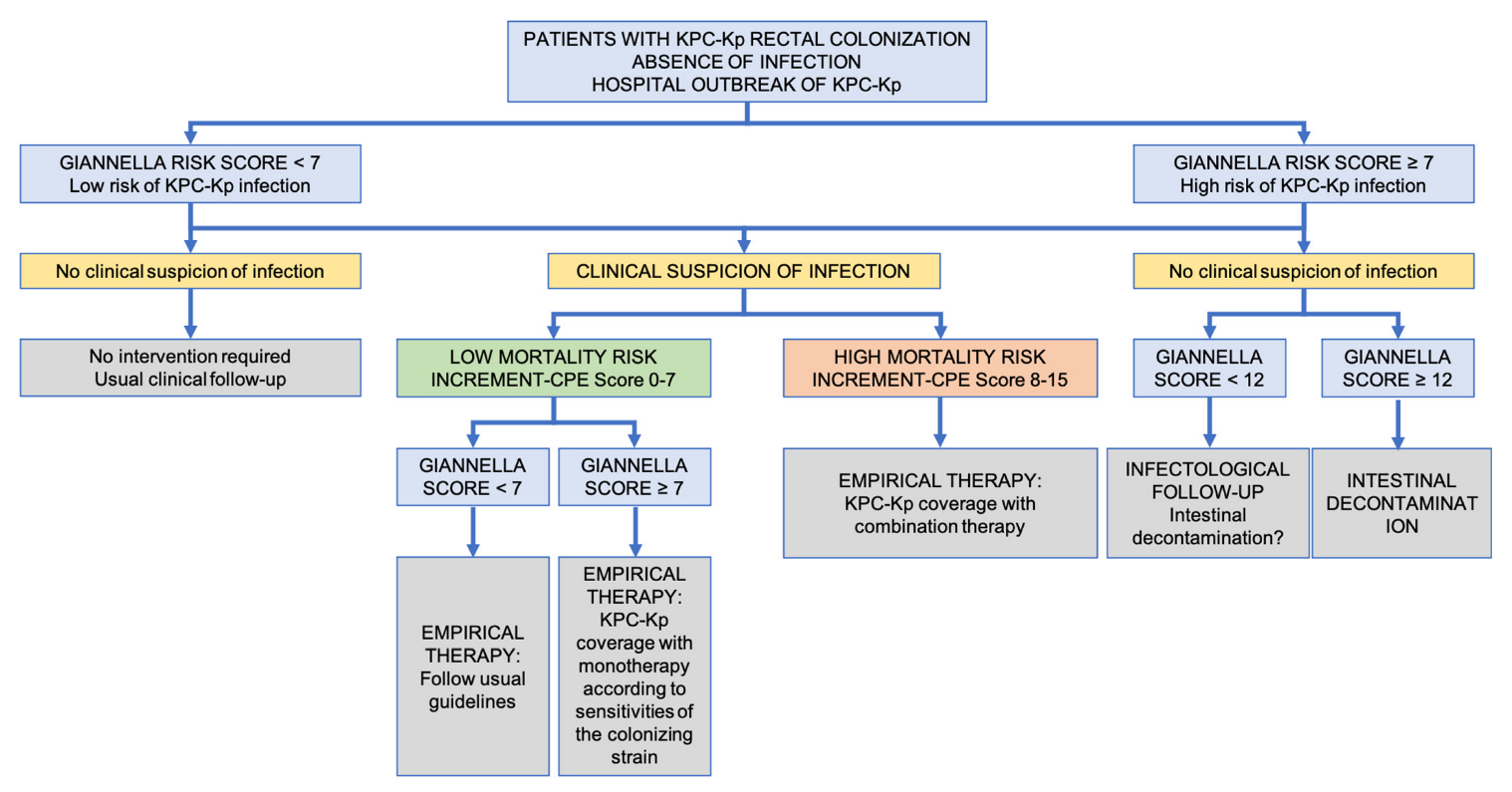
- Ferrer, R.; Soriano, A.; Cantón, R.; Del Pozo, J.L.; García-Vidal, C.; Garnacho-Montero, J.; Larrosa, N.; Rascado, P.; Salavert, M.; Pintado, V.; et al. A systematic literature review and expert consensus on risk factors associated to infection progression in adult patients with respiratory tract or rectal colonisation by carbapenem-resistant Gram-negative bacteria. Rev. Esp. Quimioter. 2022. [Google Scholar] [CrossRef]
- Freire, M.P.; Oshiro, I.C.; Pierrotti, L.C.; Bonazzi, P.R.; de Oliveira, L.M.; Song, A.T.; Camargo, C.H.; van der Heijden, I.M.; Rossi, F.; Costa, S.F.; et al. Carbapenem-Resistant Enterobacteriaceae Acquired Before Liver Transplantation: Impact on Recipient Outcomes. Transplantation 2017, 101, 811–820. [Google Scholar] [CrossRef]
- McConville, T.H.; Sullivan, S.B.; Gomez-Simmonds, A.; Whittier, S.; Uhlemann, A.-C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS ONE 2017, 12, e0186195. [Google Scholar] [CrossRef]
- Girmenia, C.; Bertaina, A.; Piciocchi, A.; Perruccio, K.; Algarotti, A.; Busca, A.; Cattaneo, C.; Raiola, A.M.; Guidi, S.; Iori, A.P.; et al. Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey. Clin. Infect. Dis. 2017, 65, 1884–1896. [Google Scholar] [CrossRef]
- Demiraslan, H.; Cevahir, F.; Berk, E.; Metan, G.; Cetin, M.; Alp, E. Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am. J. Infect. Control. 2017, 45, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Giannella, M.; Trecarichi, E.; De Rosa, F.G.; Del Bono, V.; Bassetti, M.; Lewis, R.; Losito, A.R.; Corcione, S.; Saffioti, C.; Bartoletti, M.; et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin. Microbiol. Infect. 2014, 20, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Cano, Á.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Pérez-Nadales, E.; Causse, M.; Castón, J.J.; Guzman-Puche, J.; Torre-Giménez, J.; Kindelán, L.; et al. Risks of Infection and Mortality Among Patients Colonized with Klebsiella pneumoniae Carbapenemase-Producing, K. pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Song, N.; Chen, Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: A meta-analysis. J. Glob. Antimicrob. Resist. 2020, 21, 306–313. [Google Scholar] [CrossRef]
- Namikawa, H.; Niki, M.; Niki, M.; Yamada, K.; Nakaie, K.; Sakiyama, A.; Oinuma, K.-I.; Tsubouchi, T.; Tochino, Y.; Takemoto, Y.; et al. Clinical and virulence factors related to the 30-day mortality of Klebsiella pneumoniae bacteremia at a tertiary hospital: A case–control study. Eur. J. Clin. Microbiol. 2019, 38, 2291–2297. [Google Scholar] [CrossRef]
- Kaur, A.; Gandra, S.; Gupta, P.; Mehta, Y.; Laxminarayan, R.; Sengupta, S. Clinical outcome of dual colistin- and car-bapenem-resistant Klebsiella pneumoniae bloodstream infections: A single-center retrospective study of 75 cases in India. Am. J. Infect. Control 2017, 45, 1289–1291. [Google Scholar] [CrossRef]
- Tseng, C.-P.; Wu, H.-S.; Wu, T.-H.; Lin, Y.-T.; Fung, C.-P. Clinical characteristics and outcome of patients with community-onset Klebsiella pneumoniae bacteremia requiring intensive care. J. Microbiol. Immunol. Infect. 2013, 46, 217–223. [Google Scholar] [CrossRef]
- Man, M.Y.; Shum, H.; Chan, Y.; Chan, K.; Yan, W.; Lee, R.; Lau, S. Clinical predictors and outcomes of Klebsiella pneumoniae bacteraemia in a regional hospital in Hong Kong. J. Hosp. Infect. 2017, 97, 35–41. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Fligou, F.; Bartzavali, C.; Zotou, A.; Spyropoulou, A.; Koutsileou, K.; Vamvakopoulou, S.; Sioulas, N.; Karamouzos, V.; Anastassiou, E.D.; et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: Risk factors and predictors of mortality. Eur. J. Clin. Microbiol. 2017, 36, 1125–1131. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Sun, L.; Wang, Z.; Sun, L.; Xu, J.; Zeng, L.; Sun, T. Clinical Observation and Prognostic Analysis of Patients with Klebsiella pneumoniae Bloodstream Infection. Front. Cell. Infect. Microbiol. 2020, 10, 577244. [Google Scholar] [CrossRef]
- Juan, C.H.; Chuang, C.; Chen, C.H.; Li, L.; Lin, Y.T. Clinical characteristics, antimicrobial resistance and capsular types of commu-nity-acquired, healthcare-associated, and nosocomial Klebsiella pneumoniae bacteremia. Antimicrob. Resist. Infect. Control. 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospi-talization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, A.; Mendez, R.; Ewig, S.; de la Torre, M.C.; Cilloniz, C.; Gabarrus, A.; Prina, E.; Ranzani, O.T.; Ferrer, M.; Almirall, J.; et al. Validation of a Prediction Score for Drug-Resistant Microorganisms in Community-acquired Pneumonia. Ann. Am. Thorac. Soc. 2021, 18, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Dominedò, C.; Torres, A. Multidrug Resistant Gram-Negative Bacteria in Community-Acquired Pneumonia. Crit. Care 2019, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Oh, W.S.; Kang, C.-I.; Chung, D.R.; Peck, K.R.; Ko, K.S.; Yeom, J.S.; Kim, C.K.; Kim, S.W.; Chang, H.-H.; et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: A prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents 2008, 31, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rafat, C.; Messika, J.; Barnaud, G.; Dufour, N.; Magdoud, F.; Billard-Pomarès, T.; Gaudry, S.; Dreyfuss, D.; Branger, C.; Decré, D.; et al. Hypervirulent Klebsiella pneumoniae, a 5-year study in a French ICU. J. Med. Microbiol. 2018, 67, 1083–1089. [Google Scholar] [CrossRef]
- Villafuerte, D.; Aliberti, S.; Soni, N.J.; Faverio, P.; Marcos, P.J.; Wunderink, R.G.; Rodriguez, A.; Sibila, O.; Sanz, F.; Martin-Loeches, I.; et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology 2020, 25, 543–551. [Google Scholar] [CrossRef]
- Hwang, J.H.; Handigund, M.; Hwang, J.H.; Cho, Y.G.; Kim, D.S.; Lee, J. Clinical Features and Risk Factors Associated with 30-Day Mortality in Patients with Pneumonia Caused by Hypervirulent Klebsiella pneumoniae (hvKP). Ann. Lab. Med. 2020, 40, 481–487. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Samsa, G.P.; Brown, V.; Niederman, M.S. Microbiology of Ventilator–Associated Pneumonia Compared with That of Hospital-Acquired Pneumonia. Infect. Control Hosp. Epidemiol. 2007, 28, 825–831. [Google Scholar] [CrossRef]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.-F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extend-ed-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Bergen, P.J.; Rao, G.G.; Nation, R.L.; Landersdorfer, C.B. A systematic review and meta-analysis of treatment out-comes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents 2020, 55, 105833. [Google Scholar] [CrossRef] [PubMed]
- Meatherall, B.L.; Gregson, D.; Ross, T.; Pitout, J.D.; Laupland, K.B. Incidence, Risk Factors, and Outcomes of Klebsiella pneumoniae Bacteremia. Am. J. Med. 2009, 122, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Skerk, V.; Krhen, I.; Schonwald, S.; Cajic, V.; Markovinovic, L.; Roglic, S.; Zekan, S.; Andracevic, A.T.; Kruzic, V. The role of unusual pathogens in prostatitis syndrome. Int. J. Antimicrob. Agents 2004, 24, 53–56. [Google Scholar] [CrossRef]
- Liu, K.-H.; Lee, H.-C.; Chuang, Y.-C.; Tu, C.-A.; Chang, K.; Lee, N.-Y.; Kos, W.-C. Prostatic abscess in southern Taiwan: Another invasive infection caused predominantly by Klebsiella pneumoniae. J. Microbiol. Immunol. Infect. 2003, 36, 31–36. [Google Scholar] [PubMed]
- Yilmaz, E.; Akalin, H.; Ozbey, S.; Kordan, Y.; Sinirtaş, M.; Gürcüoglu, E.; Ozakin, C.; Heper, Y.; Mistik, R.; Helvaci, S. Risk factors in community-acquired/onset urinary tract infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J. Chemother. 2008, 20, 581–585. [Google Scholar] [CrossRef]
- Koksal, E.; Tulek, N.; Sonmezer, M.C.; Temocin, F.; Bulut, C.; Hatipoglu, C.; Erdinc, F.S.; Ertem, G. Investigation of risk factors for community-acquired urinary tract infections caused by extended-spectrum beta-lactamase Escherichia coli and Klebsiella species. Investig. Clin. Urol. 2019, 60, 46–53. [Google Scholar] [CrossRef]
- Molton, J.S.; Chan, M.; Kalimuddin, S.; Oon, J.; Young, B.E.; Low, J.G.; Salada, B.M.A.; Lee, T.H.; Wijaya, L.; Fisher, D.A.; et al. Oral vs Intravenous Antibiotics for Patients with Klebsiella pneumoniae Liver Abscess: A Randomized, Controlled Noninferiority Study. Clin. Infect. Dis. 2020, 71, 952–959. [Google Scholar] [CrossRef]
- Rahimian, J.; Wilson, T.; Oram, V.; Holzman, R.S. Pyogenic Liver Abscess: Recent Trends in Etiology and Mortality. Clin. Infect. Dis. 2004, 39, 1654–1659. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Chen, M.; Wang, C.; Kang, Y.; Lou, J. Causative agents and outcome of spontaneous bacterial peritonitis in cirrhotic patients: Community-acquired versus nosocomial infections. BMC Infect. Dis. 2019, 19, 463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Song, Y.; Kang, J.; Duan, S.; Li, Q.; Feng, F.; Duan, J. Epidemiology of patients with central nervous system infections, mainly neurosurgical patients: A retrospective study from 2012 to 2019 in a teaching hospital in China. BMC Infect. Dis. 2021, 21, 826. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-T.; Lai, S.-Y.; Yi, W.-C.; Hsueh, P.-R.; Liu, K.-L.; Chang, S.-C. Klebsiella pneumoniae Genotype K1: An Emerging Pathogen That Causes Septic Ocular or Central Nervous System Complications from Pyogenic Liver Abscess. Clin. Infect. Dis. 2007, 45, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [PubMed]
- Daikos, G.L.; Tsaousi, S.; Tzouvelekis, L.S.; Anyfantis, I.; Psichogiou, M.; Argyropoulou, A.; Stefanou, I.; Sypsa, V.; Miriagou, V.; Nepka, M.; et al. Carbapenemase-Producing Klebsiella pneumoniae Bloodstream Infections: Lowering Mortality by Antibiotic Combination Schemes and the Role of Carbapenems. Antimicrob. Agents Chemother. 2014, 58, 2322–2328. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.-R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Cantón, R.; et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): A retrospective cohort study. Lancet Infect. Dis. 2017, 17, 726–734. [Google Scholar] [CrossRef]
- Machuca, I.; Gutiérrez-Gutiérrez, B.; Gracia-Ahufinger, I.; Rivera Espinar, F.; Cano, Á.; Guzmán-Puche, J.; Pérez-Nadales, E.; Natera, C.; Rodríguez, M.; León, R.; et al. Mortality Associated with Bacteremia Due to Colistin-Resistant Klebsiella pneumoniae with High-Level Meropenem Resistance: Importance of Combi-nation Therapy without Colistin and Carbapenems. Antimicrob. Agents Chemother. 2017, 61, e00406-17. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Paterson, D.L.; Potoski, B.A.; Kilayko, M.C.; Sandovsky, G.; Sordillo, E.; Polsky, B.; Adams-Haduch, J.M.; Doi, Y. Treatment Outcome of Bacteremia Due to KPC-Producing Klebsiella pneumoniae: Superiority of Combination Antimicrobial Regimens. Antimicrob. Agents Chemother. 2012, 56, 2108–2113. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.-R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Pintado, V.; et al. A Predictive Model of Mortality in Patients with Bloodstream Infections due to Carbapenemase-Producing Enterobacteriaceae. Mayo Clin. Proc. 2016, 91, 1362–1371. [Google Scholar] [CrossRef]
- Machuca, I.; Gutiérrez-Gutiérrez, B.; Rivera-Espinar, F.; Cano, Á.; Gracia-Ahufinger, I.; Guzman-Puche, J.; Marfil-Pérez, E.; Pérez-Nadales, E.; Castón, J.J.; Bonomo, R.A.; et al. External validation of the INCREMENT-CPE mortality score in a carbapenem-resistant Klebsiella pneumoniae bacteraemia cohort: The prognostic significance of colistin resistance. Int. J. Antimicrob. Agents 2019, 54, 442–448. [Google Scholar] [CrossRef]
- Rodriguez-Gómez, J.; Pérez-Nadales, E.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Martinez-Martinez, L.; Rivera, F.; Cano, Á.; Castón, J.J.; Robles, J.C.; de la Fuente, C.; et al. Prognosis of urinary tract infection caused by KPC-producing Klebsiella pneumoniae: The impact of inappropriate empirical treatment. J. Infect. 2019, 79, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Espinar, F.; Machuca, I.; Tejero, R.; Rodríguez, J.; Mula, A.; Marfil, E.; Cano, Á.; Gutiérrez-Gutiérrez, B.; Rodríguez, M.; Pozo, J.C.; et al. Impact of KPC Production and High-Level Meropenem Resistance on All-Cause Mortality of Ventilator-Associated Pneumonia in Association with Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e02164-19. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Viale, P.; Viscoli, C.; Trecarichi, E.M.; Tumietto, F.; Marchese, A.; Spanu, T.; Ambretti, S.; Ginocchio, F.; Cristini, F.; et al. Predictors of mortality in bloodstream infec-tions caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin. Infect. Dis. 2012, 55, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Scorzolini, L.; Cipolla, A.; Mascellino, M.T.; Cancelli, F.; Castaldi, D.; D’Abramo, A.; D’Agostino, C.; Russo, G.; Ciardi, M.R.; et al. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: The activity of the double-carbapenem regimen is related to meropenem MIC value. J. Antimicrob. Chemother. 2017, 72, 1981–1984. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Martucci, G.; Montini, L.; Panarello, G.; Cutuli, S.L.; Di Carlo, D.; Di Gravio, V.; Di Stefano, R.; Capitanio, G.; Vallecoccia, M.S.; et al. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: A two-center, matched case-control study. Crit. Care 2017, 21, 173. [Google Scholar] [CrossRef] [PubMed]
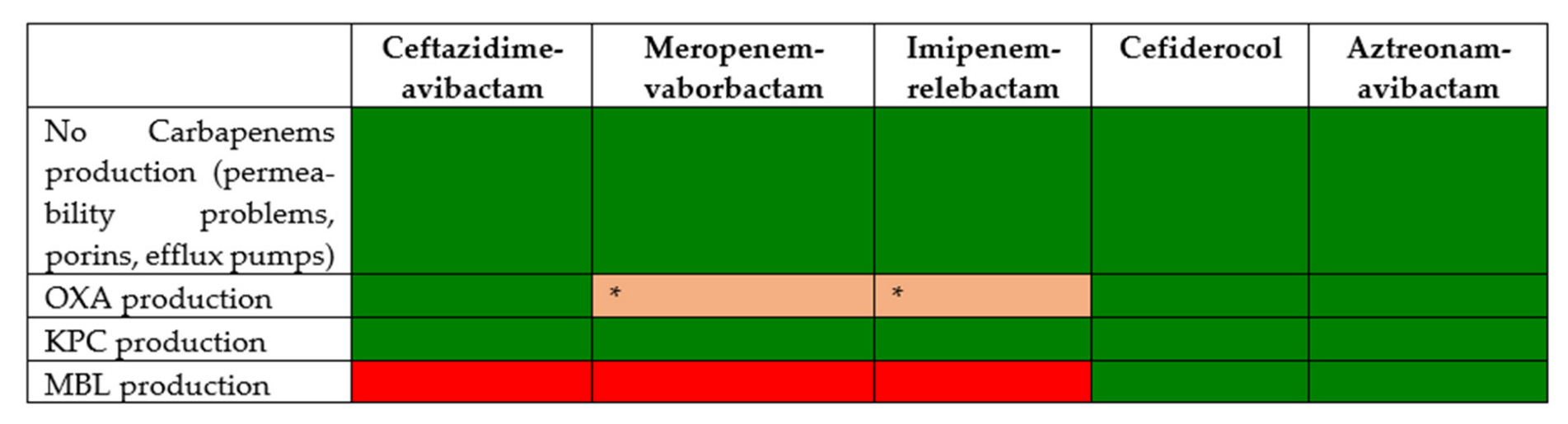
- Shields, R.K.; Clancy, C.J.; Hao, B.; Chen, L.; Press, E.G.; Iovine, N.M.; Kreiswirth, B.N.; Nguyen, M.H. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum β-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against car-bapenem-resistant K. pneumoniae. Antimicrob. Agents Chemother. 2015, 59, 5793–5797. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-Relebactam and Mero-penem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. Emergence of Ceftazidime-Avibactam Resistance and Restoration of Carbapenem Susceptibility in Klebsiella pneumoniae Carbapenemase-Producing K pneumoniae: A Case Report and Review of Literature. Open Forum Infect. Dis. 2017, 4, ofx101. [Google Scholar] [CrossRef]
- Cano, Á.; Guzmán-Puche, J.; García-Gutiérrez, M.; Castón, J.J.; Gracia-Ahufinger, I.; Perez-Nadales, E.; Recio, M.; Natera, A.M.; Marfil-Pérez, E.; Martínez-Martínez, L.; et al. Use of carbapenems in the combined treatment of emerging ceftazidime/avibactam-resistant and carbapenem-susceptible KPC-producing Klebsiella pneumoniae infections: Report of a case and review of the literature. J. Glob. Antimicrob. Resist. 2020, 22, 9–12. [Google Scholar] [CrossRef]
- Hernández-García, M.; Castillo-Polo, J.A.; Cordero, D.G.; Pérez-Viso, B.; García-Castillo, M.; de la Fuente, J.S.; Morosini, M.I.; Cantón, R.; Ruiz-Garbajosa, P. Impact of Ceftazidime-Avibactam Treatment in the Emergence of Novel KPC Variants in the ST307-Klebsiella pneumoniae High-Risk Clone and Consequences for Their Routine Detection. J. Clin. Microbiol. 2022, 60, e0224521. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Pérez-Rodríguez, M.T.; Soto, A.; Rodríguez, L.; Perez-Landeiro, A.; Martínez-Lamas, L.; Nodar, A.; Crespo, M. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Carvalhaes, C.G.; Arends, S.J.R.; Castanheira, M.; Mendes, R.E. Aztreonam/avibactam activity against clinical isolates of Enterobacterales collected in Europe, Asia and Latin America in 2019. J. Antimicrob. Chemother. 2021, 76, 659–666. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-17. [Google Scholar] [CrossRef] [PubMed]
- Castón, J.J.; Lacort-Peralta, I.; Martín-Dávila, P.; Loeches, B.; Tabares, S.; Temkin, L.; Torre-Cisneros, J.; Paño-Pardo, J.R. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int. J. Infect. Dis. 2017, 59, 118–123. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; DE Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients with Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Temkin, E.; Torre-Cisneros, J.; Beovic, B.; Benito, N.; Giannella, M.; Gilarranz, R.; Jeremiah, C.; Loeches, B.; Machuca, I.; Jiménez-Martín, M.J.; et al. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Carbapenem-Resistant Organisms. Antimicrob. Agents Chemother. 2017, 61, e01964-16. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and Safety of Mero-penem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients with Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections: Table 1. Clin. Infect. Dis. 2016, 63, 1615–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e01694-17. [Google Scholar] [CrossRef]
- Gomez-Simmonds, A.; Stump, S.; Giddins, M.J.; Annavajhala, M.K.; Uhlemann, A.C. Clonal Background, Resistance Gene Profile, and Porin Gene Mutations Modulate In Vitro Susceptibility to Imipenem-Relebactam in Diverse Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00573-18. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteri-aceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [PubMed]
- Athans, V.; Neuner, E.A.; Hassouna, H.; Richter, S.S.; Keller, G.; Castanheira, M.; Brizendine, K.D.; Mathers, A.J. Meropenem-Vaborbactam as Salvage Therapy for Ceftazidime-Avibactam-Resistant Klebsiella pneumoniae Bacteremia and Abscess in a Liver Transplant Recipient. Antimicrob. Agents Chemother. 2019, 63, e01551-18. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Ambretti, S.; Lazzarotto, T.; Gaibani, P. In vitro activity of imipenem-relebactam against KPC-producing Klebsiella pneumoniae resistant to ceftazidime-avibactam and/or meropenem-vaborbactam. Clin. Microbiol. Infect. 2022, 28, 749–751. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Barnes, M.D.; Alsop, J.; Taracila, M.A.; Bethel, C.R.; Becka, S.A.; van Duin, D.; Kreiswirth, B.N.; Kaye, K.S.; Bonomo, R.A. Relebactam Is a Potent Inhibitor of the KPC-2 β-Lactamase and Restores Imipenem Susceptibility in KPC-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00174-18. [Google Scholar] [CrossRef]
- Zusman, O.; Altunin, S.; Koppel, F.; Benattar, Y.D.; Gedik, H.; Paul, M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 29–39. [Google Scholar] [CrossRef]
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodríguez-Hernández, M.J.; Tallón-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: Results from the REJUVENATE study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef]
- Golden, A.R.; Adam, H.J.; Baxter, M.; Walkty, A.; Lagacé-Wiens, P.; Karlowsky, J.A.; Zhanel, G.G. In Vitro Activity of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated from Patients in Canadian Intensive Care Units. Diagn. Microbiol. Infect. Dis. 2020, 97, 115012. [Google Scholar] [CrossRef]
- Shaw, E.; Rombauts, A.; Tubau, F.; Padullés, A.; Càmara, J.; Lozano, T.; Cobo-Sacristán, S.; Sabe, N.; Grau, I.; Rigo-Bonnin, R.; et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018, 73, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
| High Risk Clone | Mechanism of Antimicrobial Resistance | Co-Resistance |
|---|---|---|
| ST11 ST15 ST101 ST147 | ESBL (CTX-M-15, …) Carbapenemases (KPC, VIM, NDM, OXA-48) | Fluoroquinolones (topoisomerases mutations, qnr, aac(6′)-Ib-cr…), plasmidic AmpC beta-lactamases (DHA-1), aminoglycosides (ArmA, RmtB methylases) |
| ST258 | Carbapenemases (KPC) | Colistin (mutations in pmrB), ceftazidime-avibactam |
| ST307 | ESBL Carbapenemases (KPC-like, NDM) | Colistin (mutations in mgrB and pmrB), ceftazidime-avibactam resistance |
| ST383 | Carbapenemases (KPC, VIM, OXA-48) | ESBL (CTX-M-15) |
| ST392 | Carbapenemases (KPC) | Multidrug resistance |
| ST405 | Carbapenemases (OXA-48) | Cephalosporins (ESBLs: CTX-M-14, CTX-M-15) |
| ST512 | Carbapenemases (KPC-like) | Ceftazidime-avibactam |
| Reference and Patients | Prediction | Prognostic Factor | OR (95% CI) p-Value |
|---|---|---|---|
| Namikawa et al. [47] 129 patients with bacteremia | 30-day mortality | Sepsis | 7.46 (1.85–30.1) <0.01 |
| iutA gen | 4.47 (1.03–19.5) <0.05 | ||
| Tseng et al. [49] 309 patients with community-onset bacteremia admitted to ICU | Infection-related mortality in ICU | APACHE II score | 1.43 (1.12–2.01) 0.04 |
| Cancer | 35.48 (2.54–495.57) <0.01 | ||
| 1-year mortality | Cancer | 3.14 (1.36–7.26) <0.01 | |
| Man et al. [50] 853 patients with bacteremia (20.9% admitted to ICU) | 30-day mortality | Respiratory tract infection | 2.99 (2.06–4.34) <0.01 |
| Intra-abdominal infection, excluding hepatobiliary | 2.76 (1.76–4.34) <0.01 | ||
| Mechanical ventilation | 2.20 (1.50–3.22) <0.01 | ||
| Medical ward | 1.83 (1.25–2.67) <0.01 | ||
| No diabetes mellitus | 1.76 (1.24–2.50) <0.01 | ||
| Inappropriate empirical antibiotic treatment | 1.71 (1.27–2.32) <0.01 | ||
| Female sex | 1.70 (1.25–2.31) <0.01 | ||
| Age > 65 years | 1.69 (1.16–2.47) <0.01 | ||
| Solid tumour | 1.46 (1.05–2.01) 0.02 | ||
| Papadimitriou-Olivgeris et al. [51] 139 patients with ICU-acquired bacteremia (CP-Kp) | 30-day mortality | Septic shock | 6.5 (2.2–19.5) <0.01 |
| SAPS II | 1.1 (1.0–1.2) <0.01 | ||
| Corticosteroids during bacteremia treatment | 3.1 (1.1–8.6) 0.02 | ||
| Parenteral nutrition | 2.8 (1.0–7.7) 0.04 | ||
| Combination antibiotic treatment | 0.24 (0.07–0.75) 0.01 |
| References | OR (95% CI) | p-Value | |
|---|---|---|---|
| Extended-spectrum beta-lactamases | |||
| Man et al. [50] | No hepatobiliary site | 2.231 (1.341–3.712) | 0.002 |
| Corticosteroids in the previous 30 days | 1.957 (1.061–3.610) | 0.032 | |
| Solid cancer | 1.851 (1.214–2.823) | 0.004 | |
| CVC carrier in the current admission | 1.686 (1.051–2.705) | 0.030 | |
| Resistance to carbapenems | |||
| Prior admission to ICU | 2.32 (1.22–4.4) | 0.01 | |
| Zhang et al. [52] | Surgery | 2.33 (1.26–4.32) | 0.007 |
| Prior antibiotics | 2.02 (1.1–3.74) | 0.024 | |
| Mechanical ventilation | 3.3 (1.56–6.97) | 0.002 | |
| APACHE II > 10 points | 1.9 (1.06–3.42) | 0.031 | |
| Hospital stay > 14 days | 4.34 (2.21–8.55) | <0.001 | |
| Population | Variables | Score |
|---|---|---|
| General a | Severe sepsis or septic shock | 5 |
| Pitt score ≥ 6 | 4 | |
| Charlson comorbidity index | 3 | |
| Source other than urinary or biliary tract | 3 | |
| Inappropriate early targeted therapy | 2 | |
| Maximum score | 17 | |
| Solid-organ transplant b | INCREMENT-CPE score ≥ 8 | 8 |
| Cytomegalovirus disease (previous 3 days) | 7 | |
| Lymphocytes ≤ 600 mm3 | 4 | |
| No source control | 3 | |
| Inappropriate empirical therapy | 2 | |
| Interaction INCREMENT-CPE score ≥ 8 * Cytomegalovirus disease (previous 3 days) | –7 | |
| Maximum score c | 17 |
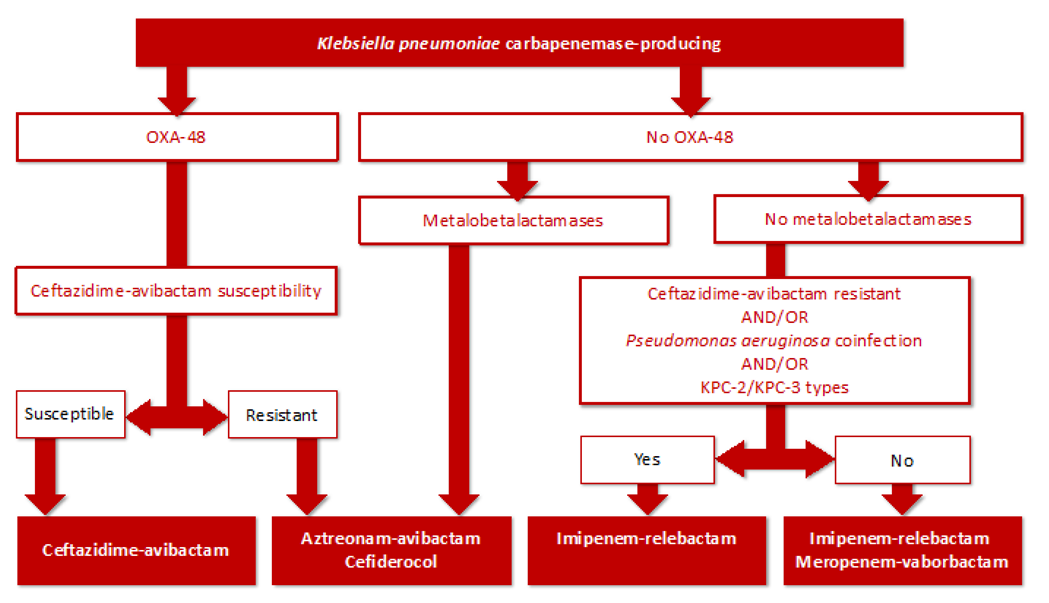
| Carbapenem-Resistant Klebsiella pneumoniae | Recommended Treatment | Alternative Therapy |
|---|---|---|
| No carbapenemase production or not available | ||
| Resistant to ertapenem and susceptible to meropenem | Meropenem (extended infusion) | Ceftazidime-avibactam Quinolones, trimethropim-sulfamethoxazole, nitrofurantoin, aminoglycosides can be used in cystitis if susceptible |
| Resistant to Ertapenem and meropenem | Ceftazidime-avibactam, Meropenem-vaborbactam, Imipenem-relebactam, Cefiderocol | Aztreonam-avibactam b Tigecycline (in combination therapy), Eravacycline (only with abdominal source) Quinolones, trimethropim-sulfamethoxazole, nitrofurantoin, aminoglycoside can be used in cystitis if susceptible |
| Carbapenemase production | ||
| Oxacillinase carbapenemases (OXAs) | Ceftazidime-avibactam Cefiderocol | Aztreonam-avibactam b Tigecycline (in combination therapy), Eravacycline (only with abdominal source), Aminoglycoside (urinary tract infections, catheter-related infections, combined therapy (including Plazomycin), Fosfomycin (urinary tract infections) |
| Klebsiella pneumoniae carbapenemases (KPCs) | Ceftazidime-avibactam, Imipenem-relebactam, Meropenem-vaborbactam, Cefiderocol | Aztreonam-avibactam b Tigecycline (in combination therapy), Eravacycline (only with abdominal source) Amynoglicoside (urinary tract infections, catheter-related infections), combined therapy (including Plazomycin), Fosfomycin (urinary tract infections) |
| Metallo-b-lactamases a (MBLs) | Aztreonam (when susceptible) Aztreonam-avibactam b | Tigecycline (in combination therapy), Eravacycline (only with abdominal source) Amynoglicoside (urinary tract infections, catheter-related infections, combined therapy (including Plazomycin), Fosfomycin (urinary tract infections) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Cortés, P.; Martin-Loeches, I.; Rodríguez, A.; Bou, G.; Cantón, R.; Diaz, E.; De la Fuente, C.; Torre-Cisneros, J.; Nuvials, F.X.; Salavert, M.; et al. Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults. Antibiotics 2022, 11, 1160. https://doi.org/10.3390/antibiotics11091160
Vidal-Cortés P, Martin-Loeches I, Rodríguez A, Bou G, Cantón R, Diaz E, De la Fuente C, Torre-Cisneros J, Nuvials FX, Salavert M, et al. Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults. Antibiotics. 2022; 11(9):1160. https://doi.org/10.3390/antibiotics11091160
Chicago/Turabian StyleVidal-Cortés, Pablo, Ignacio Martin-Loeches, Alejandro Rodríguez, Germán Bou, Rafael Cantón, Emili Diaz, Carmen De la Fuente, Julián Torre-Cisneros, Francisco Xavier Nuvials, Miguel Salavert, and et al. 2022. "Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults" Antibiotics 11, no. 9: 1160. https://doi.org/10.3390/antibiotics11091160
APA StyleVidal-Cortés, P., Martin-Loeches, I., Rodríguez, A., Bou, G., Cantón, R., Diaz, E., De la Fuente, C., Torre-Cisneros, J., Nuvials, F. X., Salavert, M., Aguilar, G., Nieto, M., Ramírez, P., Borges, M., Soriano, C., Ferrer, R., Maseda, E., & Zaragoza, R. (2022). Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults. Antibiotics, 11(9), 1160. https://doi.org/10.3390/antibiotics11091160










