Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis
Abstract
:1. Introduction
2. Materials and Methods
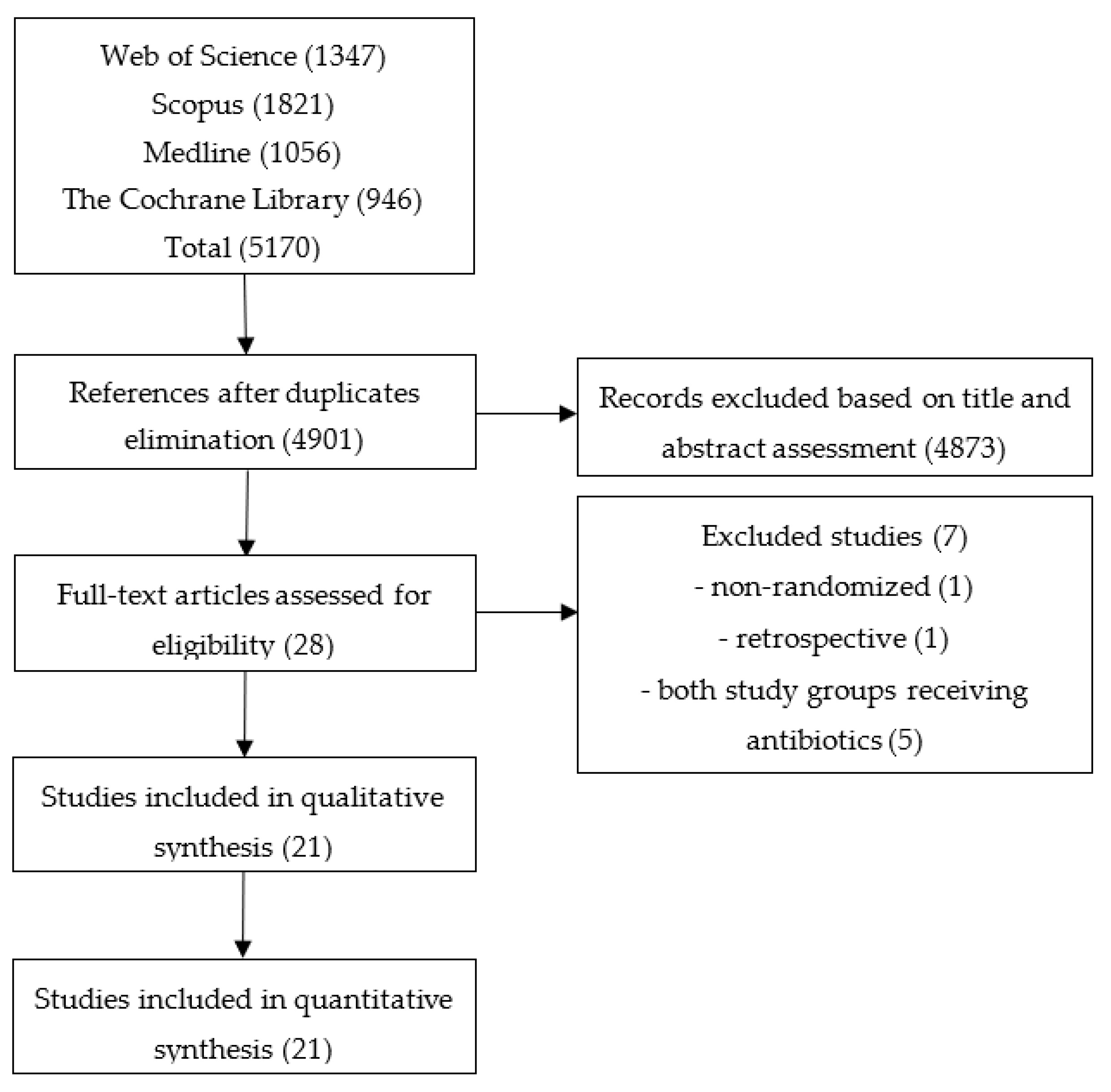
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
2.5. Trial Sequential Analysis
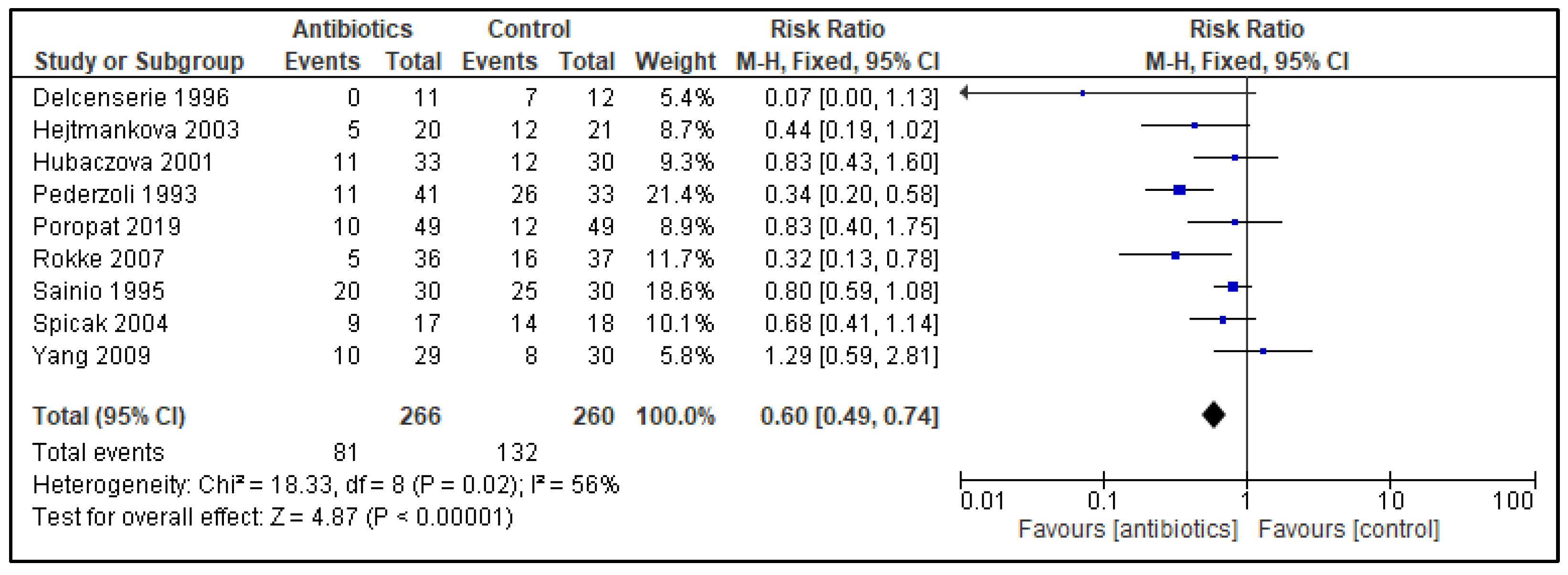
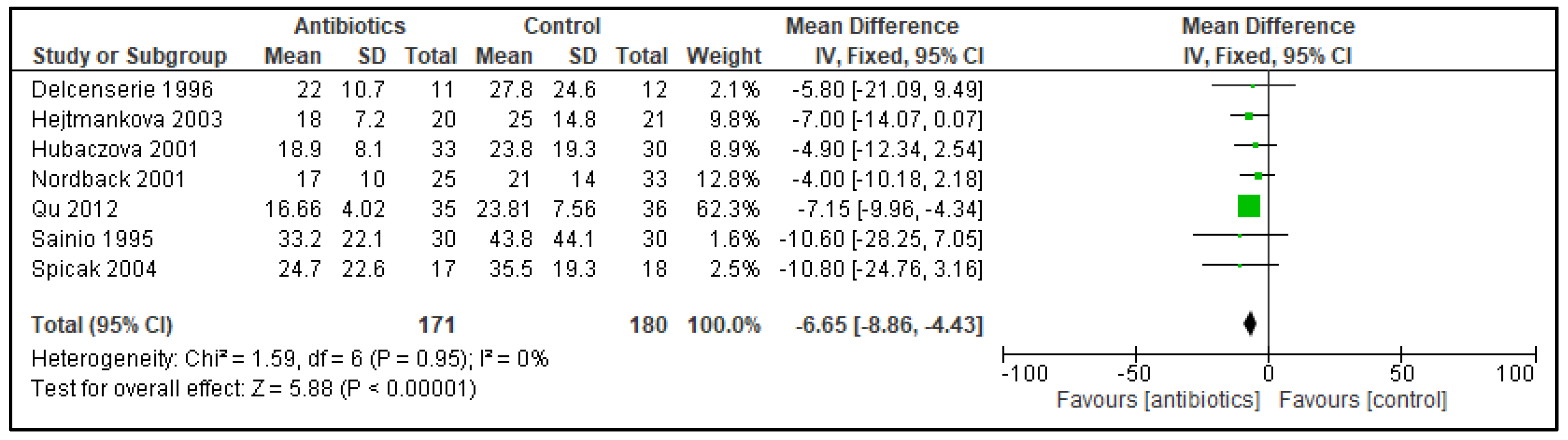
3. Results
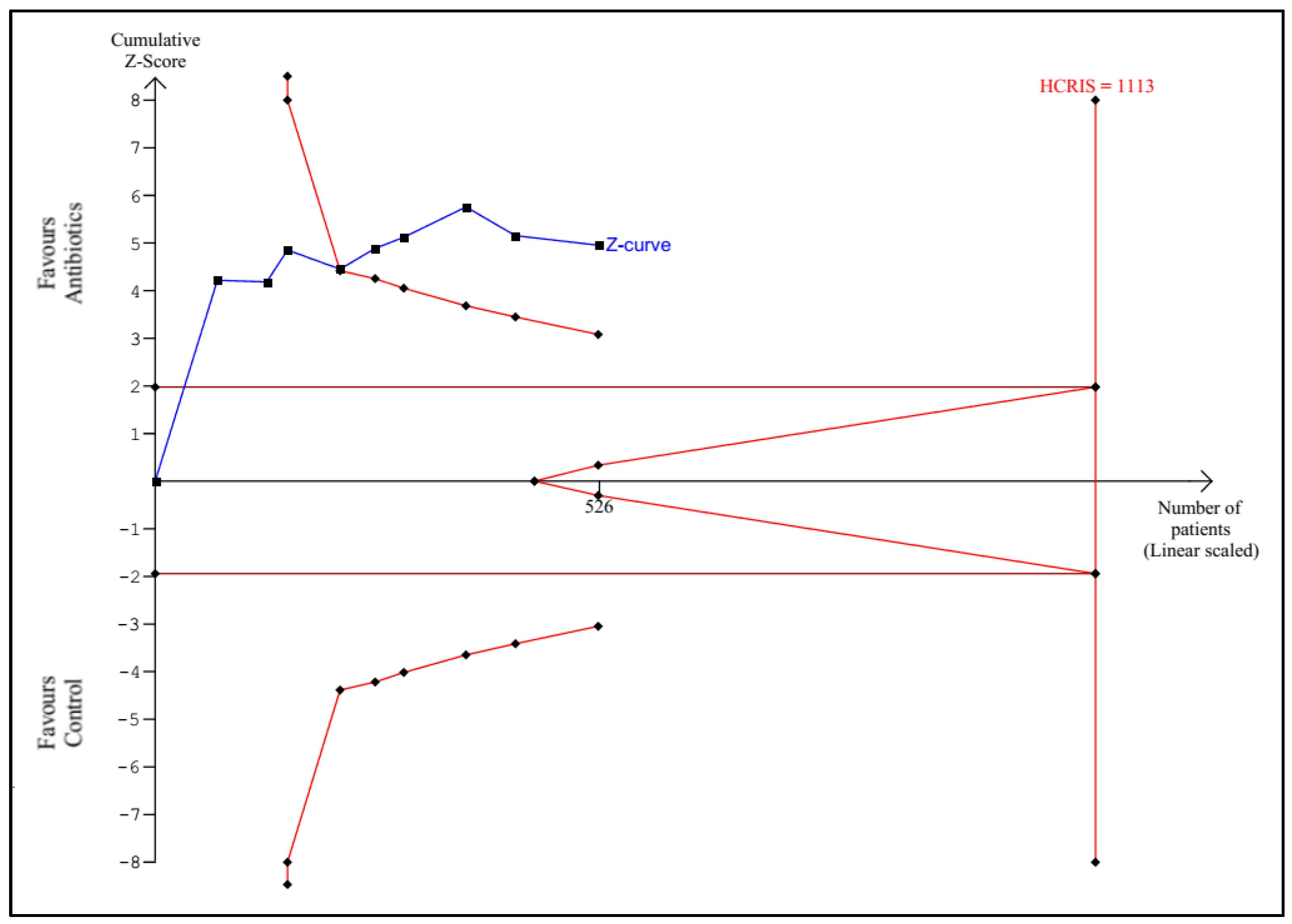
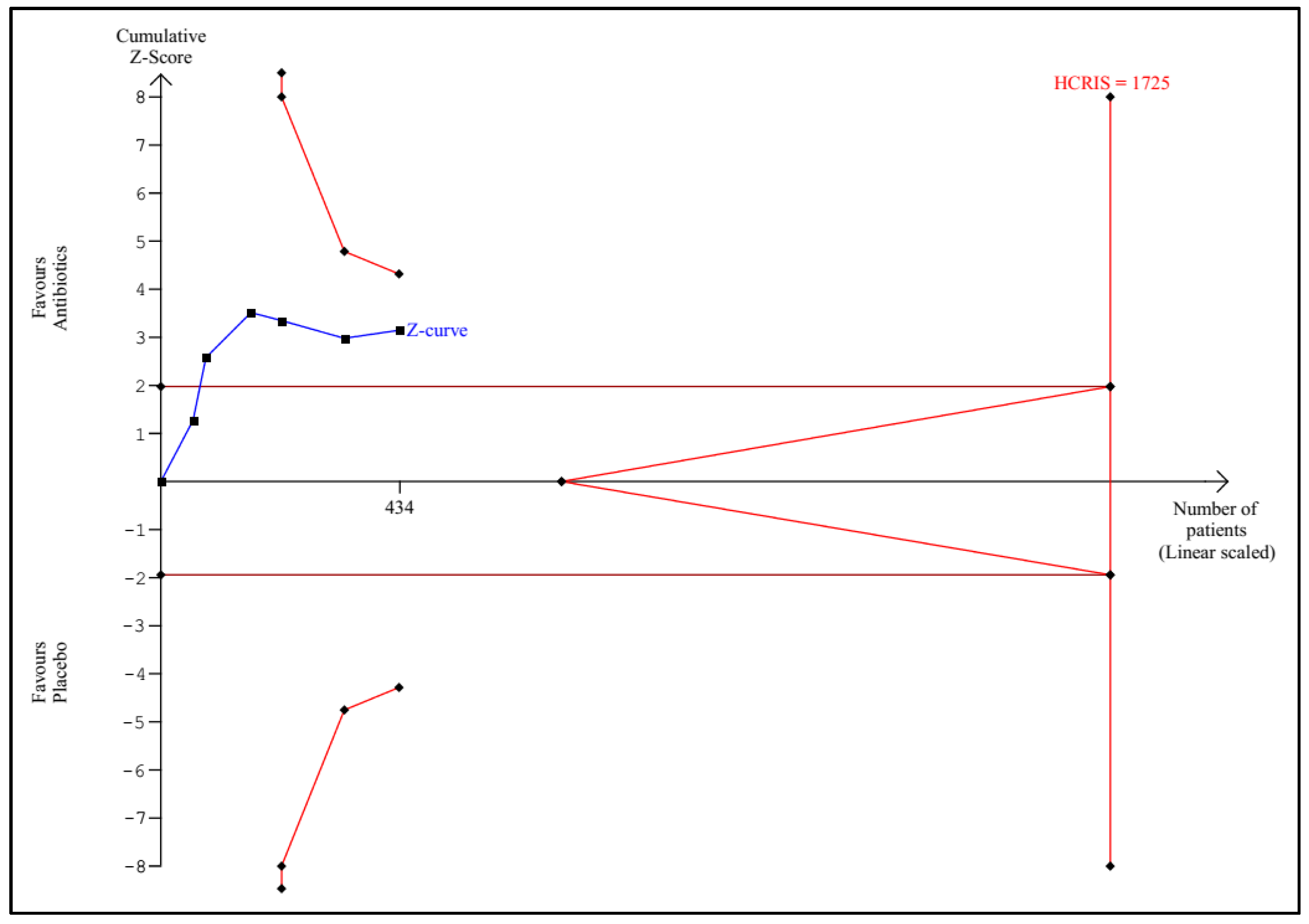
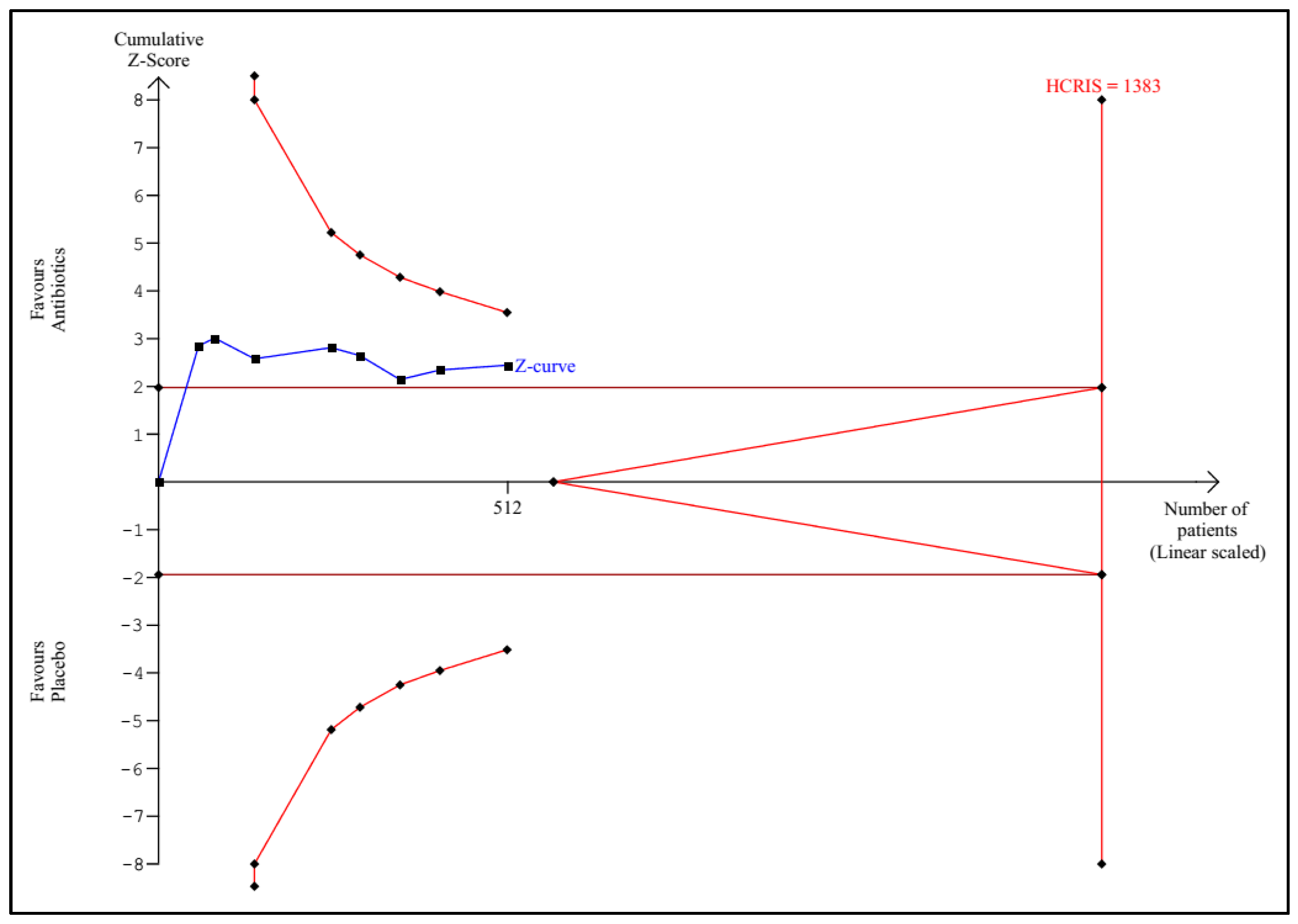
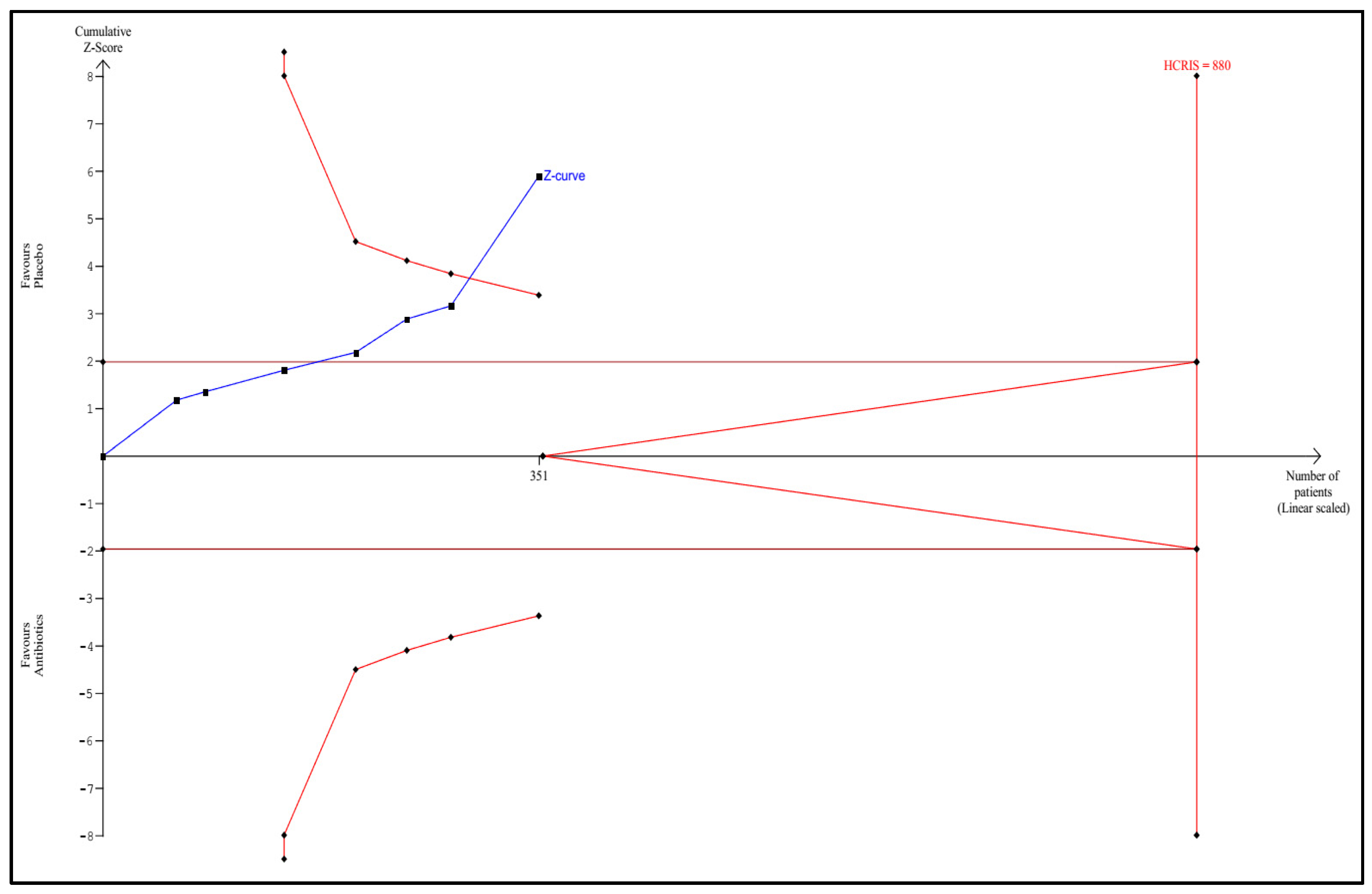
3.1. Trial Sequential Analysis
3.2. Assessment of Risk of Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategies for Electronic Databases
| MEDLINE: 1. exp Pancreas/ 2. exp Pancreatitis/ 3. pancrea*.mp. 4. or/1–3 5. exp antibacterial agents/ 6. antibacteria*.mp. 7. exp antibiotic prophylaxis/ 8. (antibiotic* or prophyla*).mp. 9. cephalosporin*.mp. 10. ceftriaxone.mp. 11. cefepime.mp. 12. cefuroxime.mp. 13. cefazolin.mp. 14. carbapenem*.mp. 15. imipenem.mp. 16. imipenem-cilastatin.mp. 17. meropenem.mp. 18. ertapenem.mp. 19. gentam*cin*.mp. 20. amikacin*.mp. 21. metronidazole.mp. 22. beta-lactam*.mp. 23. amoxicillin.mp. 24. ampicillin.mp. 25. penicillin.mp. 26. (tazobactam or piperacillin).mp. 27. clavulanic acid.mp. 28. sulbactam.mp. 29. vancom*cin.mp. 30. or/5–29 31. (random* or blind* or placebo* or meta-analysis).mp. 32. exp randomized controlled trial/ 33. or/31–32 34. 4 and 30 and 33 |
| WEB OF SCIENCE: TS = (pancreas OR pancreatitis) TS = pancrea* #1 or #2 TS = (antibacterial agents) TS = antibacteria* TS = (antibiotic* or prophyla*) TS = cephalosporin* TS = ceftriaxone TS = cefepime TS = cefuroxime TS = cefazolin TS = carbapenem* TS = imipenem TS = imipenem-cilastatin TS = meropenem TS = ertapenem TS = gentam*cin* TS = amikacin* TS = metronidazole TS = beta-lactam* TS = amoxicillin TS = ampicillin TS = penicillin TS = (tazobactam or piperacillin) TS = (clavulanic acid) TS = sulbactam TS = vancom*cin or/#4-#27 TS = (random* or blind* or placebo* or meta-analysis) TS = (randomized controlled trial) |
| SCOPUS: ALL (“pancreas”) OR ALL (“pancreatitis”) OR ALL (“pancreas*”) AND ALL (“antibacterial agents”) OR ALL (“antibacterial”) OR ALL (“antibiotic prophylaxis”) OR ALL (“antibiotic* OR prophyla*”) OR ALL (“cephalosporin*”) OR ALL (“ceftriaxone”) OR ALL (“cefepime”) OR ALL (“cefuroxime”) OR ALL (“cefazolin”) OR ALL (“carbapenem*”) OR ALL (“imipenem”) OR ALL (“imipene mcilastatin”) OR ALL (“meropenem”) OR ALL (“ertapenem”) OR ALL (“gentam*cin”) OR ALL (“amikacin*”) OR ALL (“metronidazole”) OR ALL (“betalactam*”) OR ALL (“amoxicillin”) OR ALL (“ampicillin”) OR ALL (“penicillin”) OR ALL (“tazobactam or piperacillin”) OR ALL (“clavulanic acid”) OR ALL (“sulbactam”) OR ALL (“vancom*cin”) AND ALL (“random* or blind* or placebo* or meta-analysis”) OR ALL (“randomized controlled trial”) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMITTO (DOCTYPE, “cp”)) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMITTO (SUBJAREA, “NURS”) OR LIMIT-TO (SUBJAREA, “HEAL”) OR LIMITTO (UBJAREA, “BIOC”) OR LIMIT-TO (SUBJAREA, “PHAR”) OR LIMIT-TO (SUBJAREA, “IMMU”)) |
| CENTRAL: #1 MeSH descriptor: [Pancreatitis] explode all trees #2 MeSH descriptor: [Pancreas] explode all trees #3 pancrea* in Trials #4 #1 OR #2 OR #3 #5 MeSH descriptor: [Anti-Bacterial Agents] explode all trees #6 antibacteria* in Trials #7 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees #8 (antibiotic* OR prophyla*) in Trials #9 cephalosporin* in Trials #10 cefepime in Trials #11 ceftriaxone in Trials #12 cefuroxime in Trials #13 cefazolin in Trials #14 carbapenem* in Trials #15 imipenem in Trials #16 imipenem-cilastatin in Trials #17 meropenem in Trials #18 ertapenem in Trials #19 gentam*cin* in Trials #20 amikacin* in Trials #21 metronidazole in Trials #22 beta-lactam* in Trials #23 amoxicillin in Trials #24 ampicillin in Trials #25 penicillin in Trials #26 (tazobactam or piperacillin) in Trials #27 clavulanic acid in Trials #28 sulbactam in Trials #29 vancom*cin in Trials #30 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 #31 (random* OR blind* OR placebo*) #32 MeSH descriptor: [Randomized Controlled Trial] explode all trees #33 #31 OR #32 #34 #4 AND #30 AND #33 |
References
- Xiao, A.Y.; Tan, M.L.Y.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Schepers, N.J.; Bakker, O.J.; Besselink, M.G.; Ali, U.A.; Bollen, T.L.; Gooszen, H.G.; Van Santvoort, H.C.; Bruno, M.J. Impact of characteristics of organ failure and infected pancreatic necrosis on mortality in necrotising pancreatitis. Gut 2019, 68, 1044–1051. [Google Scholar] [CrossRef]
- Dellinger, E.P.; Tellado, J.M.; Soto, N.E.; Ashley, S.W.; Barie, P.S.; Dugernier, T.; Imrie, C.W.; Johnson, C.D.; Knaebel, H.P.; Laterre, P.F.; et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: A randomized, double-blind, placebo-controlled study. Ann. Surg. 2007, 245, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, R.; Rünzi, M.; Kron, M.; Kahl, S.; Kraus, D.; Jung, N.; Maier, L.; Malfertheiner, P.; Goebell, H.; Beger, H.G. Prophylactic Antibiotic Treatment in Patients with Predicted Severe Acute Pancreatitis: A Placebo-Controlled, Double-Blind Trial. Gastroenterology 2004, 126, 997–1004. [Google Scholar] [CrossRef]
- Poropat, G.; Radovan, A.; Peric, M.; Mikolasevic, I.; Giljaca, V.; Hauser, G.; Milic, S.; Stimac, D. Prevention of infectious complications in acute pancreatitis: Results of a single-center, randomized, controlled trial. Pancreas 2019, 48, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Deng, L.-H.; Zhang, Z.-D.; Yang, X.-N.; Wan, M.-H.; Song, B.; Xia, Q. Effect of antibiotic prophylaxis on acute necrotizing pancreatitis: Results of a randomized controlled trial. J. Gastroenterol. Hepatol. 2009, 24, 736–742. [Google Scholar] [CrossRef]
- Villatoro, E.; Mulla, M.; Larvin, M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst. Rev. 2010, 5, CD002941. [Google Scholar] [CrossRef]
- Wittau, M.; Mayer, B.; Scheele, J.; Henne-Bruns, D.; Dellinger, E.P.; Isenmann, R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand. J. Gastroenterol. 2011, 46, 261–270. [Google Scholar] [CrossRef]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Lohse, H.A.S.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.R.J.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Feuerstein, J.; Flamm, S.; Gellad, Z.; Gerson, L.; Gupta, S.; et al. American Gastroenterological Association Institute Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Dumonceau, J.-M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Ferreira, A.O.; Gyökeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013, 13 (Suppl. 2), e1–e15. [Google Scholar] [CrossRef] [PubMed]
- Párniczky, A.; Lantos, T.; Tóth, E.M.; Szakács, Z.; Gódi, S.; Hágendorn, R.; Illés, D.; Koncz, B.; Márta, K.; Mikó, A.; et al. Antibiotic therapy in acute pancreatitis: From global overuse to evidence based recommendations. Pancreatology 2019, 19, 488–499. [Google Scholar] [CrossRef]
- Horibe, M.; Sanui, M.; Sasaki, M.; Honda, H.; Ogura, Y.; Namiki, S.; Sawano, H.; Goto, T.; Ikeura, T.; Takeda, T.; et al. Impact of Antimicrobial Prophylaxis for Severe Acute Pancreatitis on the Development of Invasive Candidiasis: A Large Retrospective Multicenter Cohort Study. Pancreas 2019, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- Shah, A.; Smith, A.F. Trial sequential analysis: Adding a new dimension to meta-analysis. Anaesthesia 2020, 75, 15–20. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Golub, R.; Siddiqi, F.; Pohl, D. Role of Antibiotics in Acute Pancreatitis: A Meta-Analysis. J. Gastrointest Surg. 1998, 2, 496–503. [Google Scholar] [CrossRef]
- Sharma, V.K.; Howden, C.W. Prophylactic Antibiotic Administration Reduces Sepsis and Mortality in Acute Necrotizing Pancreatitis: A Meta-Analysis. Pancreas 2001, 22, 28–31. [Google Scholar] [CrossRef]
- Villatoro, E.; Larvin, M.; Bassi, C. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis (Review). Cochrane Database Syst. Rev. 2003, 4, CD002941. [Google Scholar]
- Dambrauskas, Z.; Gulbinas, A.; Pundzius, J.; Barauskas, G. Meta-analysis of prophylactic parenteral antibiotic use in acute necrotizing pancreatitis. Medicina 2007, 43, 291–300. [Google Scholar] [CrossRef] [PubMed]
- García-Barrasa, A.; Borobia, F.G.; Pallares, R.; Jorba, R.; Poves, I.; Busquets, J.; Fabregat, J. A Double-blind, Placebo-controlled Trial of Ciprofloxacin Prophylaxis in Patients with Acute Necrotizing Pancreatitis. J. Gastrointest. Surg. 2009, 13, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gao, J.; Zou, D.W.; Li, Z.S. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: Evidence from a meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 2008, 103, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.S.; Mahid, S.S.; Idstein, S.R.; Hornung, C.A.; Galandiuk, S. Antibiotic prophylaxis is not protective in severe acute pancreatitis: A systematic review and meta-analysis. Am. J. Surg. 2009, 197, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sun, Y.-H.; Wen, L.-M.; Wang, J.-H.; Cheng, K.; Lin, H.; Chen, Q.-L. Assessment of prophylactic antibiotics administration for acute pancreatitis: A meta-analysis of randomized controlled trials. Chin. Med. J. 2020, 133, 212–220. [Google Scholar] [CrossRef]
- De Vries, A.C.; Besselink, M.G.H.; Buskens, E.; Ridwan, B.U.; Schipper, M.; van Erpecum, K.J.; Gooszen, H.G. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: Relationship between methodological quality and outcome. Pancreatology 2007, 7, 531–538. [Google Scholar] [CrossRef]
- Claire, R.; Gluud, C.; Berlin, I.; Coleman, T.; Leonardi-Bee, J. Using Trial Sequential Analysis for estimating the sample sizes of further trials: Example using smoking cessation intervention. BMC Med. Res. Methodol. 2020, 20, 284. [Google Scholar] [CrossRef]
- Brown, L.A.; Hore, T.A.; Phillips, A.R.; Windsor, J.A.; Petrov, M.S. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology 2014, 14, 436–443. [Google Scholar] [CrossRef]
- Marstrand-Joergensen, M.R.; Bertilsson, S.; Kalaitzakis, E. Extrapancreatic infections are common in acute pancreatitis and they are related to organ failure: A population-based study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1293–1300. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, J.Y.; Wang, X.Y.; Hu, Y.; Cui, Y.F. The impacts of infectious complications on outcomes in acute pancreatitis: A retrospective study. Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pando, E.; Alberti, P.; Hidalgo, J.; Vidal, L.; Dopazo, C.; Caralt, M.; Blanco, L.; Gómez-Gavara, C.; Bilbao, I.; Balsells, J.; et al. The role of extra-pancreatic infections in the prediction of severity and local complications in acute pancreatitis. Pancreatology 2018, 18, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Schwender, B.J.; Gordon, S.R.; Timothy, G.B. Risk Factors for the Development of Intra-Abdominal Fungal Infections in Acute Pancreatitis. Pancreas 2015, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Reuken, P.A.; Albig, H.; Rödel, J.; Hocke, M.; Will, U.; Stallmach, A.; Bruns, T. Fungal Infections in Patients with Infected Pancreatic Necrosis and Pseudocysts: Risk Factors and Outcome. Pancreas 2018, 47, 92–98. [Google Scholar] [CrossRef]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017, 17, 39. [Google Scholar] [CrossRef]
- Schwarz, M.; Isenmann, R.; Meyer, H.; Beger, H.G. Antibiotika bei nekrotisierender Pankreatitis: Ergebnisse einer kontrollierten Studie. Dtsch Med. Wochenschr. 1997, 122, 356–361. [Google Scholar] [CrossRef]
- Sainio, V.; Kemppainen, E.; Puolakkainen, P.; Haapiainen, R.; Schröder, T.; Kivilaakso, E.; Valtonen, V.; Taavitsainen, M.; Kivisaarl, L. Early antibiotic treatment in acute necrotising pancreatitis. Lancet 1995, 346, 663–667. [Google Scholar] [CrossRef]
- Qu, R.; Ji, Y.; Ling, Y.; Ye, C.-Y.; Yang, S.-M.; Liu, Y.-Y.; Yang, R.-Y.; Luo, Y.-F.; Guo, Z. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med. J. 2012, 33, 382–387. [Google Scholar]
- Luiten, E.J.T.; Hop, W.C.J.; Lange, J.F.; Bruining, H.A. Controlled Clinical Trial of Selective Decontamination for the Treatment of Severe Acute Pancreatitis. Ann. Surg. 1995, 222, 57–65. [Google Scholar] [CrossRef]
- Røkke, O.; Harbitz, T.B.; Liljedal, J.; Pettersen, T.; Fetvedt, T.; Heen, L.; Skreden, K.; Viste, A. Early treatment of severe pancreatitis with imipenem: A prospective randomized clinical trial. Scand. J. Gastroenterol. 2007, 42, 771–776. [Google Scholar] [CrossRef]
- Nordback, I.; Sand, J.; Saaristo, R.; Paajanen, H. Early Treatment with Antibiotics Reduces the Need for Surgery in Acute Necrotizing Pancreatitis-A Single-Center Randomized Study. J. Gastrointest Surg. 2001, 5, 113–119. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poropat, G.; Goričanec, K.; Lacković, A.; Kresović, A.; Lončarić, A.; Marušić, M. Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis. Antibiotics 2022, 11, 1191. https://doi.org/10.3390/antibiotics11091191
Poropat G, Goričanec K, Lacković A, Kresović A, Lončarić A, Marušić M. Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis. Antibiotics. 2022; 11(9):1191. https://doi.org/10.3390/antibiotics11091191
Chicago/Turabian StylePoropat, Goran, Karla Goričanec, Alojzije Lacković, Andrea Kresović, Antun Lončarić, and Martina Marušić. 2022. "Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis" Antibiotics 11, no. 9: 1191. https://doi.org/10.3390/antibiotics11091191
APA StylePoropat, G., Goričanec, K., Lacković, A., Kresović, A., Lončarić, A., & Marušić, M. (2022). Systematic Review with Trial Sequential Analysis of Prophylactic Antibiotics for Acute Pancreatitis. Antibiotics, 11(9), 1191. https://doi.org/10.3390/antibiotics11091191





