Carbapenem Combinations for Infections Caused by Carbapenemase-Producing Pseudomonas aeruginosa: Experimental In Vitro and In Vivo Analysis
Abstract
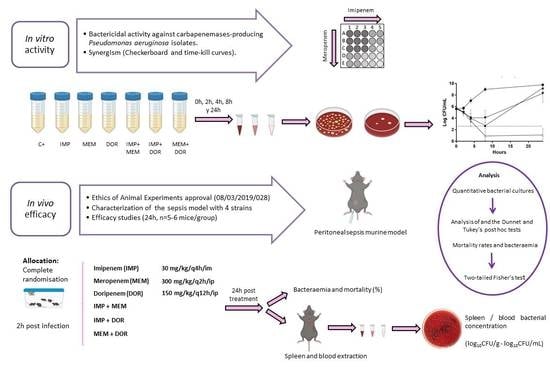
:1. Introduction
2. Results
2.1. In Vitro Results
2.1.1. Isolates’ Carbapenemase Production, Molecular Typing, Antimicrobial Susceptibility Testing, and FICi of Dual Carbapenem Combinations
2.1.2. Time-Kill Assays
2.2. In Vivo Results
2.2.1. Peritoneal Sepsis Model
2.2.2. Efficacy Studies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. In Vitro Studies
5.1.1. Bacterial Isolates’ Characterization and Molecular Typing
5.1.2. Antimicrobials
5.1.3. Antimicrobial Susceptibility Testing
5.1.4. Synergy Studies
Checkerboard Assays
Time-Kill Assays
5.2. In Vivo Studies
5.2.1. Animals
5.2.2. Peritoneal Sepsis Model
5.2.3. Efficacy Studies
5.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Qu, J.; Cai, Z.; Liu, Y.; Duan, X.; Han, S.; Liu, J.; Zhu, Y.; Jiang, Z.; Zhang, Y.; Zhuo, C.; et al. Persistent Bacterial Coinfection of a COVID-19 Patient Caused by a Genetically Adapted Pseudomonas aeruginosa Chronic Colonizer. Front. Cell. Infect. Microbiol. 2021, 11, 641920. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.Á.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Marquez-Valdelamar, L.M.; Bravata-Alcantara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control 2022. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Healthcare-Associated Infections in Intensive Care Units (Annual Epidemiological Report for 2016). Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2016-HAI_0.pdf (accessed on 4 May 2018).
- Zilberberg, M.D.; Shorr, A.F. Prevalence of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009. J. Hosp. Med. 2013, 8, 559–563. [Google Scholar] [CrossRef]
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G., Jr.; van Duin, D. Results from a 13-Year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob. Agents Chemother. 2017, 61, e02671-16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 27 February 2017).
- European Centre for Disease Prevention and Control (ECDC). European Antimicrobial Resistance Surveillance Network. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 18 November 2020).
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Khodare, A.; Kale, P.; Pindi, G.; Joy, L.; Khillan, V. Incidence, microbiological profile, and impact of preventive measures on central line-associated bloodstream infection in liver care Intensive Care Unit. Indian J. Crit. Care Med. 2020, 24, 17–22. [Google Scholar] [PubMed]
- Verma, N.; Prahraj, A.K.; Mishra, B.; Behera, B.; Gupta, K. Detection of carbapenemase-producing Pseudomonas aeruginosa by phenotypic and genotypic methods in a tertiary care hospital of East India. J. Lab. Phys. 2019, 11, 287–291. [Google Scholar] [CrossRef]
- Hopman, J.; Meijer, C.; Kenters, N.; Coolen, J.P.M.; Ghamati, M.R.; Mehtar, S.; van Crevel, R.; Morshuis, W.J.; Verhagen, A.; van den Heuvel, M.M.; et al. Risk Assessment after a severe hospital-acquired infection associated with carbapenemase-producing Pseudomonas aeruginosa. JAMA Netw. Open 2019, 2, e187665. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and characteristics of metallo-β-lactamasepProducing Pseudomonas aeruginosa. Infect. Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vázquez, M.; Sola-Campoy, P.J.; Zurita, Á.M.; Ávila, A.; Gómez-Bertomeu, F.; SolÍs, S.; López-Urrutia, L.; Gónzalez-BarberÁ, E.M.; Cercenado, E.; Bautista, V.; et al. Carbapenemase-producing Pseudomonas aeruginosa in Spain: Interregional dissemination of the high-risk clones ST175 and ST244 carrying bla(VIM-2), bla(VIM-1), bla(IMP-8), bla(VIM-20) and bla(KPC-2). Int. J. Antimicrob. Agents 2020, 56, 106026. [Google Scholar] [CrossRef]
- Del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 2015, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Estrada, S.; Borgatta, B.; Rello, J. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect. Drug Resist. 2016, 9, 7–18. [Google Scholar]
- Nordmann, P.; Perler, J.; Kieffer, N.; Poirel, L. In-vitro evaluation of a dual carbapenem combination against carbapenemase-producing Acinetobacter baumannii. J. Infect. 2020, 80, 121–142. [Google Scholar] [CrossRef]
- Cebrero-Cangueiro, T.; Nordmann, P.; Carretero-Ledesma, M.; Pachón, J.; Pachón-Ibáñez, M.E. Efficacy of dual carbapenem treatment in a murine sepsis model of infection due to carbapenemase-producing Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 76, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; D’Abramo, A.; D’Agostino, C.; Iannetta, M.; Mascellino, M.T.; Gallinelli, C.; Mastroianni, C.M.; Vullo, V. Synergistic activity and effectiveness of a double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae bloodstream infections. J. Antimicrob. Chemother. 2014, 69, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Galani, L.; Baziaka, F.; Karaiskos, I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 2388–2390. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Cipolla, A.; Gizzi, F.; D’Abramo, A.; Favaro, M.; De Angelis, M.; Ferretti, G.; Russo, G.; Iannetta, M.; Mastroianni, C.M.; et al. Severe Bloodstream Infection due to KPC-Producer E. coli in a renal transplant recipient treated with the double-carbapenem regimen and analysis of in vitro synergy testing: A case report. Medicine 2016, 95, e2243. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Tofiño, E.; Zamorano, L.; Cortes-Lara, S.; López-Causapé, C.; Sánchez-Diener, I.; Cabot, G.; Bou, G.; Martínez-Martínez, L.; Oliver, A. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J. Antimicrob. Chemother. 2019, 74, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.; Tarazi, Z.; Nolan, T.; Parkinson, J.; Rubio-Aparicio, D.; Lomovskaya, O.; Dudley, M.N.; Griffith, D.C. Activity of meropenem-vaborbactam in mouse models of infection due to KPC-Producing carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01446-17. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wu, Y.; Cao, L.; Yao, D.; Long, M. Is Meropenem as a Monotherapy Truly Incompetent for Meropenem-Nonsusceptible Bacterial Strains? A Pharmacokinetic/Pharmacodynamic Modeling with Monte Carlo Simulation. Front. Microbiol. 2019, 10, 2777. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11.0. Available online: http://www.eucast.org (accessed on 31 January 2021).
- Parra Millán, R.; Jiménez Mejías, M.E.; Sánchez Encinales, V.; Ayerbe Algaba, R.; Gutiérrez Valencia, A.; Pachón Ibáñez, M.E.; Díaz, C.; Pérez Del Palacio, J.; López Cortés, L.F.; Pachón, J.; et al. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob. Agents Chemother. 2016, 60, 4464–4470. [Google Scholar] [CrossRef]
- Vila, J.; Pachón, J. Therapeutic options for Acinetobacter baumannii infections: An update. Expert Opin. Pharmacother. 2012, 13, 2319–2336. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Scorzolini, L.; Cipolla, A.; Mascellino, M.T.; Cancelli, F.; Castaldi, D.; D’Abramo, A.; D’Agostino, C.; Russo, G.; Ciardi, M.R.; et al. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: The activity of the double-carbapenem regimen is related to meropenem MIC value. J. Antimicrob. Chemother. 2017, 72, 1981–1984. [Google Scholar] [CrossRef]
- Fredborg, M.; Sondergaard, T.E.; Wang, M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2017, 88, 355–360. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Martucci, G.; Montini, L.; Panarello, G.; Cutuli, S.L.; Di Carlo, D.; Di Gravio, V.; Di Stefano, R.; Capitanio, G.; Vallecoccia, M.S.; et al. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: A two-center, matched case-control study. Crit. Care 2017, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.; Nafplioti, K.; Chatzikonstantinou, M.; Souli, M. In vitro evaluation of double-carbapenem combinations against OXA-48-producing Klebsiella pneumoniae isolates using time-kill studies. J. Med. Microbiol. 2018, 67, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Nazer, L.O.; Le, J. Critical review of double-Carbapenem therapy for the treatment of Carbapenemase-producing Klebsiella pneumoniae. Ann. Pharmacother. 2019, 53, 70–81. [Google Scholar]
- White, B.P.; Patel, S.; Tsui, J.; Chastain, D.B. Adding double carbapenem therapy to the armamentarium against carbapenem-resistant Enterobacteriaceae bloodstream infections. Infect. Dis. 2019, 51, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.C.; Nicolau, D.P. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 3002–3004. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Mulet, X.; Fernández-Esgueva, M.; Norte, C.; Zamorano, L.; Del Barrio-Tofiño, E.; Oliver, A. Validation of MALDI-TOF for the early detection of the ST175 high-risk clone of Pseudomonas aeruginosa in clinical isolates belonging to a Spanish nationwide multicenter study. Enferm. Infecc. Microbiol. Clin. 2021, 39, 279–282. [Google Scholar] [CrossRef]
- Bedenić, B.; Meštrović, T. Mechanisms of resistance in gram-negative urinary pathogens: From country-specific molecular insights to global clinical relevance. Diagnostics 2021, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Nicolau, D.P.; Gill, C.M. Carbapenemase-producing Pseudomonas aeruginosa—An emerging challenge. Emerg. Microbes Infect. 2022, 11, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.K.; Smith, C.A.; Frase, H.; Black, D.J.; Vakulenko, S.B. Kinetic and structural requirements for carbapenemase activity in GES-type β-lactamases. Biochemistry 2015, 54, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Canut Blasco, A.; Collazos Blanco, A.; Díez Aguilar, M.; Morosini Reilly, M.I.; Rodríguez-Gascón, A.; Seral-García, C. Métodos Microbiológicos para la Determinación In Vitro de la Actividad de Combinaciones de Antimicrobianos; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC): Madrid, Spain, 2020. [Google Scholar]
- Pachón-Ibáñez, M.E.; Labrador-Herrera, G.; Cebrero-Cangueiro, T.; Díaz, C.; Smani, Y.; Del Palacio, J.P.; Rodríguez-Baño, J.; Pascual, A.; Pachón, J.; Conejo, M.C. Efficacy of colistin and its combination with rifampin in vitro and in experimental models of infection caused by carbapenemase-producing clinical isolates of Klebsiella pneumoniae. Front. Microbiol. 2018, 9, 912. [Google Scholar] [CrossRef]
- National Academies Press (NAP). Guide for the Care and Use of Laboratory Animals; Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council, Eds.; National Academies Press (NAP): Washington, DC, USA, 2010. [Google Scholar]
- O’Reilly, T.; Cleeland, R.; Squires, E. Evaluation of antimicrobials in experimental animal infections. In Antibiotics in Laboratory Medicine, 4th ed.; Lorian, V., Ed.; William & Wilkins: New York, NY, USA, 1996; pp. 604–765. [Google Scholar]
- Dinc, G.; Demiraslan, H.; Elmali, F.; Ahmed, S.S.; Alp, E.; Doganay, M. Antimicrobial efficacy of doripenem and its combinations with sulbactam, amikacin, colistin, tigecycline in experimental sepsis of carbapenem-resistant Acinetobacter baumannii. New Microbiol. 2015, 38, 67–73. [Google Scholar]
- Bretonnière, C.; Jacqueline, C.; Caillon, J.; Guitton, C.; Le Mabecque, V.; Miégeville, A.F.; Villers, D.; Potel, G.; Boutoille, D. Efficacy of doripenem in the treatment of Pseudomonas aeruginosa experimental pneumonia versus imipenem and meropenem. J. Antimicrob. Chemother. 2010, 65, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Banevicius, M.A.; Nicolau, D.P. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 2008, 52, 2497–2502. [Google Scholar] [CrossRef] [Green Version]




| P. aeruginosa | ST | Carbapenemases | MIC (mg/L) | ΣFICi (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|
| IMP | MEM | DOR | IMP + MEM | IMP + DOR | MEM + DOR | |||
| ARA01-015 | 235 | VIM-2 | 128 | 16 | 64 | 1.06 | 0.56 | 1.00 |
| ARA01-045 | 973 | VIM-2 | 64 | 32 | 32 | 0.75 | 1.06 | 0.63 |
| CAT05-004 | 253 | VIM-1 | 128 | 128 | 128 | 1.00 | 0.50 | 0.50 |
| CLE02-006 | 664 | IMP-1 | 128 | 64 | 64 | 0.75 | 1.00 | 0.75 |
| CVA03-019 | 175 | OXA-2/VIM-20 | 128 | 32 | 32 | 0.75 | 0.75 | 0.75 |
| MAD02-005 | 175 | GES-5 | 64 | 128 | 64 | 0.75 | 0.75 | 0.63 |
| MAD02-007 | 175 | VIM-2 | 128 | 32 | 32 | 0.50 | 1.25 | 1.00 |
| MAD02-021 | 235 | GES-5 | 64 | 128 | 64 | 0.63 | 0.75 | 0.63 |
| MAD04-041 | 155 | IMP-8 | 128 | 16 | 16 | 0.75 | 1.50 | 0.63 |
| MAD05-041 | 111 | VIM-2 | 128 | 16 | 16 | 1.00 | 0.75 | 0.50 |
| MUR01-018 | 111 | IMP-33 | 256 | 128 | 128 | 1.06 | 1 | 0.25 |
| Hours after Infection | Isolates | Carbapenemases | ST | Spleen (log10 CFU/g) | Blood (log10 CFU/mL) | Bacteremia (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|
| 2 | CVA03-019 | OXA-2/VIM-20 | 175 | 5.62 ± 0.51 | 3.35 ± 0.49 | 100 | - |
| MAD02-007 | VIM-2 | 175 | 7.38 ± 0.12 | 5.69 ± 0.55 | 100 | - | |
| MAD02-021 | GES-5 | 235 | 5.64 ± 0.49 | 3.50 ± 0.28 | 100 | - | |
| MUR01-018 | IMP-33 | 111 | 5.90 ± 0.79 | 2.67 ± 0.46 | 100 | - | |
| 24 | CVA03-019 | OXA-2/VIM-20 | 175 | 8.7 ± 0.1 | 7.2 ± 1.1 | 100 | 83 |
| MAD02-007 | VIM-2 | 175 | 8.6 ± 1.1 | 7.1 ± 1.1 | 100 | 83 | |
| MAD02-021 | GES-5 | 235 | 8.3 ± 0.5 | 7.4 ± 0.6 | 100 | 100 | |
| MUR01-018 | IMP-33 | 111 | 6.5 ± 0.4 a,b,c | 2.8 ± 1.0 a,b,c | 100 | 17 c |
| Isolates; Clone (Carbapenemase) | Therapy | n | Doses (mg/kg) | Spleen (log10 CFU/g) | Blood (log10 CFU/mL) | Mortality (%) |
|---|---|---|---|---|---|---|
| IMP plus MEM | ||||||
| CVA03-019; ST175 (OXA-2/VIM-20) | Control | 6 | - | 8.7 ± 0.1 | 7.2 ± 1.1 | 83 |
| IMP | 6 | 30 | 7.6 ± 1.2 | 4.9 ± 1.4 | 17 | |
| MEM | 6 | 300 | 4.0 ± 0.9 a,b | 1.5 ± 1.6 a | 17 | |
| IMP + MEM | 5 | 4.4 ± 0.5 a,b | 1.6 ± 1.1 a,b | 0 a | ||
| MAD02-007; ST175 (VIM-2) | Control | 6 | - | 8.6 ± 1.1 | 7.1 ± 1.1 | 83 |
| IMP | 6 | 30 | 8.5 ± 0.4 | 6.2 ± 0.8 | 50 | |
| MEM | 6 | 300 | 6.6 ± 0.6 a, b | 3.4 ± 0.6 a,b | 33 | |
| IMP + MEM | 6 | 6.3 ± 0.9 a,b | 3.1 ± 0.8 a,b | 50 | ||
| MAD02-021; ST235 (GES-5) | Control | 6 | - | 8.3 ± 0.5 | 7.4 ± 0.6 | 100 |
| IMP | 6 | 30 | 6.9 ± 1.4 | 4.1 ± 1.0 a | 17 a | |
| MEM | 6 | 300 | 3.4 ± 1.1 a,b | 1.5 ± 1.3 a,b | 17 a | |
| IMP + MEM | 5 | 0.5 ± 1.1 a,b,c | 0.0 ± 0.0 a,b | 0 a | ||
| MUR01-018; ST111 (IMP-33) | Control | 6 | - | 6.5 ± 0.4 | 2.8 ± 1.0 | 17 |
| IMP | 6 | 30 | 3.6 ± 0.5 a | 0.7 ± 0.9 a | 0 | |
| MEM | 6 | 300 | 3.4 ± 0.6 a | 1.5 ± 1.4 | 0 | |
| IMP + MEM | 5 | 3.6 ± 0.3 a | 0.7 ± 1.2 | 0 | ||
| IMP plus DOR | ||||||
| CVA03-019; ST175 (OXA-2/VIM-20) | Control | 6 | - | 8.7 ± 0.1 | 7.2 ± 1.1 | 83 |
| IMP | 6 | 30 | 7.6 ± 1.2 | 4.9 ± 1.4 | 17 | |
| DOR | 5 | 150 | 7.3 ± 1.5 | 4.8 ± 2.2 | 40 | |
| IMP + DOR | 5 | 5.9 ± 1.4 a | 2.2 ± 0.5 a | 0 a | ||
| MAD02-007; ST175 (VIM-2) | Control | 6 | - | 8.6 ± 1.1 | 7.1 ± 1.1 | 83 |
| IMP | 6 | 30 | 8.5 ± 0.4 | 6.2 ± 0.8 | 50 | |
| DOR | 5 | 150 | 7.8 ± 0.6 | 5.6 ± 0.7 | 20 | |
| IMP + DOR | 5 | 6.2 ± 1.3 | 3.0 ± 2.2 a | 20 | ||
| MAD02-021; ST235 (GES-5) | Control | 6 | - | 8.3 ± 0.5 | 7.4 ± 0.6 | 100 |
| IMP | 6 | 30 | 6.9 ± 1.4 | 4.1 ± 1.0 a | 17 a | |
| DOR | 5 | 150 | 4.5 ± 1.7 a | 3.0 ± 2.7 | 0 a | |
| IMP + DOR | 5 | 2.3 ± 1.3 a,b | 2.4 ± 1.8 a | 0 a | ||
| MUR01-018; ST111 (IMP-33) | Control | 6 | - | 6.5 ± 0.4 | 2.8 ± 1.0 | 17 |
| IMP | 6 | 30 | 3.6 ± 0.5 a,d | 0.7 ± 0.9 a | 0 | |
| DOR | 6 | 150 | 4.8 ± 0.6 a | 2.2 ± 1.9 | 10 | |
| IMP + DOR | 5 | 3.0 ± 1.7 a | 0.0 ± 0.0 a | 0 | ||
| MEM plus DOR | ||||||
| CVA03-019; ST175 (OXA-2/VIM-20) | Control | 6 | - | 8.7 ± 0.1 | 7.2 ± 1.1 | 83 |
| MEM | 6 | 300 | 4.0 ± 0.9 a,d | 1.5 ± 1.6 a | 17 | |
| DOR | 5 | 150 | 7.3 ± 1.5 | 4.8 ± 2.2 | 40 | |
| MEM + DOR | 5 | 4.8 ± 1.0 a | 2.0 ± 1.6 a | 20 | ||
| MAD02-007; ST175 (VIM-2) | Control | 6 | - | 8.6 ± 1.1 | 7.1 ± 1.1 | 83 |
| MEM | 6 | 300 | 6.6 ± 0.6 a | 3.4 ± 0.6 a,d | 33 | |
| DOR | 5 | 150 | 7.8 ± 0.6 | 5.6 ± 0.7 | 20 | |
| MEM + DOR | 5 | 6.8 ± 1.0 | 4.0 ± 1.2 a | 20 | ||
| MAD02-021; ST235 (GES-5) | Control | 6 | - | 8.3 ± 0.5 | 7.4 ± 0.6 | 100 |
| MEM | 6 | 300 | 3.4 ± 1.1 a | 1.5 ± 1.3 a | 17 a | |
| DOR | 5 | 150 | 4.5 ± 1.7 a | 3.0 ± 2.7 | 0 a | |
| MEM + DOR | 5 | 2.4 ± 1.4 a | 0.0 ± 0.0 a | 0 a | ||
| MUR01-018; ST111 (IMP-33) | Control | 6 | - | 6.5 ± 0.4 | 2.8 ± 1.0 | 17 |
| MEM | 6 | 300 | 3.4 ± 0.6 a,d | 1.5 ± 1.4 | 0 | |
| DOR | 6 | 150 | 4.8 ± 0.6 a | 2.2 ± 1.9 | 10 | |
| MEM + DOR | 5 | 4.0 ± 0.5 a | 0.5 ± 0.9 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Espejo, S.; Del Barrio-Tofiño, E.; Cebrero-Cangueiro, T.; López-Causapé, C.; Álvarez-Marín, R.; Cisneros, J.M.; Pachón, J.; Oliver, A.; Pachón-Ibáñez, M.E. Carbapenem Combinations for Infections Caused by Carbapenemase-Producing Pseudomonas aeruginosa: Experimental In Vitro and In Vivo Analysis. Antibiotics 2022, 11, 1212. https://doi.org/10.3390/antibiotics11091212
Herrera-Espejo S, Del Barrio-Tofiño E, Cebrero-Cangueiro T, López-Causapé C, Álvarez-Marín R, Cisneros JM, Pachón J, Oliver A, Pachón-Ibáñez ME. Carbapenem Combinations for Infections Caused by Carbapenemase-Producing Pseudomonas aeruginosa: Experimental In Vitro and In Vivo Analysis. Antibiotics. 2022; 11(9):1212. https://doi.org/10.3390/antibiotics11091212
Chicago/Turabian StyleHerrera-Espejo, Soraya, Ester Del Barrio-Tofiño, Tania Cebrero-Cangueiro, Carla López-Causapé, Rocío Álvarez-Marín, José Miguel Cisneros, Jerónimo Pachón, Antonio Oliver, and María Eugenia Pachón-Ibáñez. 2022. "Carbapenem Combinations for Infections Caused by Carbapenemase-Producing Pseudomonas aeruginosa: Experimental In Vitro and In Vivo Analysis" Antibiotics 11, no. 9: 1212. https://doi.org/10.3390/antibiotics11091212
APA StyleHerrera-Espejo, S., Del Barrio-Tofiño, E., Cebrero-Cangueiro, T., López-Causapé, C., Álvarez-Marín, R., Cisneros, J. M., Pachón, J., Oliver, A., & Pachón-Ibáñez, M. E. (2022). Carbapenem Combinations for Infections Caused by Carbapenemase-Producing Pseudomonas aeruginosa: Experimental In Vitro and In Vivo Analysis. Antibiotics, 11(9), 1212. https://doi.org/10.3390/antibiotics11091212