Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste
Abstract
:1. Introduction
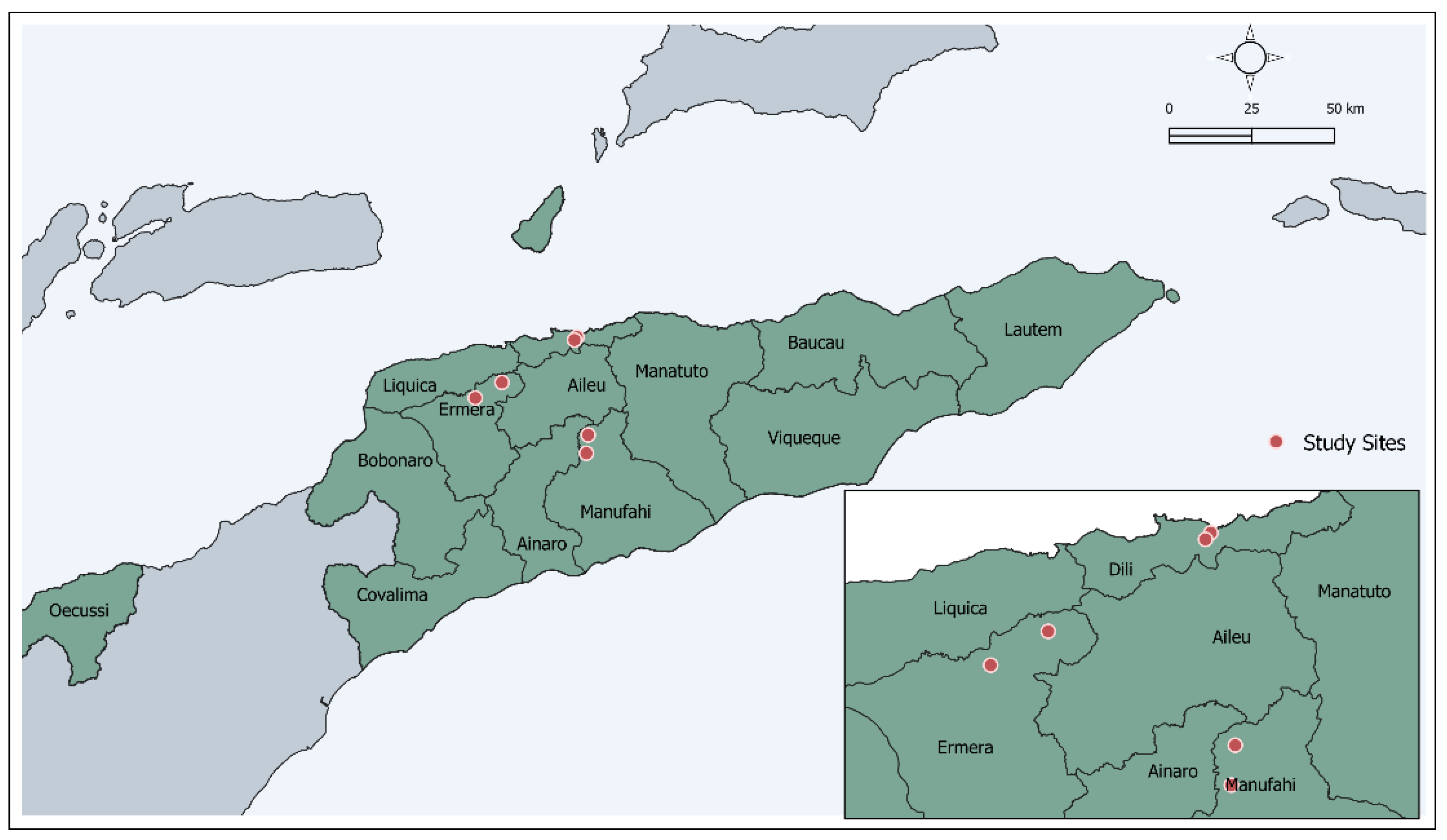
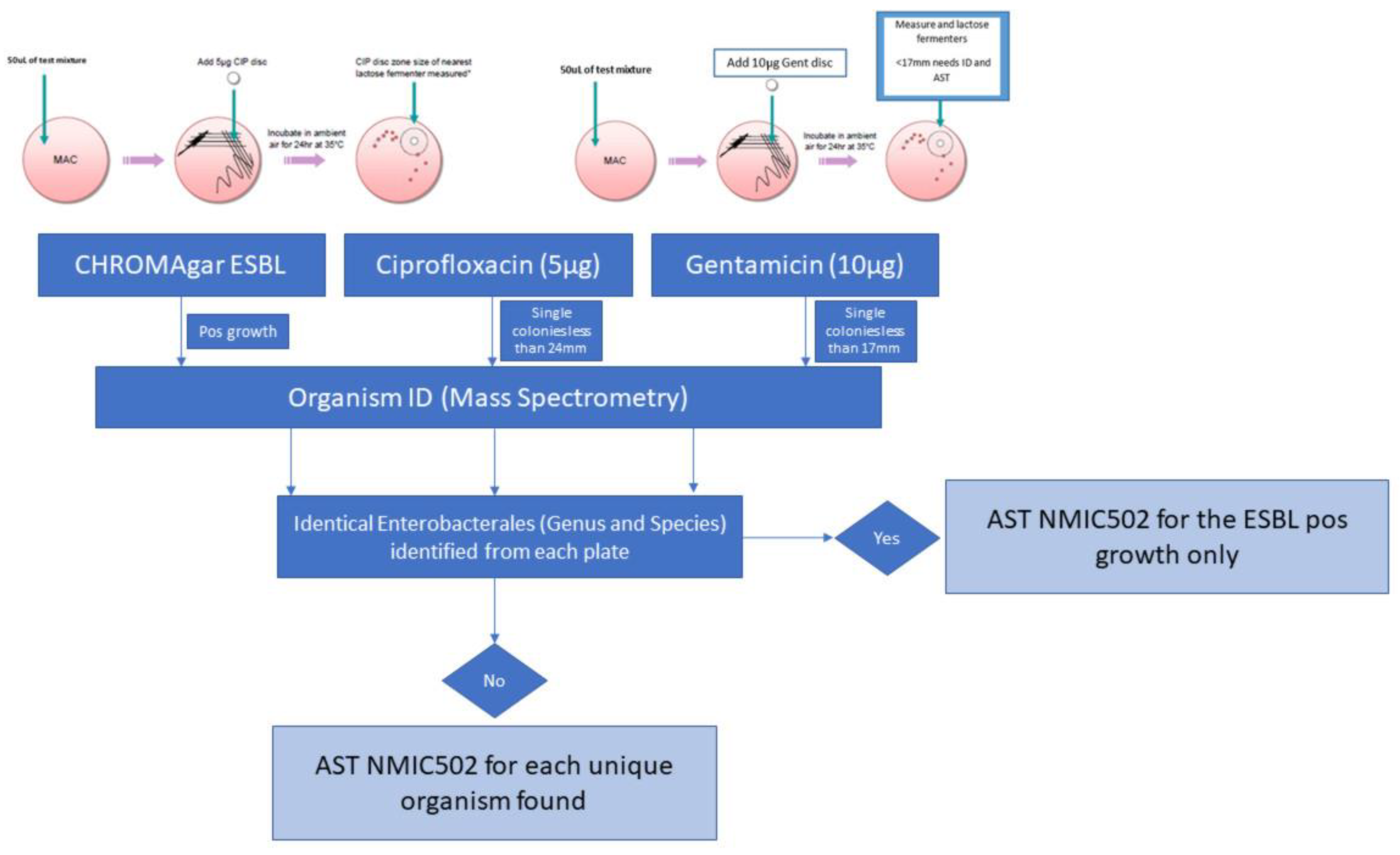
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population, Total-Timor-Leste. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=TL (accessed on 19 May 2022).
- Fox-Lewis, A.; Takata, J.; Miliya, T.; Lubell, Y.; Soeng, S.; Sar, P.; Rith, K.; McKellar, G.; Wuthiekanun, V.; McGonagle, E.; et al. Antimicrobial Resistance in Invasive Bacterial Infections in Hospitalized Children, Cambodia, 2007–2016. Emerg. Infect. Dis. 2018, 24, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Sugianli, A.K.; Ginting, F.; Kusumawati, R.L.; Pranggono, E.H.; Pasaribu, A.P.; Gronthoud, F.; Geerlings, S.; Parwati, I.; de Jong, M.D.; van Leth, F.; et al. Antimicrobial Resistance in Uropathogens and Appropriateness of Empirical Treatment: A Population-Based Surveillance Study in Indonesia. J. Antimicrob. Chemother. 2017, 72, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef]
- Chereau, F.; Opatowski, L.; Tourdjman, M.; Vong, S. Risk Assessment for Antibiotic Resistance in South East Asia. BMJ 2017, 358, 2–8. [Google Scholar] [CrossRef]
- Temkin, E.; Fallach, N.; Almagor, J.; Gladstone, B.P.; Tacconelli, E.; Carmeli, Y. Estimating the Number of Infections Caused by Antibiotic-Resistant Escherichia Coli and Klebsiella Pneumoniae in 2014: A Modelling Study. Lancet Glob. Health 2018, 6, e969–e979. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine: 6th Revision: Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 17 June 2022).
- Pitout, J.D.; Laupland, K.B. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: An Emerging Public-Health Concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Reuland, E.A.; Overdevest, I.T.M.A.; al Naiemi, N.; Kalpoe, J.S.; Rijnsburger, M.C.; Raadsen, S.A.; Ligtenberg-Burgman, I.; van der Zwaluw, K.W.; Heck, M.; Savelkoul, P.H.M.; et al. High Prevalence of ESBL-Producing Enterobacteriaceae Carriage in Dutch Community Patients with Gastrointestinal Complaints. Clin. Microbiol. Infect. 2013, 19, 542–549. [Google Scholar] [CrossRef]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic Resistance in Enterobacteriaceae: Mechanisms and Clinical Implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef]
- Donskey, C.J. The Role of the Intestinal Tract as a Reservoir and Source for Transmission of Nosocomial Pathogens. Clin. Infect. Dis. 2004, 39, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Juárez, P.; Suárez-Cuenca, J.A.; Volkow-Fernández, P.; Silva-Sánchez, J.; Barrios-Camacho, H.; Nájera-León, E.; Velázquez-Acosta, C.; Vilar-Compte, D. Fecal ESBL Escherichia Coli Carriage as a Risk Factor for Bacteremia in Patients with Hematological Malignancies. Support. Care Cancer 2016, 24, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Malczynski, M.; Obias, A.; Reiner, S.; Jin, N.; Huang, J.; Noskin, G.A.; Zembower, T. Screening for Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae among High-Risk Patients and Rates of Subsequent Bacteremia. Clin. Infect. Dis. 2007, 45, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, J.; de Avila, R.A.; Safdar, N. Risk of Infection Following Colonization with Carbapenem-Resistant Enterobactericeae: A Systematic Review. Am. J. Infect. Control 2016, 44, 539–543. [Google Scholar] [CrossRef]
- Lim, C.; Ashley, E.A.; Hamers, R.L.; Turner, P.; Kesteman, T.; Akech, S.; Corso, A.; Mayxay, M.; Okeke, I.N.; Limmathurotsakul, D.; et al. Surveillance Strategies Using Routine Microbiology for Antimicrobial Resistance in Low- and Middle-Income Countries. Clin. Microbiol. Infect. 2021, 27, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Ashley, E.A.; Celhay, O.J.; Douangnouvong, A.; Hamers, R.L.; Ling, C.L.; Lubell, Y.; Miliya, T.; Roberts, T.; Soputhy, C.; et al. ACORN (A Clinically-Oriented Antimicrobial Resistance Surveillance Network): A Pilot Protocol for Case Based Antimicrobial Resistance Surveillance. Wellcome Open Res. 2020, 5, 13. [Google Scholar] [CrossRef]
- Lim, C.; Hantrakun, V.; Teerawattanasook, N.; Srisamang, P.; Teparrukkul, P.; Sumpradit, N.; Turner, P.; Day, N.P.; Cooper, B.S.; Peacock, S.J.; et al. Impact of Low Blood Culture Usage on Rates of Antimicrobial Resistance. J. Infect. 2021, 82, 355–362. [Google Scholar] [CrossRef]
- Deen, J.; von Seidlein, L.; Andersen, F.; Elle, N.; White, N.J.; Lubell, Y. Community-Acquired Bacterial Bloodstream Infections in Developing Countries in South and Southeast Asia: A Systematic Review. Lancet Infect. Dis. 2012, 12, 480–487. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Carrique-Mas, J.; Limmathurotsakul, D.; Day, N.P.J.; Thwaites, G.E.; Baker, S.; Ashley, E.; de Balogh, K.; Baird, K.; Basnyat, B.; et al. A Current Perspective on Antimicrobial Resistance in Southeast Asia. J. Antimicrob. Chemother. 2017, 72, 2963–2972. [Google Scholar] [CrossRef]
- Huang, I.F.; Lee, W.Y.; Wang, J.L.; Hung, C.H.; Hu, H.H.; Hung, W.Y.; Hung, Y.J.; Chen, W.C.; Shen, Y.T.; Cheng, M.F. Fecal Carriage of Multidrug-Resistant Escherichia Coli by Community Children in Southern Taiwan. BMC Gastroenterol. 2018, 18, 86. [Google Scholar] [CrossRef]
- Moremi, N.; Claus, H.; Vogel, U.; Mshana, S.E. Faecal Carriage of CTX-M Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae among Street Children Dwelling in Mwanza City, Tanzania. PLoS ONE 2017, 12, e0184592. [Google Scholar] [CrossRef] [PubMed]
- Marr, I.; Sarmento, N.; O’Brien, M.; Lee, K.; Gusmao, C.; de Castro, G.; Janson, S.; Tong, S.Y.C.; Baird, R.W.; Francis, J.R. Antimicrobial Resistance in Urine and Skin Isolates in Timor-Leste. J. Glob. Antimicrob. Resist. 2018, 13, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, S.; Saidani, M.; Hamzaoui, Z.; Alonso, C.A.; Torres, C.; Maamar, E.; Slim, A.F.; Boutiba, B.B.I. Community Fecal Carriage of Broad-Spectrum Cephalosporin-Resistant Escherichia Coli in Tunisian Children. Diagn. Microbiol. Infect. Dis. 2017, 87, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gurnee, E.A.; Ndao, I.M.; Johnson, J.R.; Johnston, B.D.; Gonzalez, M.D.; Burnham, C.A.D.; Hall-Moore, C.M.; McGhee, J.E.; Mellmann, A.; Warner, B.B.; et al. Gut Colonization of Healthy Children and Their Mothers with Pathogenic Ciprofloxacin-Resistant Escherichia Coli. J. Infect. Dis. 2015, 212, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Le, B.; Amaral, S.; Arkell, P.; Monteiro, M.; Clarke, N.; Barros, T.; de Jesus Mendonça, J.; Gusmão, S.M.E.; dos Reis Seixas, L.M.; et al. Prevalence of Scabies and Impetigo in School-Age Children in Timor-Leste. Parasit Vectors 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Clinical Breakpoints and Dosing of Antibiotics; Version 10.0; EUCAST: Växjö, Sweden, 2020. [Google Scholar]
- Karanika, S.; Karantanos, T.; Arvanitis, M.; Grigoras, C.; Mylonakis, E. Fecal Colonization with Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae and Risk Factors among Healthy Individuals: A Systematic Review and Metaanalysis. Clin. Infect. Dis. 2016, 63, 310–318. [Google Scholar] [CrossRef]
- Harris, L.; Bongers, A.; Yan, J.; Francis, J.R.; Marr, I.; Lake, S.; Martins, S. Estimates of Antibacterial Consumption in Timor-Leste Using Distribution Data and Variation in Municipality Usage Patterns. Antibiotics 2021, 10, 1468. [Google Scholar] [CrossRef]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- Liss, M.A.; Nakamura, K.K.; Peterson, E.M. Comparison of Broth Enhancement to Direct Plating for Screening of Rectal Cultures for Ciprofloxacin-Resistant Escherichia Coli. J. Clin. Microbiol. 2013, 51, 249–252. [Google Scholar] [CrossRef]
- Dellgren, L.; Claesson, C.; Högdahl, M.; Forsberg, J.; Hanberger, H.; Nilsson, L.E.; Hällgren, A. Phenotypic Screening for Quinolone Resistance in Escherichia Coli. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
| Sample Size N | Ciprofloxacin Resistance n (%) | Gentamicin Resistance n (%) | ESBL n (%) | ESBL with Ciprofloxacin Resistance n (%) | ESBL with Gentamicin Resistance n (%) | ESBL with Ciprofloxacin and Gentamicin Co-Resistance n (%) | |
|---|---|---|---|---|---|---|---|
| Sex * | |||||||
| Male | 273 | 49 (17.9) | 26 (9.5) | 32 (11.7) | 15 (5.5) | 5 (1.8) | 1 (0.4) |
| Female | 330 | 59 (17.9) | 16 (4.8) | 29 (8.8) | 9 (2.7) | 1 (0.3) | 0 |
| Age group (years) * | |||||||
| ≤10 | 339 | 56 (16.5) | 26 (7.7) | 38 (11.2) | 14 (4.1) | 5 (1.5) | 0 |
| >10 | 264 | 52 (19.7) | 16 (6.1) | 23 (8.7) | 10 (3.8) | 1 (0.4) | 1 (0.4) |
| Municipality | |||||||
| Dili | 313 | 83 (26.5) | 33 (10.5) | 37 (11.8) | 18 (5.7) | 3 (1.0) | 1 (0.3) |
| Ermera | 262 | 30 (11.4) | 12 (4.6) | 26 (9.9) | 7 (2.7) | 3 (1.1) | 0 |
| Manufahi | 46 | 2 (4.3) | 1 (2.2) | 0 | 0 | 0 | 0 |
| Total | 621 | 115 (18.5) | 46 (7.4) | 63 (10.1) | 25 (4.0) | 6 (1.0) | 1 (0.2) |
| Sample N | Ciprofloxacin Resistance | Gentamicin Resistance | ESBL | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | AOR (95% CI) | % (95% CI) | AOR (95% CI) | % (95% CI) | AOR (95% CI) | ||
| Dili | 313 | 36.09 (27.03–45.14) | Ref | 11.79 (7.53–16.04) | Ref | 13.41 (8.81–18.01) | Ref |
| Ermera | 262 | 12.93 (8.01–17.84) | 0.38 (0.24–0.60) p < 0.001 | 5.20 (0.83–9.57) | 0.40 (0.20–0.81) p = 0.011 | 8.39 (0–16.88) | 0.80 (0.47–1.38) p = 0.429 |
| Manufahi | 46 | 4.54 (0–10.99) | 0.07 (0.01–0.51) p = 0.009 | 2.22 (0–6.63) | NA, n = 1 | 0 | NA |
| Total | 621 | 16.52 (6.14–26.91) | 6.76 (2.83–10.69) | 8.30 (1.56–15.05) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oakley, T.; Le, B.; da Conceicao, V.; Marr, I.; Maia, C.; Soares, M.; Belo, J.C.; Sarmento, N.; da Silva, E.; Amaral, S.; et al. Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste. Antibiotics 2022, 11, 1262. https://doi.org/10.3390/antibiotics11091262
Oakley T, Le B, da Conceicao V, Marr I, Maia C, Soares M, Belo JC, Sarmento N, da Silva E, Amaral S, et al. Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste. Antibiotics. 2022; 11(9):1262. https://doi.org/10.3390/antibiotics11091262
Chicago/Turabian StyleOakley, Tessa, Brandon Le, Virginia da Conceicao, Ian Marr, Carolina Maia, Messias Soares, Joana Correia Belo, Nevio Sarmento, Endang da Silva, Salvador Amaral, and et al. 2022. "Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste" Antibiotics 11, no. 9: 1262. https://doi.org/10.3390/antibiotics11091262
APA StyleOakley, T., Le, B., da Conceicao, V., Marr, I., Maia, C., Soares, M., Belo, J. C., Sarmento, N., da Silva, E., Amaral, S., Vaz Nery, S., Lynar, S., Francis, J. R., & Yan, J. (2022). Gastrointestinal Carriage of Antimicrobial Resistance in School-Aged Children in Three Municipalities of Timor-Leste. Antibiotics, 11(9), 1262. https://doi.org/10.3390/antibiotics11091262






