In Vitro Activity of New β-Lactamase Inhibitor Combinations against blaNDM, blaKPC, and ESBL-Producing Enterobacteriales Uropathogens
Abstract
:1. Introduction
2. Results
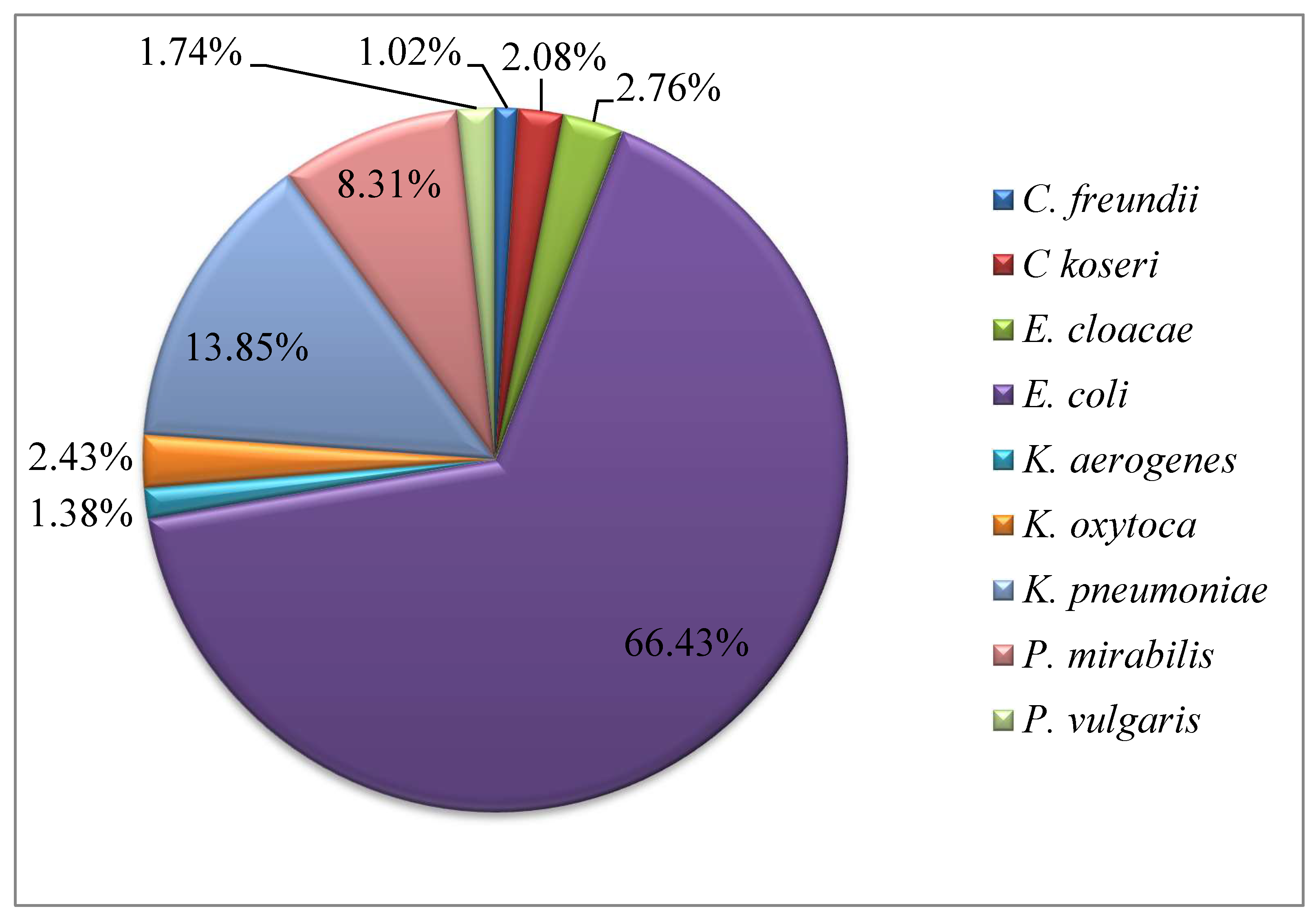
2.1. Frequency of Enterobacteriales Species and Antimicrobial Susceptibility
2.2. Comparison of Antimicrobial Resistance Pattern in OPD and Ward Patients
2.3. Phenotypic and Genotypic Detection of ESBL Producers and Carbapenem-Resistant (CR) Enterobacteriales
2.4. Plasmid Analysis
2.5. Susceptibility Patterns to Newer Drugs
2.6. Prevalence of Multi-Drug-Resistant (MDR), Extensively Drug-Resistant (XDR), and Pan Drug-Resistant (PDR) Isolates
3. Materials and Methods
3.1. Study Setting and Period
3.2. Sample Collection and Processing
3.3. Phenotypic Detection of Extended Spectrum β-Lactamases (ESBLs) and Carbapenemases
3.4. Genotypic Detection of Extended Spectrum β-Lactamases (ESBLs) and Carbapenemase
3.5. Plasmid Analysis
3.6. Conjugation and Transformation Assays
3.7. Susceptibility of ESBL Producers and CRE Isolates to Newer β-Lactam/β-Lactamase Inhibitor Combinations
3.8. Statistical Analysis
4. Discussion
5. Conclusions
6. Limitation of Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuralayanapalya, S.P.; Patil, S.S.; Hamsapriya, S.; Shinduja, R.; Roy, P.; Amachawadi, R.G. Prevalence of extended-spectrum beta-lactamase producing bacteria from animal origin: A systematic review and meta-analysis report from India. PLoS ONE 2019, 14, e0221771. [Google Scholar] [CrossRef] [PubMed]
- Abongomera, G.; Koller, M.; Musaazi, J.; Lamorde, M.; Kaelin, M.; Tasimwa, H.B.; Eberhard, N.; Hongler, J.; Haller, S.; Kambugu, A.; et al. Spectrum of antibiotic resistance in UTI caused by Escherichia coli among HIV-infected patients in Uganda: A cross-sectional study. BMC Infect. Dis. 2021, 21, 1179. [Google Scholar] [CrossRef] [PubMed]
- Khah, A.N.; Hakemi-Vala, M.; Samavat, S.; Nasiri, M.J. Prevalence, serotyping and drug susceptibility patterns of Escherichia coli isolates from kidney transplanted patients with urinary tract infections. World J. Biol. Chem. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Giamarellou, H. Carbapenem-Sparing Strategies for ESBL Producers: When and How. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Alraddadi, B.M.; Saeedi, M.; Qutub, M.; Alshukairi, A.; Hassanien, A.; Wali, G. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect. Dis. 2019, 19, 772. [Google Scholar] [CrossRef]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Mendes, R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS ONE 2019, 14, e0220265. [Google Scholar] [CrossRef]
- Jean, S.; Lee, N.; Tang, H.; Lu, M.; Ko, W. Carbapenem-Resistant Enterobacteriaceae Infections: Taiwan Aspects. Front. Microbiol. 2018, 9, 2888. [Google Scholar] [CrossRef]
- Navarro, E.D.; Bulman, Z.; Chen, Y.-H.; Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Khalid, S.; Ahmad, N.; Ali, S.M.; Khan, A.U. Outbreak of efficiently transferred carbapenem-resistant bla NDM-producing gram-negative bacilli isolated from neonatal intensive care unit of an Indian hospital. Microb. Drug Resist. 2020, 26, 284–289. [Google Scholar] [CrossRef]
- Hoang, C.Q.; Nguyen, H.D.; Vu, H.Q.; Nguyen, A.T.; Pham, B.T.; Tran, T.L.; Nguyen, H.T.H.; Dao, Y.M.; Nguyen, T.S.M.; Nguyen, D.A.; et al. Emergence of New Delhi Metallo-Beta-Lactamase (NDM) and Klebsiella pneumoniae Carbapenemase (KPC) Production by Escherichia coli and Klebsiella pneumoniae in Southern Vietnam and Appropriate Methods of Detection: A Cross-Sectional Study. BioMed Res. Int. 2019, 50, 525–527. [Google Scholar] [CrossRef]
- Oli, A.N.; Itumo, C.J.; Okam, P.C.; Ezebialu, I.U.; Okeke, K.N.; Ifezulike, C.C.; Ezeobi, I.; Emechebe, G.O.; Okezie, U.M.; Adejumo, S.A.; et al. Carbapenem-resistant enterobacteriaceae posing a dilemma in effective healthcare delivery. Antibiotics 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Harris, P.N.A.; Henderson, A.; Schembri, M.A.; Paterson, D.L. Oral cephalosporin and b -lactamase inhibitor combinations for ESBL-producing Enterobacteriaceae urinary tract infections. J. Antimicrob. Chemother. 2020, 75, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; De la Rosa, J.M.O.; Sadek, M.; Nordmann, P. Impact of Acquired Broad-Spectrum β-lactamases on Susceptibility to Cefiderocol and Newly Developed b-Lactam/b-Lactamase Inhibitor Combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e00039-22. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Carvalhaes, C.; Deshpande, L.; Castanheira, M. Activity of meropenem/vaborbactam and comparators against Gram-negative isolates from Eastern and Western European patients hospitalized with pneumonia including ventilator-associated pneumonia (2014–19). J. Antimicrob. Chemother. 2021, 76, 2600–2605. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Chen, L.; Hao, B.; Press, E.G.; Alkrouk, A.; Potoski, B.A. Evaluation of the In Vitro Activity of Ceftazidime-Avibactam and aeruginosa Isolates. Antimicrob. Agents Chemother. 2016, 60, 3227–3231. [Google Scholar] [CrossRef]
- Petti, C.A.; Weinstein, M.P.; Carroll, K.C. Systems for Detection and Identification of Bacteria and Yeasts. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2011; Volume 1, pp. 15–26. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J.F. Epidemiological Expansion, Structural Studies, and Clinical Challenges of New β-Lactamases from Gram-Negative Bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Elsherif, R.; Ismail, D.; Elawady, S.; Jastaniah, S.; Al-Masaudi, S.; Harakeh, S.; Karrouf, G. Boronic acid disk diffusion for the phenotypic detection of polymerase chain reaction-confirmed, carbapenem-resistant, gram-negative bacilli isolates. BMC Microbiol. 2016, 16, 135. [Google Scholar] [CrossRef]
- Noster, J.; Thelen, P.; Hamprecht, A. Detection of Multidrug-Resistant Enterobacterales—From ESBLs to Carbapenemases. Antibiotics 2021, 10, 1140. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Shen, Z.; Xia, L.; Wang, J.; Zhao, L.; Wang, K.; Wang, W.; Hao, Z.; Liu, Z. Characterization of NDM-1-producing carbapenemase in Proteus mirabilis among broilers in China. Microorganisms 2021, 9, 2443. [Google Scholar] [CrossRef]
- Baraka, K.; Abozahra, R.; Haggag, M.M.; Abdelhamid, S.M.; Baraka, K.; Abozahra, R.; Haggag, M.M.; Abdelhamid, S.M. Genotyping and molecular investigation of plasmid-mediated carbapenem resistant clinical Klebsiella pneumoniae isolates in Egypt. AIMS Microbiol. 2023, 9, 228–244. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Sheikh, A.S.; Basheer, A.; Hafsa, H.T.; Ahmed, M.; Sabri, A.N.; Shahid, S. Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population. Antibiotics 2023, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Bullens, M.; de Cerqueira Melo, A.; Raziq, S.; Lee, J.; Khalid, G.G.; Khan, S.N.; Zada, A.; Wailly, Y.; Zeshan, S.M.; Saad, N.J. Antibiotic resistance in patients with urinary tract infections in Pakistan. Public Health Action 2022, 12, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, O.M.; Uddin, F.; Mahmoud, S.F.; Alswat, A.S.; Sohail, M.; Youssef, M. Resistance to Some New Drugs and Prevalence of ESBL- and MBL-Producing Enterobacteriaceae Uropathogens Isolated from Diabetic Patients. Life 2022, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
- Nasir, F.; Khan, M.I.; Kashif, S.; Uddin, F.; Naseer, A.; Masood, S. Prevalence of ESBLs secreting and carbapenem-resistant E. coli from urinary tract infection. RMJ 2021, 46, 518–521. [Google Scholar]
- Dona Vindya Madushika Perera, P.; Gamage, S.; Sara Melros De Silva, H.; Kushlani Jayatilleke, S.; de Silva, N.; Aydin, A.; Enne, V.I.; Marie Corea, E.; Stefani, S. Phenotypic and genotypic distribution of ESBL, AmpC β-lactamase and carbapenemase-producing Enterobacteriaceae in community-acquired and hospital-acquired urinary tract infections in Sri Lanka. J. Glob. Antimicrob. Resist. 2022, 30, 115–122. [Google Scholar] [CrossRef]
- Masoud, S.M.; Abd El-Baky, R.M.; Aly, S.A.; Ibrahem, R.A. Co-existence of certain esbls, mbls and plasmid mediated quinolone resistance genes among mdr E. coli isolated from different clinical specimens in Egypt. Antibiotics 2021, 10, 835. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Duin, D.V.; Doi, Y. The global epidemiology of carbapenemase—Producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef]
- Uddin, F.; Imam, S.H.; Khan, S.; Khan, T.A.; Ahmed, Z.; Sohail, M.; Elnaggar, A.Y.; Fallatah, A.M.; El-Bahy, Z.M. NDM Production as a Dominant Feature in Carbapenem-Resistant Enterobacteriaceae Isolates from a Tertiary Care Hospital. Antibiotics 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.F.; D’souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updates 2016, 29, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Shklyar, M.; Schwaber, M.J.; Navon-venezia, S.; Dhaher, Y.; Edgar, R.; Solter, E.; Benenson, S.; Masarwa, S.; Carmeli, Y. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J. Antimicrob. Chemother. 2011, 66, 2763–2766. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Khabra, E.; Paikin, S.; Carmeli, Y. Dissemination of the blaKPC gene by clonal spread and horizontal gene transfer: Comparative study of incidence and molecular mechanisms. J. Antimicrob. Chemother. 2016, 71, 2143–2146. [Google Scholar] [CrossRef]
- Song, Z.; Qin, Y.; Peng, Y.; Huang, M.; Hua, Y.; Jiang, H.; Hu, X.; Rui, Y. Carbapenem-resistant Klebsiella pneumoniae (CRKP) transfers conjugative plasmids containing blaNDM-5 and mcr-1 genes via outer membrane vesicles (OMVs). Res. Squ. 2022; preprint. [Google Scholar]
- Elshamy, A.A.; Saleh, S.E.; Alshahrani, M.Y.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. OXA-48 Carbapenemase-Encoding Transferable Plasmids of Klebsiella pneumoniae Recovered from Egyptian Patients Suffering from Complicated Urinary Tract Infections. Biology 2021, 10, 889. [Google Scholar] [CrossRef]
- Kettani Halabi, M.; Lahlou, F.A.; Diawara, I.; El Adouzi, Y.; Marnaoui, R.; Benmessaoud, R.; Smyej, I. Antibiotic Resistance Pattern of Extended Spectrum Beta Lactamase Producing Escherichia coli Isolated From Patients With Urinary Tract Infection in Morocco. Front. Cell. Infect. Microbiol. 2021, 11, 720701. [Google Scholar] [CrossRef]
- Castanheira, M.; Rhomberg, P.R.; Flamm, R.K.; Jones, R.N. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016, 60, 5454–5458. [Google Scholar] [CrossRef]
- Dhillon, S. Meropenem/Vaborbactam: A Review in Complicated Urinary Tract Infections. Drugs 2018, 78, 1259–1270. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam–β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef]
- Bakthavatchalam, Y.D.; Routray, A.; Mane, A.; Kamat, S.; Gupta, A.; Kumar Bari, A.; Rohit, A.; Poojary, A.; Mukherjee, N.; Sethuraman, N.; et al. In vitro activity of Ceftazidime–Avibactam and its comparators against Carbapenem resistant Enterobacterales collected across India: Results from ATLAS surveillance 2018 to 2019. Diagn. Microbiol. Infect. Dis. 2022, 103, 115652. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell. Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Both, A.; Bü, T.H.; Huang, J.; Perbandt, M.; Belmar Campos, C.; Christner, M.; Maurer, F.P.; Kluge, S.; König, C.; Aepfelbacher, M.; et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J. Antimicrob. Chemother. 2017, 72, 2483–2488. [Google Scholar] [CrossRef]
- Mavroidi, A.; Katsiari, M.; Likousi, S.; Palla, E.; Roussou, Z.; Nikolaou, C.; Mathas, C.; Merkouri, E.; Platsouka, E.D. Changing Characteristics and In Vitro Susceptibility to Ceftazidime/Avibactam of Bloodstream Extensively Drug-Resistant Klebsiella pneumoniae from a Greek Intensive Care Unit. Microb. Drug Resist. 2020, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Doyle, T.B.; Collingsworth, T.D.; Sader, H.S.; Mendes, R.E. Increasing frequency of OXA-48-producing Enterobacterales world-wide and activity of ceftazidime/avibactam, meropenem/vaborbactam and comparators against these isolates. J. Antimicrob. Chemother. 2021, 76, 3125–3134. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of ceftolozane/tazobactam against phenotypically defined extended-spectrum β-lactamase (ESBL)-positive isolates of Escherichia coli and Klebsiella pneumoniae isolated from hospitalized patients (SMART 2016). Diagn. Microbiol. Infect. Dis. 2020, 96, 114925. [Google Scholar] [CrossRef]


| Antibiotics | E. coli n = 192 (%) | K. pneumoniae n = 40 (%) | P. mirabilis n = 24 (%) | Other Species * n = 33 (%) | Total 289 (%) |
|---|---|---|---|---|---|
| Cefazolin | 163 (84.8) | 35 (87.5) | 23 (95.8) | 31 (93.9) | 252 (87.1) |
| Cefuroxime | 163 (84.8) | 35 (87.5) | 23 (95.8) | 31 (93.9) | 252 (87.1) |
| Ampicillin | 163 (84.8) | 35 (87.5) | 23 (95.8) | 29 (87.8) | 250 (86.5) |
| Piperacillin | 163 (84.8) | 35 (87.5) | 23 (95.8) | 31 (93.9) | 252 (87.2) |
| Amoxicillin/clavulanic acid | 162 (84.8) | 35 (87.5) | 23 (95.8) | 29 (87.8) | 249 (85.1) |
| Piperacillin/tazobactam | 156 (81.3) | 33 (82.5) | 22 (91.6) | 29 (87.8) | 240 (59.8) |
| Ceftaroline | 126 (65) | 29 (72.5) | 15 (62.5) | 23 (69.6) | 193 (66.8) |
| Cefepime | 133 (69.7) | 30 (75) | 16 (66.6) | 25 (75.7) | 204 (70.6) |
| Cefoxitin | 95 (49.4) | 22 (55) | 16 (66.6) | 16 (48.4) | 147 (54.3) |
| Ceftriaxone | 142 (73.9) | 31 (77.5) | 19 (79.1) | 28 (84.8) | 220 (76.1) |
| Ceftazidime | 136 (70.9) | 31 (77.5) | 16 (66.6) | 27 (81.8) | 210 (72.6) |
| Aztreonam | 133 (70.3) | 30 (75) | 16 (66.6) | 26 (78.7) | 205 (71.6) |
| Meropenem | 48 (25) | 12 (30) | 10 (41.6) | 12 (36.3) | 82 (28.3) |
| Imipenem | 58 (30.2) | 16 (37.5) | 12 (50) | 13 (39.3) | 99 (34.25) |
| Amikacin | 56 (29.1) | 19 (47.5) | 13 (54.1) | 15 (45.4) | 103 (35.6) |
| Gentamicin | 99 (51.5) | 24 (60) | 15 (62.5) | 22 (66.6) | 160 (55.3) |
| Nalidixic acid | 135 (70.3) | 30 (75) | 15 (62.3) | 24 (72.7) | 204 (70.5) |
| Norfloxacine | 134 (69.7) | 29 (72.5) | 15 (62.5) | 23 (69.6) | 201 (69.5) |
| Ciprofloxacin | 129 (67.1) | 29 (72.5) | 17 (70.8) | 24 (72.7) | 199 (68.8) |
| Fosfomycin | 61 (31.7) | - | - | - | - |
| Trimethoprim/sulfamethoxazole | 146 (76) | 28 (70) | 19 (79.1) | 23 (69.6) | 215 (74.3) |
| Nitrofurantoin | 64 (33.3) | 17 (42.5) | 24 (100) | 15 (45.4) | 120 (41.5) |
| Trimethoprim | 151 (78.6) | 28 (70) | 23 (95.8) | 25 (75.7) | 227 (78.5) |
| Tetracycline | 124 (64.5) | 31 (77.5) | 24 (100) | 23 (69.6) | 202 (69.8) |
| Antibiotics | Ward Patients 138 (%) | OPD Patients 151 (%) | Total 289 (%) | Z Value | p Value * |
|---|---|---|---|---|---|
| Cefazolin | 132 (95.65%) | 120 (79.47%) | 252 (87.1) | 4.1125 | <0.00001 |
| Cefuroxime | 132 (95.65%) | 120 (79.47%) | 252 (87.1) | 4.1125 | <0.00001 |
| Ampicillin | 130 (94.20%) | 120 (79.47%) | 250 (86.5) | 3.6615 | 0.00026 |
| Piperacillin | 132 (95.65%) | 120 (79.47%) | 252 (87.2) | 4.1125 | <0.00001 |
| Amoxicillin/clavulanic acid | 122 (88.40%) | 127 (84.10%) | 249 (85.1) | 1.0573 | 0.28914 |
| Piperacillin/tazobactam | 125 (90.57%) | 115 (76.15%) | 240 (59.8) | 3.2633 | 0.00112 |
| Ceftaroline | 98 (71.01%) | 95 (62.91%) | 193 (66.8) | 1.4604 | 0.1443 |
| Cefepime | 111 (83.43%) | 93 (61.58%) | 204 (70.6) | 3.512 | 0.00044 |
| Cefoxitin | 96 (69.56%) | 51 (33.77%) | 147 (54.3) | 6.0791 | <0.00001 |
| Ceftriaxone | 120 (86.95%) | 100 (66.22%) | 220 (76.1) | 4.1292 | <0.00001 |
| Ceftazidime | 111 (83.43%) | 99 (65.56%) | 210 (72.6) | 2.8335 | 0.00466 |
| Aztreonam | 104 (75.36%) | 101 (66.89%) | 205 (71.6) | 1.5849 | 0.1141 |
| Meropenem | 80 (57.97%) | 2 (1.32%) | 82 (28.3) | 10.6698 | <0.00001 |
| Imipenem | 92 (92.92%) | 7 (4.63%) | 99 (34.25) | 11.0992 | <0.00001 |
| Amikacin | 83 (60.14%) | 20 (13.24%) | 103 (35.6) | 8.3152 | <0.00001 |
| Gentamicin | 86 (62.31%) | 74 (49.0%) | 160 (55.3) | 2.0509 | 0.04036 |
| Nalidixic acid | 123 (89.13%) | 85 (56.29%) | 204 (70.5) | 6.2086 | <0.00001 |
| Norfloxacine | 101 (73.18%) | 100 (66.2%) | 201 (69.5) | 1.2848 | 0.20054 |
| Ciprofloxacin | 100 (72.4%) | 99 (65.56%) | 199 (68.8) | 1.2654 | 0.20408 |
| Fosfomycin | 42 (30.43%) | 19 (12.59%) | - | 3.7148 | 0.0002 |
| Trimethoprim/sulfamethoxazole | 128 (92.75%) | 87 (56.61%) | 215 (74.3) | 6.8362 | <0.00001 |
| Nitrofurantoin | 86 (62.31%) | 34 (22.51%) | 120 (41.5) | 6.8588 | <0.00001 |
| Trimethoprim | 120 (86.95%) | 107 (70.86%) | 227 (78.5) | 3.3295 | <0.00086 |
| Tetracycline | 125 (90.57%) | 77 (50.99%) | 202 (69.8) | 7.328 | <0.00001 |
| Genotypic Resistant Isolates | Susceptibility Pattern | |||||
|---|---|---|---|---|---|---|
| MEV n (%) | CAZ | C/T | ||||
| S | R | S | R | S | R | |
| ESBL-types (71) | 71 (100) | - | 71 (100) | - | 71 (100) | - |
| Coexistence ESBL + ESBLs (54) | 54 (100) | - | 54 (100) | - | 54 (100) | - |
| ESBLs + NDM (44) | - | 44 (100) | - | 44 (100) | - | 44 (100) |
| NDM (33) | - | 33 (100) | - | 33 (100) | - | 33 (100) |
| OXA (1) | - | 1 (100) | 1 (100) | - | - | 1 (100) |
| OXA + CTX-M (9) | 4 (44.44) | 5 (55.55) | 2 (22.22) | 7 (77.77) | 2 (22.22) | 7 (77.77) |
| OXA + NDM (4) | - | 4 (100) | - | 4 (100) | - | 4 (100) |
| KPC + SHV (1) | 1 (100) | 1 (100) | 1 (100) | |||
| KPC (4) | 4 (100) | - | 4 (100) | - | 4 (100) | |
| Total | 134 | 87 | 128 | 93 | 127 | 94 |
| Antibiotic | Non ESBL n = 65 (%) | ESBL n = 125 (%) | p Value | CS n = 190 (%) | CR n = 99 (%) | p Value * |
|---|---|---|---|---|---|---|
| Ampicillin | 65 (100) | 97 (77.6) | <0.0001 | 162 (85.26) | 24 (24.24) | <0.0001 |
| Fosfomycin | 65 (100) | 86 (68.8) | <0.0001 | 115 (60.52) | 43 (43.43) | <0.0001 |
| Norfloxacine | 59 (90.76) | 37 (12.80) | <0.0001 | 96 (50.52) | 1 (1.01) | <0.0001 |
| Nitrofurantoin | 60 (92.30) | 85 (68) | <0.0001 | 145 (76.31) | 35 (35.35) | <0.0001 |
| Ciprofloxacin | 59 (90.76) | 38 (30.4) | <0.0001 | 97 (51.05) | 3 (3.03) | <0.0001 |
| Trimethoprim/sulfamethoxazole | 51 (78.46) | 21 (16.8) | <0.0001 | 72 (37.89) | 3 (3.03) | <0.0001 |
| Nalidixic acid | 57 (87.69) | 27 (21.6) | <0.0001 | 84 (44.21) | 1 (1.01) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razaq, L.; Uddin, F.; Ali, S.; Abbasi, S.M.; Sohail, M.; Yousif, N.E.; Abo-Dief, H.M.; El-Bahy, Z.M. In Vitro Activity of New β-Lactamase Inhibitor Combinations against blaNDM, blaKPC, and ESBL-Producing Enterobacteriales Uropathogens. Antibiotics 2023, 12, 1481. https://doi.org/10.3390/antibiotics12101481
Razaq L, Uddin F, Ali S, Abbasi SM, Sohail M, Yousif NE, Abo-Dief HM, El-Bahy ZM. In Vitro Activity of New β-Lactamase Inhibitor Combinations against blaNDM, blaKPC, and ESBL-Producing Enterobacteriales Uropathogens. Antibiotics. 2023; 12(10):1481. https://doi.org/10.3390/antibiotics12101481
Chicago/Turabian StyleRazaq, Lubna, Fakhur Uddin, Shahzad Ali, Shah Muhammad Abbasi, Muhammad Sohail, Nabila E. Yousif, Hala M. Abo-Dief, and Zeinhom M. El-Bahy. 2023. "In Vitro Activity of New β-Lactamase Inhibitor Combinations against blaNDM, blaKPC, and ESBL-Producing Enterobacteriales Uropathogens" Antibiotics 12, no. 10: 1481. https://doi.org/10.3390/antibiotics12101481
APA StyleRazaq, L., Uddin, F., Ali, S., Abbasi, S. M., Sohail, M., Yousif, N. E., Abo-Dief, H. M., & El-Bahy, Z. M. (2023). In Vitro Activity of New β-Lactamase Inhibitor Combinations against blaNDM, blaKPC, and ESBL-Producing Enterobacteriales Uropathogens. Antibiotics, 12(10), 1481. https://doi.org/10.3390/antibiotics12101481





