Drug-Induced Acute Pancreatitis in Adults: Focus on Antimicrobial and Antiviral Drugs, a Narrative Review
Abstract
1. Introduction
2. Drug-Induced Pancreatitis
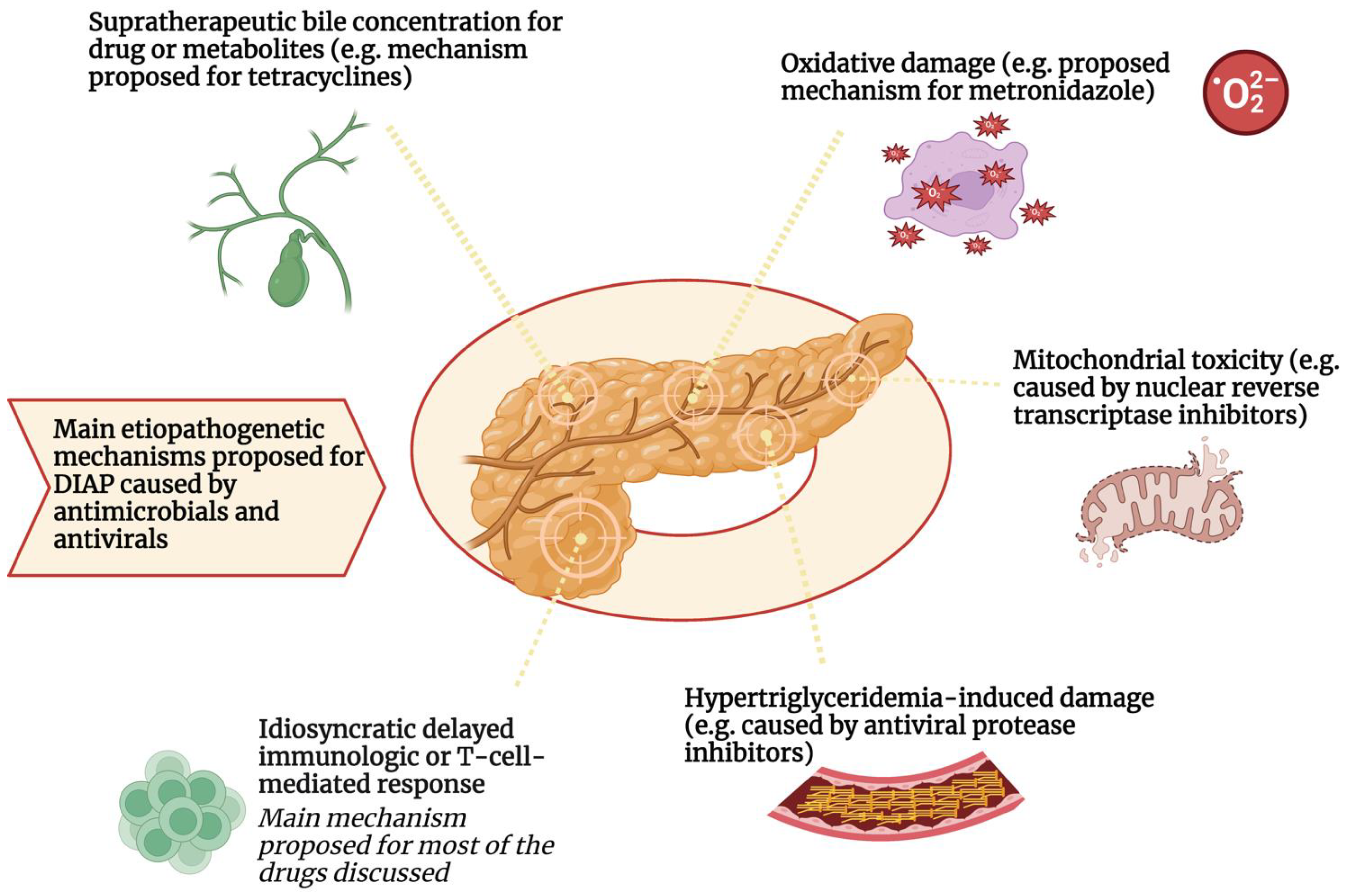
Main Mechanisms of Drug-Induced Pancreatitis
3. Antibiotics
3.1. Nucleic Acid Synthesis Inhibitors
3.1.1. Metronidazole and 5-Nitroinidazoles
3.1.2. Fluoroquinolones
3.1.3. Nitrofurantoin
3.1.4. Rifampicin
3.2. Protein Synthesis Inhibitors
3.2.1. Tetracyclines
3.2.2. Macrolides
3.3. Trimethoprim/Sulfamethoxazole—Inhibitors of Cellular Metabolism
3.4. Inhibitors of Cell Wall Synthesis
3.4.1. Beta-Lactams
3.4.2. Isoniazid
4. Antivirals
4.1. Protease Inhibitors
4.2. Interferon α2b/Ribavirin
4.3. Remdesivir
4.4. Reverse Transcriptase Inhibitors
4.4.1. Didanosine
4.4.2. Lamivudine
4.4.3. Less Associated Reverse Transcriptase Inhibitors
4.5. DNA Polymerase Inhibitors
5. Antifungals and Antiparasitics
5.1. Pentamidine
5.2. Meglumine Antimoniate
5.3. Paromomycin
5.4. Stibogluconate
5.5. Triazoles
5.5.1. Itraconazole
5.5.2. Voriconazole
5.6. Artesunate
6. Materials and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.; Appelgren, B.; Sjödin, V.; Ansari, D.; Nilsson, J.; Persson, U.; Tingstedt, B.; Andersson, R. Acute Pancreatitis—Costs for Healthcare and Loss of Production. Scand. J. Gastroenterol. 2013, 48, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Szatmary, P.; Grammatikopoulos, T.; Cai, W.; Huang, W.; Mukherjee, R.; Halloran, C.; Beyer, G.; Sutton, R. Acute Pancreatitis: Diagnosis and Treatment. Drugs 2022, 82, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Rompianesi, G.; Hann, A.; Komolafe, O.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Serum Amylase and Lipase and Urinary Trypsinogen and Amylase for Diagnosis of Acute Pancreatitis. Cochrane Database Syst. Rev. 2017, 4, CD012010. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Gabata, T.; Takada, T.; Hirata, K.; Yoshida, M.; Mayumi, T.; Hirota, M.; Kadoya, M.; Yamanouchi, E.; Hattori, T.; et al. New Diagnostic Criteria of Acute Pancreatitis. J. Hepatobiliary Pancreat. Sci. 2010, 17, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.-A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Petrov, M.S.; Yadav, D. Global Epidemiology and Holistic Prevention of Pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Niinomi, I.; Hosohata, K.; Oyama, S.; Inada, A.; Wakabayashi, T.; Iwanaga, K. Pharmacovigilance Assessment of Drug-Induced Acute Pancreatitis Using a Spontaneous Reporting Database. Int. J. Toxicol. 2019, 38, 487–492. [Google Scholar] [CrossRef]
- Saini, J.; Marino, D.; Badalov, N.; Vugelman, M.; Tenner, S. Drug-Induced Acute Pancreatitis: An Evidence-Based Classification (Revised). Clin. Transl. Gastroenterol. 2023, 14, e00621. [Google Scholar] [CrossRef]
- Sosnowski, K.; Nehring, P.; Przybyłkowski, A. Pancreas and Adverse Drug Reactions: A Literature Review. Drug Saf. 2022, 45, 929–939. [Google Scholar] [CrossRef]
- Wolfe, D.; Kanji, S.; Yazdi, F.; Barbeau, P.; Rice, D.; Beck, A.; Butler, C.; Esmaeilisaraji, L.; Skidmore, B.; Moher, D.; et al. Drug Induced Pancreatitis: A Systematic Review of Case Reports to Determine Potential Drug Associations. PLoS ONE 2020, 15, e0231883. [Google Scholar] [CrossRef]
- Roberto Simons-Linares, C.; Elkhouly, M.A.; Salazar, M.J. Drug-Induced Acute Pancreatitis in Adults: An Update. Pancreas 2019, 48, 1263–1273. [Google Scholar] [CrossRef]
- Trivedi, C.D.; Pitchumoni, C.S. Drug-Induced Pancreatitis. J. Clin. Gastroenterol. 2005, 39, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Badalov, N.; Baradarian, R.; Iswara, K.; Li, J.; Steinberg, W.; Tenner, S. Drug-Induced Acute Pancreatitis: An Evidence-Based Review. Clin. Gastroenterol. Hepatol. 2007, 5, 648–661.e3. [Google Scholar] [CrossRef] [PubMed]
- Dhir, R.; Brown, D.K.; Olden, K.W. Drug-Induced Pancreatitis: A Practical Review. Drugs Today 2007, 43, 499. [Google Scholar] [CrossRef] [PubMed]
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases—A Prospective Study on Incidence and Severity. J. Crohns Colitis 2016, 10, 61–68. [Google Scholar] [CrossRef]
- Pavlos, R.; Mallal, S.; Phillips, E. HLA and Pharmacogenetics of Drug Hypersensitivity. Pharmacogenomics 2012, 13, 1285–1306. [Google Scholar] [CrossRef]
- Gerstner, T.; Büsing, D.; Bell, N.; Longin, E.; Kasper, J.-M.; Klostermann, W.; Hebing, B.; Hanefeld, F.; Eckel, U.; Hoffmann, R.; et al. Valproic Acid-Induced Pancreatitis: 16 New Cases and a Review of the Literature. J. Gastroenterol. 2007, 42, 39–48. [Google Scholar] [CrossRef]
- Cappell, M.S.; Marks, M. Acute Pancreatitis in HIV-Seropositive Patients: A Case Control Study of 44 Patients. Am. J. Med. 1995, 98, 243–248. [Google Scholar] [CrossRef]
- Qin, W.; Zhao, B.; Shang, Y.; Zhang, L. Clinical Profile of Acute Pancreatitis Following Treatment with Protease Inhibitors: A Real-World Analysis of Post-Marketing Surveillance Data. Expert Opin. Drug Saf. 2021, 20, 1109–1115. [Google Scholar] [CrossRef]
- Li, C.; Jiang, M.; Pan, C.; Li, J.; Xu, L. The Global, Regional, and National Burden of Acute Pancreatitis in 204 Countries and Territories, 1990–2019. BMC Gastroenterol. 2021, 21, 332. [Google Scholar] [CrossRef]
- Foli, A.; Benvenuto, F.; Piccinini, G.; Bareggi, A.; Cossarizza, A.; Lisziewicz, J.; Lori, F. Direct Analysis of Mitochondrial Toxicity of Antiretroviral Drugs. AIDS 2001, 15, 1687–1694. [Google Scholar] [CrossRef]
- Manfredi, R.; Calza, L. HIV Infection and the Pancreas: Risk Factors and Potential Management Guidelines. Int. J. STD AIDS 2008, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Rizvi, S.F.A.; Anwar, U. Tetracycline: Classification, Structure Activity Relationship and Mechanism of Action as a Theranostic Agent for Infectious Lesions—A Mini Review. Biomed. J. Sci. Tech. Res. 2018, 7, 5787–5796. [Google Scholar] [CrossRef]
- Gilson, M.; Moachon, L.; Jeanne, L.; Dumaine, V.; Eyrolle, L.; Morand, P.; Ben M’rad, M.; Salmon, D. Acute Pancreatitis Related to Tigecycline: Case Report and Review of the Literature. Scand. J. Infect. Dis. 2008, 40, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Sura, M.E.; Heinrich, K.A.; Suseno, M. Metronidazole-Associated Pancreatitis. Ann. Pharmacother. 2000, 34, 1152–1155. [Google Scholar] [CrossRef]
- Wu, S.-D.; Zhang, Z.-H.; Jin, J.-Z.; Kong, J.; Wang, W.; Zhang, Q.; Li, D.-Y.; Wang, M.-F. Effects of Narcotic Analgesic Drugs on Human Oddi’s Sphincter Motility. World J. Gastroenterol. 2004, 10, 2901–2904. [Google Scholar] [CrossRef]
- O’Rourke, A.; Beyhan, S.; Choi, Y.; Morales, P.; Chan, A.P.; Espinoza, J.L.; Dupont, C.L.; Meyer, K.J.; Spoering, A.; Lewis, K.; et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 2020, 64, 207–219. [Google Scholar] [CrossRef]
- Sanford, K.A.; Mayle, J.E.; Dean, H.A.; Greenbaum, D.S. Metronidazole-Associated Pancreatitis. Ann. Intern. Med. 1988, 109, 756–757. [Google Scholar] [CrossRef]
- Qian, C.; Abourizk, N.; Rizk, M.-A.; Kim, J.; Smith, D.L.; Westra, K.C.; McLaughlin, J.P. A Rare Case of Metronidazole Induced Recurrent Pancreatitis. Pancreatology 2021, 21, 318–319. [Google Scholar] [CrossRef]
- Barbulescu, A.; Oskarsson, V.; Lindblad, M.; Ljung, R.; Brooke, H.L. Oral Metronidazole Use and Risk of Acute Pancreatitis: A Population-Based Case-Control Study. Clin. Epidemiol. 2018, 10, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, M.; Ratanajamit, C.; Jacobsen, J.; Skriver, M.V.; Pedersen, L.; Sorensen, H.T. Metronidazole and Risk of Acute Pancreatitis: A Population-Based Case-Control Study. Aliment. Pharmacol. Ther. 2005, 21, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Raether, W.; Hänel, H. Nitroheterocyclic Drugs with Broad Spectrum Activity. Parasitol. Res. 2003, 90, S19–S39. [Google Scholar] [CrossRef] [PubMed]
- Slim, R.; Salem, C.B.; Zamy, M.; Fathallah, N.; Raynaud, J.-J.; Bouraoui, K.; Biour, M. Secnidazole-Induced Acute Pancreatitis: A New Side-Effect for an Old Drug? JOP 2010, 11, 85–86. [Google Scholar]
- Hegazi, M.O.; Saleh, F.; John, J.E. Is It Tinidazole-Induced Pancreatitis? J. Clin. Pharm. Ther. 2015, 40, 607–608. [Google Scholar] [CrossRef]
- Kefeli, A.; Aktürk, A.; Yeniova, A.Ö.; Basyigit, S. Ciprofloxacin Induced Pancreatitis: Has This Condition Been Overlooked? Acta Gastroenterol. Belg. 2016, 79, 65–66. [Google Scholar]
- Sunga, H.Y.; Kim, J., II; Lee, H.J.; Cho, H.J.; Cheung, D.Y.; Kim, S.S.; Cho, S.H.; Kim, J.K. Acute Pancreatitis Secondary to Ciprofloxacin Therapy in Patients with Infectious Colitis. Gut Liver 2014, 8, 265–270. [Google Scholar] [CrossRef][Green Version]
- Jiménez Moreno, M.A.; Hontoria Bautista, G.; Pereda García, R. Acute Pancreatitis Associated with Levofloxacin. Rev. Española Enfermedades Dig. 2020, 112, 510. [Google Scholar] [CrossRef]
- Bush, N.; Sharma, V.; Chandrahasan, K.; Patil, A. Ofloxacin-Ornidazole Fixed-Dose Combination Medication-Induced Pancreatitis with Positive Rechallenge. J. Fam. Med. Prim. Care 2020, 9, 3157–3159. [Google Scholar] [CrossRef]
- Drabo, Y.J.; Niakara, A.; Ouedraogo, H. Pancréatite Aiguë Secondaire à Une Prise de Norfloxacine. Ann. Fr. Anesth. Reanim. 2002, 21, 68–69. [Google Scholar] [CrossRef]
- Nelis, G.F. Nitrofurantoin-Induced Pancreatitis: Report of a Case. Gastroenterology 1983, 84, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Christophe, J.L. Pancreatitis Induced by Nitrofurantoin. Gut 1994, 35, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Mouallem, M.; Sirotin, T.; Farfel, Z. Nitrofurantoin-Induced Pancreatitis. Isr. Med. Assoc. J. 2003, 5, 754–755. [Google Scholar] [PubMed]
- Rawla, P.; Raj, J.P. Doxycycline-Induced Acute Pancreatitis: A Rare Adverse Event. Gastroenterol. Res. 2017, 10, 244–246. [Google Scholar] [CrossRef]
- Elmore, M.F.; Rogge, J.D. Tetracycline-Induced Pancreatitis. Gastroenterology 1981, 81, 1134–1136. [Google Scholar] [CrossRef]
- Wachira, J.K.; Jensen, C.H.; Rhone, K. Doxycycline-Induced Pancreatitis: A Rare Finding. S D Med. 2013, 66, 227–229. [Google Scholar]
- Torosis, J.; Vender, R. Tetracycline-Induced Pancreatitis. J. Clin. Gastroenterol. 1987, 9, 580–581. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Mengedoht, D.E.; Kline, J.J. Tetracycline-Induced Pancreatitis. Am. J. Gastroenterol. 1991, 86, 1669–1671. [Google Scholar]
- Ocal, S.; Selçuk, H.; Korkmaz, M.; Unal, H.; Yilmaz, U. Acute Pancreatitis Following Doxycycline and Ornidazole Coadministration. JOP 2010, 11, 614–616. [Google Scholar]
- Achecar Justo, L.; Rivero Fernández, M.; Cobo Reinoso, J.; Ruiz Del Árbol Olmos, L. Pancreatitis Aguda Inducida Por Doxiciclina. Med. Clin. 2010, 134, 705–706. [Google Scholar] [CrossRef]
- Inayat, F.; Virk, H.H.; Yoon, D.; Riaz, I. Drug-Induced Pancreatitis: A Rare Manifestation of Doxycycline Administration. N. Am. J. Med. Sci. 2016, 8, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.T.; Kapila, N. Probable Doxycycline-Induced Acute Pancreatitis. Am. J. Health-Syst. Pharm. 2016, 73, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Razzano, A.; Grendell, J. Doxycycline Induced Severe Acute Pancreatitis: A Rare Finding To A Common Medication. BMJ Case Rep. 2021, 14, e239640. [Google Scholar] [CrossRef] [PubMed]
- Reiche, W.; Abodunrin, F.; Destache, C.; Rangray, R.; Velagapudi, M. Doxycycline Induced Pancreatitis: An Uncommon Complication of a Common Drug. Pharmacy 2022, 10, 144. [Google Scholar] [CrossRef]
- Bassi, R.; Prakash, P.; Balakrishnan, E.; Cockey, G. Blame It on the Drug: A Rare Case of Recurrent Doxycycline-Induced Pancreatitis. Cureus 2022, 14, e29171. [Google Scholar] [CrossRef]
- Paulraj, S.; Ashok Kumar, P.; Subedi, D. A Common Medication with an Uncommon Adverse Event: A Case of Doxycycline-Induced Pancreatitis. Cureus 2020, 12, e7496. [Google Scholar] [CrossRef]
- Bernas Albeniz, A.; Aveiga Valencia, D.A.; Etxeberria Zabala, L.; Zaldibar-Gerrikagoitia Bilbao, J.; Aguilera Celorrio, L. Pancreatitis Aguda En La Unidad de Cuidados Intensivos Secundaria a Tratamiento Con Tigeciclina. Rev. Esp. Anestesiol. Reanim. 2017, 64, 46–49. [Google Scholar] [CrossRef]
- Davido, B.; Shourick, J.; Makhloufi, S.; Dinh, A.; Salomon, J. True Incidence of Tigecycline-Induced Pancreatitis: How Many Cases Are We Missing? J. Antimicrob. Chemother. 2016, 71, 2994–2995. [Google Scholar] [CrossRef]
- Hung, W.Y.; Kogelman, L.; Volpe, G.; Iafrati, M.; Davidson, L. Tigecycline-Induced Acute Pancreatitis: Case Report and Literature Review. Int. J. Antimicrob. Agents 2009, 34, 486–489. [Google Scholar] [CrossRef]
- Lipshitz, J.; Kruh, J.; Cheung, P.; Cassagnol, M. Tigecycline-Induced Pancreatitis. J. Clin. Gastroenterol. 2009, 43, 93. [Google Scholar] [CrossRef]
- Marot, J.C.; Jonckheere, S.; Munyentwali, H.; Belkhir, L.; Vandercam, B.; Yombi, J.C. Tigecycline-Induced Acute Pancreatitis: About Two Cases and Review of the Literature. Acta Clin. Belg. 2012, 67, 229–232. [Google Scholar] [PubMed]
- De Mesa, C.; Dajoyag-Mejia, M.A.; Regina, I.; Darouiche, R.O. Tigecycline-Induced Acute Pancreatitis with Rechallenge: A Case Report. J. Pharm. Technol. 2013, 29, 3–8. [Google Scholar] [CrossRef]
- Lin, J.; Wang, R.; Chen, J. Tigecycline-Induced Acute Pancreatitis in a Renal Transplant Patient: A Case Report and Literature Review. BMC Infect. Dis. 2018, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, M.T.; Jones, K.R. Tigecycline-Induced Acute Pancreatitis in a Cystic Fibrosis Patient: A Case Report and Literature Review. J. Cyst. Fibros. 2016, 15, e9–e11. [Google Scholar] [CrossRef]
- Prot-Labarthe, S.; Youdaren, R.; Benkerrou, M.; Basmaci, R.; Lorrot, M. Pediatric Acute Pancreatitis Related to Tigecycline. Pediatr. Infect. Dis. J. 2010, 29, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.P. Minocycline-Induced Pancreatitis in Cystic Fibrosis. Chest. 2001, 119, 1283–1285. [Google Scholar] [CrossRef]
- Chetaille, E.; Delcenserie, R.; Yzet, T.; Decocq, G.; Biour, M.; Andrejak, M. Minocycline Involvement in Two Cases of Acute Pancreatitis. Gastroenterol. Clin. Biol. 1998, 22, 555–556. [Google Scholar]
- Gabriel, J.G.; Bhogal, S.; Kapila, A. Minocycline-Associated Pancreatitis. Am. J. Ther. 2018, 25, e556–e557. [Google Scholar] [CrossRef]
- Anton Aranda, E.; Altuna Basurto, E. Acute Pancreatitis and Erythromycin. Med. Clin. 1991, 96, 638. [Google Scholar]
- Berger, T.M.; Cook, W.J.; O’Marcaigh, A.S.; Zimmerman, D. Acute Pancreatitis in a 12-Year-Old Girl after an Erythromycin Overdose. Pediatrics 1992, 90, 624–626. [Google Scholar] [CrossRef]
- Gonzalez Carro, P.; Ribes, F.; Garcia, M.J. Acute Pancreatitis Associated with Erythromycin [2]. Rev. Esp. Enfermedades Dig. 1995, 87, 757–758. [Google Scholar]
- Fang, C.-C.; Wang, H.-P.; Lin, J.-T. Erythromycin-Induced Acute Pancreatitis. J. Toxicol. Clin. Toxicol. 1996, 34, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Igea, F.J.; Lepe Jiménez, J.A.; Garrido Serrano, A.; Palomo Gil, S. Acute Pancreatitis Caused by Erythromycin. An. Med. Interna 2001, 18, 400. [Google Scholar] [PubMed]
- Gumaste, V.V. Erythromycin-Induced Pancreatitis. Am. J. Med. 1989, 86, 725. [Google Scholar] [CrossRef]
- Hawksworth, C.R. Acute Pancreatitis Associated with Infusion of Erythromycin Lactobionate. BMJ 1989, 298, 190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascual Velasco, F.; Goicoechea Ibarra, L.; Bichara Antanios, G. Acute Pancreatitis Induced by Erythromycin: A New Case. Med. Clin. 1991, 97, 473–474. [Google Scholar]
- Surinach, J.M.; Alegre, J.; Fernandez de Sevilla, T.; Queralt, M. Acute Pancreatitis from Erythromycin. Rev. Clin. Esp. 1993, 192, 458. [Google Scholar]
- Teillet, L.; Chaussade, S.; Mory, B.; Roche, H.; Couturier, D.; Guerre, J. Drug-Induced Acute Pancreatitis following Intravenous Erythomycin Antibiotherapy. Gastroenterol. Clin. Biol. 1991, 15, 265–266. [Google Scholar]
- Tenenbein, M.S.; Tenenbein, M. Acute Pancreatitis Due to Erythromycin Overdose. Pediatr. Emerg. Care 2005, 21, 675–676. [Google Scholar] [CrossRef]
- Acharya, G.K.; Hita, A.G.; Yeung, S.-C.J. Diabetic Ketoacidosis and Acute Pancreatitis: Serious Adverse Effects of Everolimus. Ann. Emerg. Med. 2017, 69, 666–667. [Google Scholar] [CrossRef]
- Wong, P.W.K.; Dillard, T.A.; Kroenke, K. Multiple Organ Toxicity From Addition of Erythromycin to Long-Term Lovastatin Therapy. South. Med. J. 1998, 91, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Avraam, C.; Siomos, K.; Armenaka, M.; Sion, M.L. Clarithromycin associated acute pancreatitis. Ann. Gastroenterol. 2007, 20, 35–37. [Google Scholar]
- Schouwenberg, B.J.J.W.; Deinum, J. Acute Pancreatitis after a Course of Clarithromycin. Neth. J. Med. 2003, 61, 266–267. [Google Scholar]
- Carro, P.G.; Roldán, F.P.; Huidobro, M.L.L.; de Acufia, M.M.; García, J.C.N. Acute Pancreatitis and Modified-Release Clarithromycin. Ann. Pharmacother. 2004, 38, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Pomero, F.; Fenoglio, L.; Melchio, R.; Serraino, C.; Ageno, W.; Dentali, F. Incidence and Diagnosis of Pulmonary Embolism in Northern Italy: A Population-Based Study. Eur. J. Intern. Med. 2013, 24, e77–e78. [Google Scholar] [CrossRef] [PubMed]
- Renkes, P.; Petitpain, N.; Cosserat, F.; Bangratz, S.; Trechot, P. Can Roxithromycin and Betamethasone Induce Acute Pancreatitis? A Case Report. JOP 2003, 4, 184–186. [Google Scholar] [PubMed]
- Park, T.Y.; Oh, H.-C.; Do, J.H. A Case of Recurrent Pancreatitis Induced by Trimethoprim-Sulfamethoxazole Re-Exposure. Gut Liver 2010, 4, 250–252. [Google Scholar] [CrossRef][Green Version]
- Versleijen, M.W.; Naber, A.H.; Riksen, N.P.; Wanten, G.J.; Debruyne, F.M.J. Recurrent Pancreatitis after Trimethoprim-Sulfamethoxazole Rechallenge. Neth. J. Med. 2005, 63, 275–277. [Google Scholar]
- Brett, A.S.; Shaw, S.V. Simultaneous pancreatitis and hepatitis associated with trimethoprim-sulfamethoxazole. Am. J. Gastroenterol. 1999, 94, 267–268. [Google Scholar] [CrossRef]
- Bartels, R.H.M.A.; van der Spek, J.A.N.; Oosten, H.R. Acute Pancreatitis Due to Sulfamethoxazole-Trimethoprim. South. Med. J. 1992, 85, 1006–1007. [Google Scholar] [CrossRef]
- Alberti-Flor, J.J.; Hernandez, M.E.; Ferrer, J.P.; Howell, S.; Jeffers, L. Fulminant Liver Failure and Pancreatitis Associated with the Use of Sulfamethoxazole-Trimethoprim. Am. J. Gastroenterol. 1989, 84, 1577–1579. [Google Scholar] [PubMed]
- Brazer, S.R.; Medoff, J.R. Sulfonamide-Induced Pancreatitis. Pancreas 1988, 3, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Antonow, D.R. Acute Pancreatitis Associated with Trimethoprim-Sulfamethoxazole. Ann. Intern. Med. 1986, 104, 363–365. [Google Scholar]
- Hanline, M.H. Acute Pancreatitis Caused By Ampicillin. South. Med. J. 1987, 80, 1069. [Google Scholar] [CrossRef] [PubMed]
- Chams, S.; El Sayegh, S.; Hamdon, M.; Kumar, S.; Tegeltija, V. Amoxicillin/Clavulanic Acid-Induced Pancreatitis: Case Report. BMC Gastroenterol. 2018, 18, 122. [Google Scholar] [CrossRef]
- Galindo, C.; Buenestado, J.; Rene Espinet, J.M.; Pinol, M.C. Acute Pancreatitis and Liver Injury Associated with Amoxicillin-Clavulanic Therapy. Rev. Esp. Enfermedades Dig. 1995, 87, 597–600. [Google Scholar]
- Famularo, G.; Polchi, S.; De Simone, C. Acute Cholecystitis and Pancreatitis in a Patient with Biliary Sludge Associated with the Use of Ceftriaxone: A Rare but Potentially Severe Complication. Ann. Ital. Med. Int. 1999, 14, 202–204. [Google Scholar]
- Maranan, M.C.; Gerber, S.I.; Miller, G.G. Gallstone pancreatitis caused by ceftriaxone. Pediatr. Infect. Dis. J. 1998, 17, 662–663. [Google Scholar] [CrossRef]
- Zimmermann, A.E.; Katona, B.G.; Jodhka, J.S.; Williams, R.B. Ceftriaxone-Induced Acute Pancreatitis. Ann. Pharmacother. 1993, 27, 36–37. [Google Scholar] [CrossRef]
- Zinberg, J.; Chernaik, R.; Coman, E.; Rosenblatt, R.; Brandt, L.J. Reversible Symptomatic Biliary Obstruction Associated with Ceftriaxone Pseudolithiasis. Am. J. Gastroenterol. 1991, 86, 1251–1254. [Google Scholar]
- Nakagawa, N.; Ochi, N.; Yamane, H.; Honda, Y.; Nagasaki, Y.; Urata, N.; Nakanishi, H.; Kawamoto, H.; Takigawa, N. Ceftriaxone-Associated Pancreatitis Captured on Serial Computed Tomography Scans. Radiol. Case Rep. 2018, 13, 43–46. [Google Scholar] [CrossRef]
- Pandey, A.S.; Surana, A. Isoniazid-Induced Recurrent Acute Pancreatitis. Trop. Doct 2011, 41, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Mattioni, S.; Zamy, M.; Mechai, F.; Raynaud, J.-J.; Chabrol, A.; Aflalo, V.; Biour, M.; Bouchaud, O. Isoniazid-Induced Recurrent Pancreatitis. JOP 2012, 13, 314–316. [Google Scholar] [PubMed]
- Kharibam, P.; Jithesh, G.; Ronanki, K.; Pathania, M. A Rare Case of Isoniazid Induced Recurrent Acute Pancreatitis. J. Cardiovasc. Dis. Res. 2021, 12, 1121–1124. [Google Scholar]
- Briongos-Figuero, L.S.; Bachiller-Luque, P.; Pons-Renedo, F.; Eiros-Bouza, J.M. Pancreatitis Aguda Inducida Por Isoniazida. Enfermedades Infecc. Microbiol. Clin. 2007, 25, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Chan, H.S.; Lui, S.F.; Lai, K.N. Recurrent Acute Pancreatitis Induced by Isoniazid. Tuber. Lung Dis. 1994, 75, 383–385. [Google Scholar] [CrossRef]
- Chow, K.M.; Szeto, C.C.; Leung, C.B.; Li, P.K.T. Recurrent Acute Pancreatitis after Isoniazid. Neth. J. Med. 2004, 62, 172–174. [Google Scholar]
- Izzedine, H.; Launay-Vacher, V.; Storme, T.; Deray, G. Acute Pancreatitis Induced by Isoniazid. Am. J. Gas Troenterol. 2001, 96, 3208–3209. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Larrubia, J.R.; Lana, R.; Espinos, D.; Diaz-Rubio, M. Acute Pancreatitis Induced by Isoniazid, a Casual Association. An. Med. Interna 1998, 15, 588–590. [Google Scholar]
- Rabassa, A.A. Isoniazid-Induced Acute Pancreatitis. Ann. Intern. Med. 1994, 121, 433. [Google Scholar] [CrossRef]
- Saleem, A.F.; Arbab, S.; Naz, F.Q. Isoniazid Induced Acute Pancreatitis in a Young Girl. J. Coll. Physicians Surg. Pak. 2015, 25, 299–300. [Google Scholar]
- Sanchez, A.J.; Boken, D.J. Isoniazid-Associated Pancreatitis. Infect. Med. 2004, 21, 622–623. [Google Scholar]
- Stephenson, I.; Wiselka, M.J.; Qualie, M.J. Acute Pancreatitis Induced by Isoniazid in the Treatment of Tuberculosis. Am. J. Gastroenterol. 2001, 96, 2271–2272. [Google Scholar] [CrossRef]
- Yi, P.H.; Veltre, D.R.; Kuttab, J.S.; Rangan, V.; Norton, L. Acute Groove Pancreatitis Due to Isoniazid. Neth. J. Med. 2013, 71, 104. [Google Scholar]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.J.; Cretton-Scott, E.; Teague, A.; Wensel, T.M. Protease Inhibitors for Patients with HIV-1 Infection: A Comparative Overview. Phys. Ther. Rehabil. J. 2011, 36, 332–345. [Google Scholar]
- Lin, C.; Verma, V.; Lazenby, A.; Ly, Q.P.; Berim, L.D.; Schwarz, J.K.; Madiyalakan, M.; Nicodemus, C.F.; Hollingsworth, M.A.; Meza, J.L.; et al. Phase I/II Trial of Neoadjuvant Oregovomab-Based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir For Locally Advanced Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2019, 42, 755–760. [Google Scholar] [CrossRef]
- Di Martino, V.; Ezenfis, J.; Benhamou, Y.; Bernard, B.; Opolon, P.; Bricaire, F.; Poynard, T. Severe Acute Pancreatitis Related to the Use of Nelfinavir in HIV Infection: Report of a Case with Positive Rechallenge. AIDS 1999, 13, 1421–1423. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Urich, R.; Skinner, S.; Bina, R.; Chuang, K.-Y.; Van Thiel, D.H.; Nadir, A. First Report of Telaprevir-Induced Pancreatitis. Dig. Dis. Sci. 2013, 58, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Bilar, J.M.; Mota, C.F.M.G.P.; Carvalho-Filho, R.J.; da Silva Fucuta, P.; Ferraz, M.L.C.G. Acute Pancreatitis Associated with Boceprevir: A Case Report. Braz. J. Infect. Dis. 2014, 18, 454–456. [Google Scholar] [CrossRef]
- Da Silva, J.; Giroldi, S.B.; Basso, F.d.O.; Antunes, G.N.; Borba, L.A.; de Lima, C.R.M. Acute Pancreatitis during Interferon-Alpha and Ribavirin Treatment for Hepatitis C. BMJ Case Rep. 2009, 2009, 1000–1006. [Google Scholar] [CrossRef]
- Rocca, E. Remdesivir and Pancreatic Toxicity. WHO Pharm. Newsl. 2021, 3, 16–21. Available online: https://www.who.int/publications/i/item/who-pharmaceuticals-newsletter---n-3-2021 (accessed on 1 August 2023).
- Miyazaki, K.; Yoshimura, Y.; Miyata, N.; Sasaki, H.; Shiba, A.; Aga, M.; Hamakawa, Y.; Taniguchi, Y.; Misumi, Y.; Agemi, Y.; et al. Acute Pancreatitis or Severe Increase in Pancreatic Enzyme Levels Following Remdesivir Administration in COVID-19 Patients: An Observational Study. Sci. Rep. 2022, 12, 5323. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Covino, M.; De Lucia, S.S.; Del Gaudio, A.; Fiorani, M.; Polito, G.; Settanni, C.R.; Piccioni, A.; Franceschi, F.; Gasbarrini, A. Impact of COVID-19 in Individuals with and without Pre-Existent Digestive Disorders with a Particular Focus on Elderly Patients. World J. Gastroenterol. 2023, 29, 4099–4119. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Benias, P.C.; Liu, Y.; Sejpal, D.V.; Satapathy, S.K.; Trindade, A.J. Northwell COVID-19 Research Consortium. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients with Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology 2020, 159, 2226–2228.e2. [Google Scholar] [CrossRef] [PubMed]
- Grosser, M.R.; Hale, R.E. Analysis of a COVID-19 Clinical Trial to Emphasize Experimental Design and Quantitative Reasoning in an Introductory Biology Course. J. Microbiol. Biol. Educ. 2021, 22, 10-1128. [Google Scholar] [CrossRef]
- Maxson, C.J.; Greenfield, S.M.; Turner, J.L. Acute Pancreatitis Is a Common Complication of 2′,3′-Dideoxyinosine Therapy in the Acquired Immunodeficiency Syndrome. Am. J. Gas-Troenterol. 1992, 87, 708–713. [Google Scholar]
- Shelton, M.J.; O’Donnell, A.M.; Morse, G.D. Didanosine. Ann. Pharmacother. 1992, 26, 660–670. [Google Scholar] [CrossRef]
- Seidlin, M.; Lambert, J.S.; Dolin, R.; Valentine, F.T. Pancreatitis and Pancreatic Dysfunction in Patients Taking Dideoxyinosine. AIDS 1992, 6, 831–836. [Google Scholar] [CrossRef]
- Grasela, T.H.; Walawander, C.A.; Beltangady, M.; Knupp, C.A.; Martin, R.R.; Dunkle, L.M.; Barbhaiya, R.H.; Pittman, K.A.; Dolin, R.; Valentine, F.T.; et al. Analysis of Potential Risk Factors Associated with the Development of Pancreatitis in Phase I Patients with AIDS or AIDS-Related Complex Receiving Didanosine. J. Infect. Dis. 1994, 169, 1250–1255. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Ferreira, F.A.Y.; Yonamine, R.Y.; Chehter, E.Z. Antiretroviral Drugs and Acute Pancreatitis in HIV/AIDS Patients: Is There Any Association? A Literature Review. Einstein 2014, 12, 112–119. [Google Scholar] [CrossRef]
- Sarner, L.; Fakoya, A. Acute Onset Lactic Acidosis and Pancreatitis in the Third Trimester of Pregnancy in HIV-1 Positive Women Taking Antiretroviral Medication. Sex. Transm. Infect. 2002, 78, 58–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reisler, R.B.; Murphy, R.L.; Redfield, R.R.; Parker, R.A. Incidence of Pancreatitis in HIV-1-Infected Individuals Enrolled in 20 Adult AIDS Clinical Trials Group Studies: Lessons Learned. J. Acquir. Immune Defic. Syndr. 2005, 39, 159–166. [Google Scholar] [PubMed]
- Umar, J.; Zayac, A.; Masood, U.; Rawlins, S. Lamivudine-Associated Pancreatitis: Strongest Evidence to Date. Am. J. Ther. 2017, 24, e636–e637. [Google Scholar] [CrossRef] [PubMed]
- Goffin, E.; Horsmans, Y.; Pirson, Y.; Cornu, C.; Geubel, A.; van Ypersele De Strihou, C. Acute Necrotico-Hemorrhagic Pancreatitis after Famciclovir Prescription. Transplantation 1995, 59, 1218–1219. [Google Scholar]
- Weber, A.; Carbonnel, F.; Simon, N.; Kantelip, B.; Coaquette, A.; Mantion, G.; Miguet, J.-P.; Di Martino, V. Severe Acute Pancreatitis Related to the Use of Adefovir in a Liver Transplant Recipient. Gastroenterol. Clin. Biol. 2008, 32, 247–249. [Google Scholar] [CrossRef]
- Sands, M.; Kron, M.A.; Brown, R.B. Pentamidine: A Review. Rev. Infect. Dis. 1985, 7, 625–634. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Lei, E.; Yang, A.; Li, Y.; Wen, K.; Wang, M.; Li, L.; Chen, Z.; Zhou, C.; et al. Cooperative Membrane Damage as a Mechanism for Pentamidine–Antibiotic Mutual Sensitization. ACS Chem. Biol. 2022, 17, 3178–3190. [Google Scholar] [CrossRef]
- Echinard, E.; Dupon, M.; Malou, M.; Ragnaud, J.M.; Lacut, J.Y.; Albin, H. Acute Fatal Pancreatitis Following Treatment with Pentamidine. Therapie 1986, 41, 520. [Google Scholar]
- Foisy, M.M.; Slayter, K.L.; Hewitt, R.G.; Morse, G.D. Pancreatitis during Intravenous Pentamidine Therapy in an AIDS Patient with Prior Exposure to Didanosine. Ann. Pharmacother. 1994, 28, 1025–1028. [Google Scholar] [CrossRef]
- Hart, C.C. Aerosolized Pentamidine and Pancreatitis. Ann. Intern. Med. 1989, 111, 691. [Google Scholar] [CrossRef]
- Herer, B.; Chinet, T.; Labrune, S.; Collignon, M.A.; Chretien, J.; Huchon, G. Pancreatitis Associated with Pentamidine by Aerosol. BMJ 1989, 298, 605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Schnadig, V.J.; MacGregor, M.G. Fatal Acute Pancreatitis Associated with Pentamidine Therapy. Am. J. Gastroenterol. 1989, 84, 451–453. [Google Scholar]
- Salah, A.; Lortholary, O.; Lhote, F.; Cohen, P.; Guillevin, L. Acute Pancreatitis Induced by Pentamidine Isethionate in Aerosols. Presse Med. 1994, 23, 49. [Google Scholar]
- Harris, A.G.; Caroli-Bosc, F.X.; Demarquay, J.F.; Hastier, P.; Delmont, J. Acute Pancreatitis in an Octreotide-Treated AIDS Patient—Suggested Alternative Mechanisms. Pancreas 1995, 11, 318–319. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.G.; Selub, S.E.; Hak, L.J. Pancreatitis during Pentamidine Therapy in Patients with AIDS. Clin. Pharm. 1991, 10, 56–59. [Google Scholar] [PubMed]
- Murphey, S.A.; Josephs, A.S. Acute Pancreatitis Associated With Pentamidine Therapy. Arch. Intern. Med. 1981, 141, 56–58. [Google Scholar] [CrossRef]
- Pais, J.R.; Cazorla, C.; Novo, E.; Viana, A. Massive Haemorrhage from Rupture of a Pancreatic Pseudocyst after Pentamidine-Associated Pancreatitis. Eur. J. Med. 1992, 1, 251–253. [Google Scholar]
- Pauwels, A.; Eliaszewicz, M.; Larrey, D.; Lacassin, F.; Poirier, J.M.; Meyohas, M.C.; Frottier, J. Pentamidine-Induced Acute Pancreatitis in a Patient with AIDS. J. Clin. Gastroenterol. 1990, 12, 457–459. [Google Scholar] [CrossRef]
- Salmeron, S.; Petitpretz, P.; Katlama, C.; Herve, P.; Brivet, F.; Simonneau, G.; Duroux, P.; Régnier, B. Pentamidine and Pancreatitis. Ann. Intern. Med. 1986, 105, 140. [Google Scholar] [CrossRef]
- Sauleda, J.; Gea, J.G.; Aguar, M.C.; Aran, X.; Pastó, M.; Broquetas, J.M. Probable Pentamidine-Induced Acute Pancreatitis. Ann. Pharmacother. 1994, 28, 52–53. [Google Scholar] [CrossRef]
- Singh, G.; el-Gadi, S.M.; Sparks, R.A. Pancreatitis Associated with Aerosolised Pentamidine. Sex. Transm. Infect. 1995, 71, 130–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wood, G.; Wetzig, N.; Hogan, P.; Whitby, M. Survival from Pentamidine Induced Pancreatitis and Diabetes Mellitus. Aust. N. Z. J. Med. 1991, 21, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Villamil, A.; Hammer, R.A.; Rodriguez, F.H. Edematous Pancreatitis Associated with Intravenous Pentamidine. South. Med. J. 1991, 84, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Zuger, A.; Wolf, B.Z.; El-Sadr, W.; Simberkoff, M.S.; Rahal, J.J. Pentamidine-Associated Fatal Acute Pancreatitis. JAMA 1986, 256, 2383–2385. [Google Scholar] [CrossRef]
- Murphy, R.L.; Noskin, G.A.; Ehrenpreis, E.D. Acute Pancreatitis Associated with Aerosolized Pentamidine. Am. J. Med. 1990, 88, 53N–56N. [Google Scholar]
- World Health Organization. WHO Model Formulary 2008; Stuart, M.C., Kouimtzi, M., Hill, S.R., Eds.; World Health Organization: Geneva, Switzerland, 2009; p. 183. ISBN 9789241547659. [Google Scholar]
- Moreira, V.R.; de Jesus, L.C.L.; Soares, R.-E.P.; Silva, L.D.M.; Pinto, B.A.S.; Melo, M.N.; de Andrade Paes, A.M.; Pereira, S.R.F. Meglumine Antimoniate (Glucantime) Causes Oxidative Stress-Derived DNA Damage in BALB/c Mice Infected by Leishmania (Leishmania) Infantum. Antimicrob. Agents Chemother. 2017, 61, e02360. [Google Scholar] [CrossRef]
- Barthet, M.; Brunet, P.; Bernard, J.C.; Dussol, B.; Rodor, F.; Jouglard, J.; Berland, Y.; Sahel, J. Acute Pancreatitis during Treatment with Meglumine Antimoniate (Glucantime). Gastroenterol. Clin. Biol. 1994, 18, 90–92. [Google Scholar]
- de Lalla, F.; Pellizzer, G.; Gradoni, L.; Vespignani, M.; Franzetti, M.; Stecca, C. Acute Pancreatitis Associated with the Administration of Meglumine Antimonate for the Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 1993, 16, 730–731. [Google Scholar] [CrossRef]
- Kuyucu, N.; Kara, C.; Bakirtaç, A.; Teziç, T. Successful treatment of visceral leishmaniasis with allopurinol plus ketoconazole in an infant who developed pancreatitis caused by meglumine antimoniate. Pediatr. Infect. Dis. J. 2001, 20, 455–457. [Google Scholar] [CrossRef]
- Torrús, D.; Massa, B.; Boix, V.; Portilla, J.; Pérez-Mateo, M. Meglumine Antimoniate-Induced Pancreatitis. Am. J. Gastroenterol. 1996, 91, 820–821. [Google Scholar]
- Santos, J.; Rivero, A.; Márquez, M. Acute pancreatitis with a fatal evolution due to antimonials in patients with visceral leishmaniasis and HIV infection. An. Med. Interna 2000, 17, 562–563. [Google Scholar] [PubMed]
- Tan, W.W.; Chapnick, E.K.; Abter, E.I.M.; Haddad, S.; Zimbalist, E.H.; Lutwick, L.I. Paromomycin-Associated Pancreatitis in Hiv-Related Cryptosporidiosis. Ann. Pharmacother. 1995, 29, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.D.; Herwaldt, B.L. Recommendations for Treating Leishmaniasis with Sodium Stibogluconate (Pentostam) and Review of Pertinent Clinical Studies. Am. J. Trop. Med. Hyg. 1992, 46, 296–306. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases (PDF); World Health Organization: Geneva, Switzerland, 2010; pp. 55, 186. ISBN 9789241209496. [Google Scholar]
- Berman, J.D.; Waddell, D.; Hanson, B.D. Biochemical Mechanisms of the Antileishmanial Activity of Sodium Stibogluconate. Antimicrob. Agents Chemother. 1985, 27, 916–920. [Google Scholar] [CrossRef]
- Cortes, E.; Ribera, E.; Cucurull, E.; de Otero, J.; Ocana, I.; Pahissa, A. Acute Pancreatitis Due to Antimonials in Patients with Visceral Leishmaniasis and HIV Infection. Med. Clin. 1995, 104, 578–580. [Google Scholar]
- Domingo, P. Acute Pancreatitis Associated with Sodium Stibogluconate Treatment in a Patient with Human Immunodeficiency Virus. Arch. Intern. Med. 1996, 156, 1029. [Google Scholar] [CrossRef]
- Donovan, K.L.; White, A.D.; Cooke, D.A.; Fisher, D.J. Pancreatitis and Palindromic Arthropathy with Effusions Associated with Sodium Stibogluconate Treatment in a Renal Transplant Recipient. J. Infect. 1990, 21, 107–110. [Google Scholar] [CrossRef]
- Gasser, R.A.; Magill, A.J.; Oster, C.N.; Franke, E.D.; Grogl, M.; Berman, J.D. Pancreatitis Induced by Pentavalent Antimonial Agents During Treatment of Leishmaniasis. Clin. Infect. Dis. 1994, 18, 83–90. [Google Scholar] [CrossRef]
- McCarthy, A.E.; Keystone, J.S.; Kain, K.C. Pancreatitis Occurring During Therapy with Stibogluconate: Two Case Reports. Clin. Infect. Dis. 1993, 17, 952–953. [Google Scholar] [CrossRef]
- Halim, M.A.; Alfurayh, O.; Kalin, M.E.; Dammas, S.; Al-Eisa, A.; Damanhouri, G. Successful Treatment of Visceral Leishmaniasis with Allopurinol plus Ketoconazole in a Renal Transplant Recipient after the Occurrence of Pancreatitis Due to Stibogluconate. Clin. Infect. Dis. 1993, 16, 397–399. [Google Scholar] [CrossRef]
- Valencia, M.E.; Laguna, F.; Gonzalez Lahoz, J. Nephrotic Syndrome and Acute Pancreatitis Related to Glucantime Administration. An. Med. Interna 2000, 17, 54. [Google Scholar] [PubMed]
- Meczker, Á.; Hanák, L.; Párniczky, A.; Szentesi, A.; Erőss, B.; Hegyi, P.; Hungarian Pancreatic Study Group. Analysis of 1060 Cases of Drug-Induced Acute Pancreatitis. Gastroenterology 2020, 159, 1958–1961.e8. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Hope, W.W.; Roberts, J.A. How Severe Is Antibiotic Pharmacokinetic Variability in Critically Ill Patients and What Can Be Done about It? Diagn. Microbiol. Infect. Dis. 2014, 79, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Passier, J.L.M.; van Puijenbroek, E.P.; Jonkers, G.J.P.M.; van Grootheest, A.C. Pancreatitis Associated with the Use of Itraconazole. Neth. J. Med. 2010, 68, 285–289. [Google Scholar]
- Philip, A.; Sivaprakasam, P.; Sagar, T.G.; Ganesan, P. Voriconazole-Induced Pancreatitis in a Patient of Acute Myeloid Leukemia and Invasive Aspergillosis. J. Pediatr. Hematol. Oncol. 2012, 34, 406. [Google Scholar] [CrossRef]
- Song, Q.L.; Guan, Y. A Case of Acute Pancreatitis Induced by Voriconazole during Treatment of Cryptococcal Meningitis. Br. J. Clin. Pharmacol. 2022, 88, 1925–1929. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Maseko, R.B.; Aderibigbe, B.A. Recent Advances in the Therapeutic Efficacy of Artesunate. Pharmaceutics 2022, 14, 504. [Google Scholar] [CrossRef]
- Adebayo, J.O.; Tijjani, H.; Adegunloye, A.P.; Ishola, A.A.; Balogun, E.A.; Malomo, S.O. Enhancing the Antimalarial Activity of Artesunate. Parasitol. Res. 2020, 119, 2749–2764. [Google Scholar] [CrossRef]
- Abanyie, F.; Acharya, S.D.; Leavy, I.; Bowe, M.; Tan, K.R. Safety and Effectiveness of Intravenous Artesunate for Treatment of Severe Malaria in the United States—April 2019 Through December 2020. Clin. Infect. Dis. 2021, 73, 1965–1972. [Google Scholar] [CrossRef]
- Mahdi, A.S.; Molai, M.; Chandwani, J.; Al Khalili, H.; Ibrahim, H.; Pandak, N.; Khamis, F.; Petersen, E. Late Onset Acute Pancreatitis in P. Falciparum Malar—An An adverse reaction to intravenous artesunate? IDCases 2018, 12, 124–126. [Google Scholar] [CrossRef]
- Kurth, F.; Lingscheid, T.; Steiner, F.; Stegemann, M.S.; Bélard, S.; Menner, N.; Pongratz, P.; Kim, J.; von Bernuth, H.; Mayer, B.; et al. Hemolysis after Oral Artemisinin Combination Therapy for Uncomplicated Plasmodium falciparum Malaria. Emerg. Infect. Dis. 2016, 22, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
| Class | Description |
|---|---|
| Ia | At least 1 case report with positive rechallenge, with exclusion of all other causes. |
| Ib | At least 1 case report with positive rechallenge, failing to document exclusion of other causes or other possible etiologies were available. |
| II | At least 4 cases in the literature with consistent latency. |
| III | At least 2 cases in the literature with no consistent latency among cases and no rechallenge. |
| IV | Drugs not fitting into the earlier described classes. |
| Class | Description |
|---|---|
| Ia | At least 1 case report with positive rechallenge, with exclusion of all other causes. |
| Ib | At least 1 case report with positive rechallenge, failing to document exclusion of other causes or other possible etiologies were available. |
| Ic | At least 1 case report in humans, without a positive rechallenge, other causes are ruled out. |
| II | At least 2 cases in humans reported in the literature, without a positive rechallenge, with consistent latency, and other causes, were not ruled out. |
| III | At least 2 cases in humans reported in the literature, without a positive rechallenge, with inconsistent latency, and other causes, were not ruled out. |
| IV | Drugs not fitting into the earlier described classes. |
| Class | Description |
|---|---|
| I | High quality of evidence for causation of acute pancreatitis: randomized controlled clinical trials. |
| II | Moderate quality of evidence for causation of acute pancreatitis: case–control studies and/or pharmacoepidemiology studies. |
| IIIa | Case reports showing “rechallenge and consistent latency”. |
| IIIb | Case report showing rechallenge only. |
| IIIc | Case report showing consistent latency only. |
| IV | Case Reports with no rechallenge or consistent latency. |
| Drug | At Least One Case with Positive Rechallenge | Studies Showing a High Probability of Association | Classification Class According to Simons-Linares et al. | Classification Class According to Wolfe et al. | Classification Class According to Saini et al. |
|---|---|---|---|---|---|
| Antibiotics | |||||
| Tetracicline | Yes | N/A | Not included | Ia | IIIb |
| Doxycicline | No | N/A | III | Ic | IIIc |
| Tigecycline | Yes | N/A | Ib | Ia | IV |
| Minocycline | No | N/A | III | Ic | Not included |
| Demeclocycline | No | N/A | Not included | IV | Not included |
| Erythromycin | Yes | N/A | III | Ia | IV |
| Clarithromycin | No | N/A | III | Ic | Not included |
| Roxithromycin | No | N/A | IV | IV | IV |
| Metronidazole | Yes | 2 population-based case-control studies | Ia | Ia | II |
| Secnidazole | No | N/A | IV | Ic | IV |
| Tinidazole | No | N/A | IV | Ic | Not included |
| Ciprofloxacin | Yes | N/A | III | Ib | Not included |
| Levofloxacin | Yes | N/A | Not included | Not included | Not included |
| Ofloxacin | No | N/A | Not included | Not included | IV (with ornidazole) |
| Norfloxacin | No | N/A | Not included | IV | Not included |
| Nitrofuratoin | Yes | N/A | Ib | Ia | IIIb |
| Rifampicin | Yes | N/A | Not included | Ib | Not included |
| Trimethoprim/Sulfamethoxazole | Yes | N/A | Ia | Ia | IIIb |
| Amoxicillin-clavulanate | No | N/A | IV | Ic | Not included |
| Ceftriaxone | No | N/A | III | II | Not included |
| Ampicillin | Yes | N/A | III | Ib | Not included |
| Isoniazid | Yes | N/A | |||
| Drug | At Least One Case with Positive Rechallenge | Studies Showing a High Probability of Association | Classification Class According to Simons-Linares et al. | Classification Class According to Wolfe et al. | Classification Class According to Saini et al. |
|---|---|---|---|---|---|
| Antivirals | |||||
| Adefovir | No | N/A | IV | Ic | IV |
| Famciclovir | No | N/A | IV | IV | Not included |
| Lamivudine | No | N/A | IV | Ib | Not included |
| Didanosine | Yes | Yes | II | I | I |
| Remdesevir | No | N/A | Not included | Not included | Not included |
| Interferon α2b/ribavirin | No | N/A | III | Not included | IIIc |
| Ritonavir | No | Yes | III | IV | Not included |
| Indinavir | No | Yes | Not included | Not included | Not included |
| Nelfinavir | Yes | No | Ib | Ib | Not included |
| Telaprevir | Yes | No | Ia | Ia | Not included |
| Boceprevir | No | No | IV | Ic | Not included |
| Drug | At Least One Case with Positive Rechallenge | Studies Showing a High Probability of Association | Classification Class According to Simons-Linares et al. | Classification Class According to Wolfe et al. | Classification Class According to Saini et al. |
|---|---|---|---|---|---|
| Antifungals and Antiparasitics | |||||
| Pentamidine | Yes | N/A | Ib | Ib | IV |
| Meglumine antimoniate | Yes | N/A | II | Ib | Not included |
| Paromomycin | Yes | N/A | IV | Ib | Not included |
| Stibogluconate | Yes | N/A | Ib | Ib | Not included |
| Itraconazole | Yes | N/A | Not included | Ic | IV |
| Voriconazole | Yes | N/A | Not included | Ib | Not included |
| Artesunate | No | N/A | IV | Ic | Not included |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Gaudio, A.; Covello, C.; Di Vincenzo, F.; De Lucia, S.S.; Mezza, T.; Nicoletti, A.; Siciliano, V.; Candelli, M.; Gasbarrini, A.; Nista, E.C. Drug-Induced Acute Pancreatitis in Adults: Focus on Antimicrobial and Antiviral Drugs, a Narrative Review. Antibiotics 2023, 12, 1495. https://doi.org/10.3390/antibiotics12101495
Del Gaudio A, Covello C, Di Vincenzo F, De Lucia SS, Mezza T, Nicoletti A, Siciliano V, Candelli M, Gasbarrini A, Nista EC. Drug-Induced Acute Pancreatitis in Adults: Focus on Antimicrobial and Antiviral Drugs, a Narrative Review. Antibiotics. 2023; 12(10):1495. https://doi.org/10.3390/antibiotics12101495
Chicago/Turabian StyleDel Gaudio, Angelo, Carlo Covello, Federica Di Vincenzo, Sara Sofia De Lucia, Teresa Mezza, Alberto Nicoletti, Valentina Siciliano, Marcello Candelli, Antonio Gasbarrini, and Enrico Celestino Nista. 2023. "Drug-Induced Acute Pancreatitis in Adults: Focus on Antimicrobial and Antiviral Drugs, a Narrative Review" Antibiotics 12, no. 10: 1495. https://doi.org/10.3390/antibiotics12101495
APA StyleDel Gaudio, A., Covello, C., Di Vincenzo, F., De Lucia, S. S., Mezza, T., Nicoletti, A., Siciliano, V., Candelli, M., Gasbarrini, A., & Nista, E. C. (2023). Drug-Induced Acute Pancreatitis in Adults: Focus on Antimicrobial and Antiviral Drugs, a Narrative Review. Antibiotics, 12(10), 1495. https://doi.org/10.3390/antibiotics12101495








