Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources
Abstract
:1. Introduction
2. Results
2.1. Occurrence of P. aeruginosa in Animal and Human Samples
2.2. Antimicrobial Susceptibilities of P. aeruginosa Isolates
2.3. Chemical Composition of Cinnamon Essential Oil
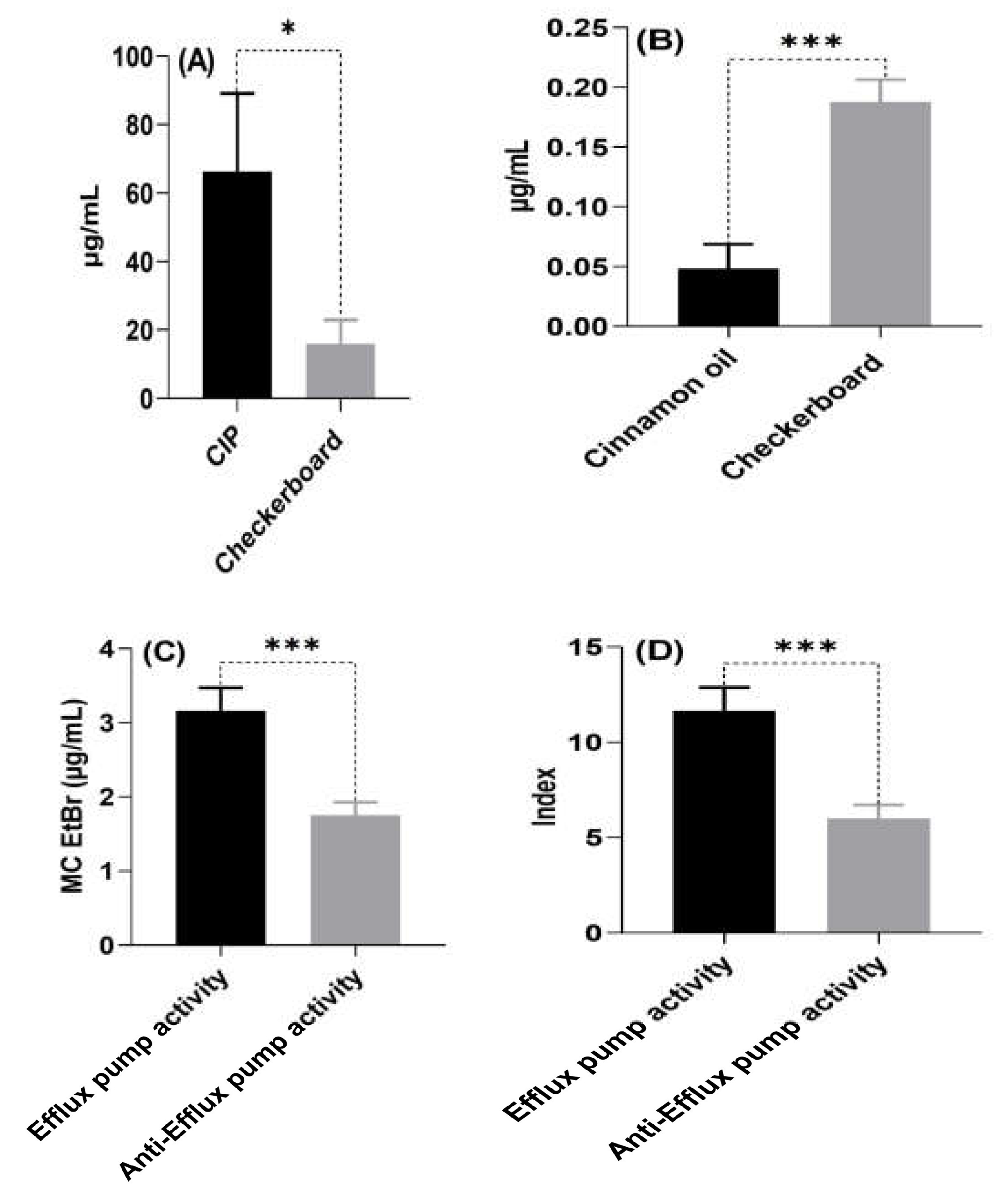
2.4. Antimicrobial Activity of Cinnamon Oil and Ciprofloxacin against XDR and PDR P. aeruginosa Isolates
2.5. Determination of the Efflux Pump’s Activity Phenotypically
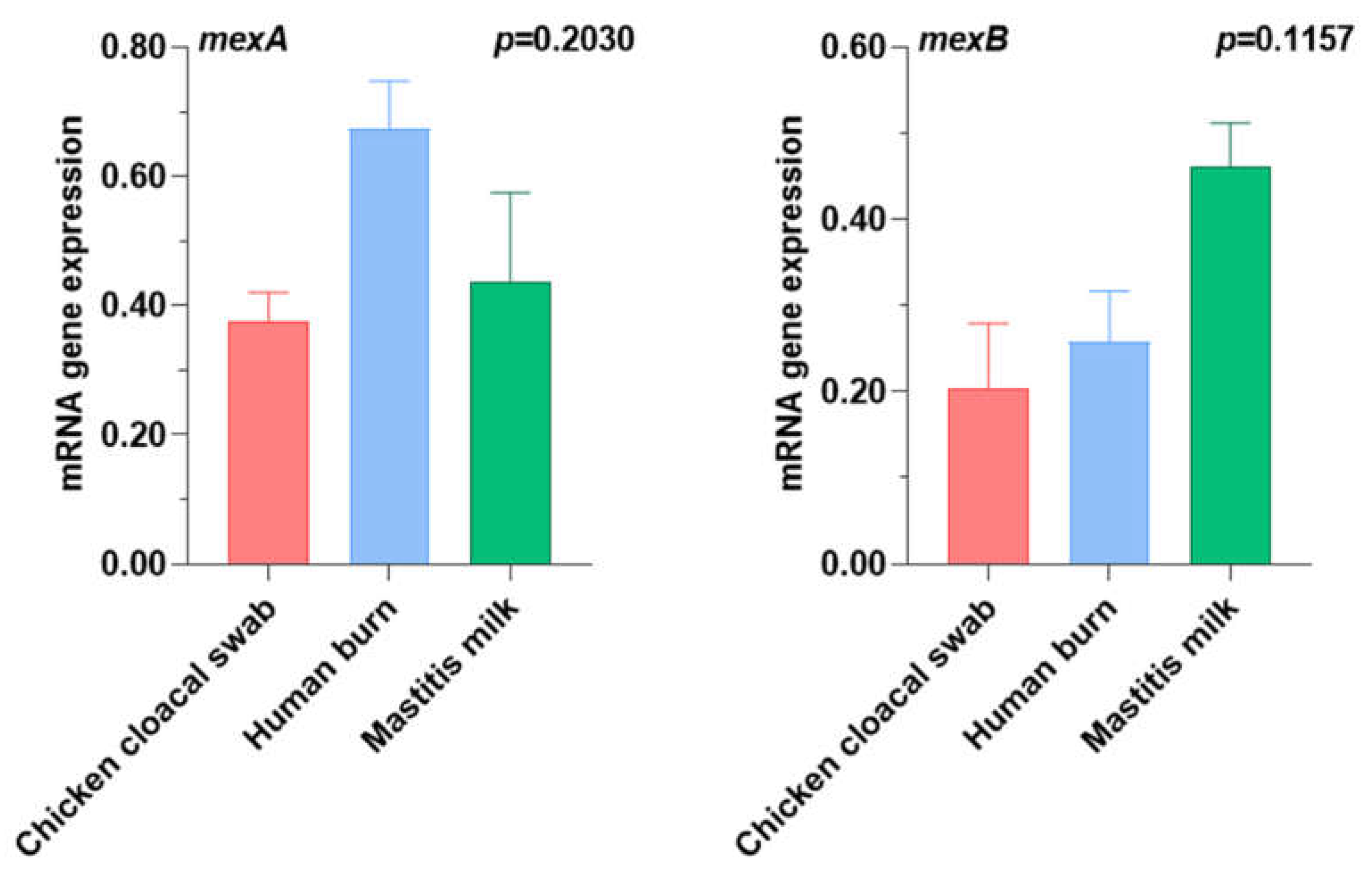
2.6. Quantification of the Expression Levels of Efflux Pump Genes Using RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Isolation and Identification of P. aeruginosa
4.3. Antimicrobial Susceptibility Testing of P. aeruginosa Isolates
4.4. Cinnamon Oil
4.5. Characterization of Cinnamon Essential Oil by Gas Chromatography-Mass Spectrometry
4.6. Antimicrobial Activities of Cinnamon Oil and Ciprofloxacin against P. aeruginosa Isolates
- The FICI = FICA + FICB;
- FICA = MIC of A in combination/MIC of A alone;
- FICB = MIC of B in combination/MIC of B alone.
4.7. Phenotypic Detection of the Efflux Pump Activity by Ethidium Bromide Cartwheel (EtBr-CW) Method
4.8. Transcriptional Analysis of the Efflux Pump Genes Using Real-Time Quantitative PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klockgether, J.; Cramer, N.; Wiehlmann, L.; Davenport, C.F.; Tümmler, B. Pseudomonas aeruginosa genomic structure and diversity. Front. Microbiol. 2011, 2, 150. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Tartor, Y.; El-Naenaeey, E. RT-PCR detection of exotoxin genes expression in multidrug resistant Pseudomonas aeruginosa. Cell. Mol. Biol. 2016, 62, 56–62. [Google Scholar]
- Arzanlou, M.; Chai, W.C.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Dreier, J.; Ruggerone, P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 660. [Google Scholar] [CrossRef]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type drug efflux pumps from Gram-negative bacteria: Molecular mechanism and inhibition. Front. Microbiol. 2015, 6, 377. [Google Scholar] [CrossRef]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Oie, S.; Fukui, Y.; Yamamoto, M.; Masuda, Y.; Kamiya, A. In vitro antimicrobial effects of aztreonam, colistin, and the 3-drug combination of aztreonam, ceftazidime and amikacin on metallo-β-lactamase-producing Pseudomonas aeruginosa. BMC Infect. Dis. 2009, 9, 123. [Google Scholar] [CrossRef]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Poole, K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 2001, 3, 255–264. [Google Scholar] [PubMed]
- Sobel, M.L.; Hocquet, D.; Cao, L.; Plesiat, P.; Poole, K. Mutations in PA3574 (nalD) lead to increased MexAB-OprM expression and multidrug resistance in laboratory and clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; Gómez-Zorrilla, S.; Suarez, C.; Dominguez, M.; Tubau, F.; Arch, O.; Oliver, A.; Pujol, M.; Ariza, J. Extensively drug-resistant Pseudomonas aeruginosa: Risk of bloodstream infection in hospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2791–2797. [Google Scholar] [CrossRef] [PubMed]
- Amirulhusni, A.N.; Palanisamy, N.K.; Mohd-Zain, Z.; Ping, L.J.; Durairaj, R. Antibacterial effect of silver nanoparticles on multi drug resistant Pseudomonas aeruginosa. Int. J. Med. Health Sci. 2012, 6, 291–294. [Google Scholar]
- Lomovskaya, O.; Zgurskaya, H.I.; Totrov, M.; Watkins, W.J. Waltzing transporters and “the dance macabre” between humans and bacteria. Nat. Rev. Drug Discov. 2007, 6, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Compagne, N.; Vieira Da Cruz, A.; Müller, R.T.; Hartkoorn, R.C.; Flipo, M.; Pos, K.M. Update on the Discovery of Efflux Pump Inhibitors against Critical Priority Gram-Negative Bacteria. Antibiotics 2023, 12, 180. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Veras, H.N.; Rodrigues, F.F.; Colares, A.V.; Menezes, I.R.; Coutinho, H.D.; Botelho, M.A.; Costa, J.G. Synergistic antibiotic activity of volatile compounds from the essential oil of Lippia sidoides and thymol. Fitoterapia 2012, 83, 508–512. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86. [Google Scholar]
- Vasconcelos, N.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Park, S.W.; Chae, S.W.; Song, J.J.; Kim, H.C. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumoniae. Acta Pathol. Microbiol. Immunol. 2013, 121, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Arora, S.; Khanna, S.; Kumar, K.H. Prevalence of multidrug-resistant, extensively drug-resistant, and pandrug-resistant Pseudomonas aeruginosa from a tertiary level intensive care unit. J. Glob. Infect. Dis. 2016, 8, 155. [Google Scholar] [PubMed]
- Abd El-Aziz, N.K.; Ammar, A.M.; El Damaty, H.M.; Abd Elkader, R.A.; Saad, H.A.; El-Kazzaz, W.; Khalifa, E. Environmental Streptococcus uberis Associated with Clinical Mastitis in Dairy Cows: Virulence Traits, Antimicrobial and Biocide Resistance, and Epidemiological Typing. Animals 2021, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Elmowalid, G.A.; Ahmad, A.A.M.; Hassan, M.N.; Abd El-Aziz, N.K.; Abdelwahab, A.M.; Elwan, S.I. Molecular Detection of New SHV β-lactamase Variants in Clinical Escherichia coli and Klebsiella pneumoniae Isolates from Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2018, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, S. Current and future status of herbal medicines. Vet. World 2008, 1, 347. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Snoeij, T.; Vandijck, D.; Lizy, C.; Hoste, E.; Lauwaert, S.; Colpaert, K.; Vandekerckhove, L.; Vogelaers, D. Morbidity and mortality of bloodstream infections in patients with severe burn injury. Am. J. Crit. Care 2010, 19, e81–e87. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Ravizzola, G.; Peroni, L.; Bonfanti, C.; Manca, N. Prevalence of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in an Italian hospital. J. Infect. Public Health 2013, 6, 179–185. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Zahran, W.A.; Hindawi, G.R.; Labib, A.Z.; Galal, R. Prevalence of multidrug-resistant Pseudomonas aeruginosa in patients with nosocomial infections at a university hospital in Egypt, with special reference to typing methods. J. Virol. Microbiol. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Shukla, S.; Mishra, P. Pseudomonas aeruginosa infection in broiler chicks in Jabalpur. Int. J. Ext. Res. 2015, 6, 37–39. [Google Scholar]
- Abd El-Ghany, W.A. Pseudomonas aeruginosa infection of avian origin: Zoonosis and one health implications. Vet. World. 2021, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H. Some studies on Pseudomonas species in chicken embryos and broilers in Assiut governorate. Ass. Univ. Bull. Environ. Res. 2004, 7, 23–30. [Google Scholar]
- Farghaly, E.; Roshdy, H.; Bakheet, A.; Abd El-Hafez, S.; Badr, H. Advanced studies on Pseudomonas aeruginosa infection in chicken. Anim. Health Res. J. 2017, 5, 207–217. [Google Scholar]
- Badr, J.; El Saidy, F.; Abdelfattah, A. Emergence of multi-drug resistant Pseudomonas aeruginosa in Broiler Chicks. Int. J. Microbiol Biotechnol. 2020, 5, 41. [Google Scholar]
- McVey, D.S.; Kennedy, M.; Chengappa, M. Veterinary Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ibrahim, N.A.; Farag, V.M.E.-M.; Abd-El-Moaty, A.M.; Atwa, S.M. Resistant gene of Pseudomonas aeruginosa in mastitic cattle with reference to some biochemical and immunological parameters. World’s Vet. J. 2017, 7, 5–13. [Google Scholar] [CrossRef]
- Dorri, K.; Modaresi, F.; Shakibaie, M.R.; Moazamian, E. Effect of gold nanoparticles on the expression of efflux pump mexA and mexB genes of Pseudomonas aeruginosa strains by Quantitative real-time PCR. Pharmacia 2022, 69, 125–133. [Google Scholar] [CrossRef]
- Gad, G.F.; El-Domany, R.A.; Zaki, S.; Ashour, H.M. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: Prevalence, antibiogram and resistance mechanisms. J. Antimicrob. Chemother. 2007, 60, 1010–1017. [Google Scholar] [CrossRef]
- Sabir, R.; Alvi, S.F.D.; Fawwad, A. Antimicrobial susceptibility pattern of aerobic microbial isolates in a clinical laboratory in Karachi-Pakistan. Pak. J. Med. Sci. 2013, 29, 851. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Gharieb, R.M.; Abd El-Aziz, N.K.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.; Abdellatif, S.S.; Ramadan, H. Virulence determinants and plasmid-mediated colistin resistance mcr genes in gram-negative bacteria isolated from bovine milk. Front. Cell. Infect. Microbiol. 2021, 11, 761417. [Google Scholar] [CrossRef]
- Inan, D.; Ogunc, D.; Gunseren, F.; Çolak, D.; Mamikoglu, L.; Gultekin, M. The resistance of Pseudomonas aeruginosa strains isolated from nosocomial infections against various antibiotics. Mikrobiyol Bul. 2000, 34, 255–260. [Google Scholar]
- Brinkman, F.S.; Bains, M.; Hancock, R.E. The amino terminus of Pseudomonas aeruginosa outer membrane protein OprF forms channels in lipid bilayer membranes: Correlation with a three-dimensional model. J. Bacteriol. 2000, 182, 5251–5255. [Google Scholar] [CrossRef]
- Colclough, A.L.; Alav, I.; Whittle, E.E.; Pugh, H.L.; Darby, E.M.; Legood, S.W.; McNeil, H.E.; Blair, J.M. RND efflux pumps in Gram-negative bacteria; regulation, structure and role in antibiotic resistance. Future Microbiol. 2020, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Krebes, K.; McNally, C.; Neshat, S. Multiple antibiotic resistance in Pseudomonas aeruginosa: Evidence for involvement of an efflux operon. J. Bacteriol. 1993, 175, 7363–7372. [Google Scholar] [CrossRef] [PubMed]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Mahadlek, J.; Charoenteeraboon, J.; Phaechamud, T. Combination effects of the antimicrobial agents and cinnamon oil. Adv. Mat. Res. 2012, 506, 246–249. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef]
- Guerra, F.Q.S.; Mendes, J.M.; Sousa, J.P.d.; Morais-Braga, M.F.; Santos, B.H.C.; Melo Coutinho, H.D.; Lima, E.d.O. Increasing antibiotic activity against a multidrug-resistant Acinetobacter spp. by essential oils of Citrus limon and Cinnamomum zeylanicum. Nat. Prod. Res. 2012, 26, 2235–2238. [Google Scholar] [CrossRef]
- Tetard, A.; Zedet, A.; Girard, C.; Plésiat, P.; Llanes, C. Cinnamaldehyde induces expression of efflux pumps and multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e01081-19. [Google Scholar] [CrossRef] [PubMed]
- Pankuch, G.A.; Lin, G.; Seifert, H.; Appelbaum, P.C. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Spilker, T.; Coenye, T.; Vandamme, P.; LiPuma, J.J. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 2004, 42, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.0. 2018. Available online: http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 4 January 2023).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Tambekar, D.; Dhanorkar, D.; Gulhane, S.; Khandelwal, V.; Dudhane, M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 17. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; dos Santos, F.R.; de Araújo Neves, I.; de Carvalho, M.G.; Sanches-Silva, A. Biological activities and major components determination in essential oils intended for a biodegradable food packaging. Ind. Crops Prod. 2017, 97, 201–210. [Google Scholar] [CrossRef]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Choi, O.; Cho, S.K.; Kim, J.; Park, C.G.; Kim, J. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac. J. Trop. Biomed. 2016, 6, 308–314. [Google Scholar] [CrossRef]
- Rankin, I. MIC Testing. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Seattle, WA, USA, 2005; pp. 53–62. [Google Scholar]
- Hamilton-Miller, J. Calculating MIC50. J. Antimicrob. Chemother. 1991, 27, 863–864. [Google Scholar] [CrossRef]
- Moody, J. Synergism testing: Broth microdilution checkerboard and broth macrodilution method. In Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2004; pp. 1–28. [Google Scholar]
- Martins, M.; Viveiros, M.; Couto, I.; Costa, S.S.; Pacheco, T.; Fanning, S.; Pages, J.-M.; Amaral, L. Identification of efflux pump-mediated multidrug-resistant bacteria by the ethidium bromide-agar cartwheel method. In Vivo 2011, 25, 171–178. [Google Scholar] [PubMed]
- Liu, W.-W.; Meng, J.; Cui, J.; Luan, Y.-S. Characterization and function of microRNA∗ s in plants. Front. Plant Sci. 2017, 8, 2200. [Google Scholar] [CrossRef] [PubMed]
- Deschaght, P.; De Baere, T.; Van Simaey, L.; De Baets, F.; De Vos, D.; Pirnay, J.-P.; Vaneechoutte, M. Comparison of the sensitivity of culture, PCR and quantitative real-time PCR for the detection of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. BMC Microbiol. 2009, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Béatrice, J.; Maud, P.; Stéphane, A.; François, C.; Frédéric, G.; Benoit, G.; Marie-Odile, H. Relative expression of Pseudomonas aeruginosa virulence genes analyzed by a real time RT-PCR method during lung infection in rats. FEMS Microbiol. Lett. 2005, 243, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, B.; Yaslianifard, S.; Yaslianifard, S.; Mahmoudi, S.; Keshavarz-Valian, S.; Mamishi, S. Evaluation of efflux pumps gene expression in resistant Pseudomonas aeruginosa isolates in an Iranian referral hospital. Iran. J. Microbiol. 2016, 8, 249. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- SAS Institute. SAS/OR 9.3 User’s Guide: Mathematical Programming Examples; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
| Sample (No.) | No. of P. aeruginosa Isolates (%) | p-Value |
|---|---|---|
| Poultry samples (110) | 80 (72.73) | |
| Cloacal swabs (20) | 15 (75) | |
| Lung and trachea (30) | 22 (73.33) | |
| Cecal contents (23) | 15 (65.22) | 0.7531 |
| Chicken heart (15) | 10 (66.67) | |
| Chicken liver (22) | 18 (81.82) | |
| Mastitis milk (24) | 18 (75) | |
| Human samples (27) | 21 (77.78) | 0.4281 |
| Burn swabs (16) | 13 (81.25) | |
| Urine (11) | 8 (72.73) | |
| Total (161) | 119 (73.91) | 0.8591 |
| Antimicrobial Class | Antimicrobial Agent | No. of Resistant P. aeruginosa Isolated from Different Sources (%) | No. (%) | p-Value | ||
|---|---|---|---|---|---|---|
| Poultry (80) | Human (21) | Cattle (18) | ||||
| Aminoglycosides | Gentamicin | 25 (31.25%) | 7 (33.33%) | 3 (16.67%) | 35 (29.41%) | 0.4281 |
| Tobramycin | 25 (31.25%) | 7 (33.33%) | 3 (16.67%) | 35 (29.41%) | 0.4281 | |
| Amikacin | 25 (31.25%) | 7 (33.33%) | 3 (16.67%) | 35 (29.41%) | 0.4281 | |
| Netilmicin | 25 (31.25%) | 7 (33.33%) | 3 (16.67%) | 35 (29.41%) | 0.4281 | |
| Carbapenems | Imipenem | 2 (2.5%) | 3 (14.28%) | 0 (0%) | 5 (4.2%) | 0.0356 |
| Meropenem | 3 (3.75%) | 3 (14.29%) | 0 (0%) | 6 (5.04%) | 0.0428 | |
| Doripenem | 3 (3.75%) | 3 (14.29%) | 0 (0%) | 6 (5.04%) | 0.0428 | |
| Cephalosporin | Ceftazidime | 27 (33.75%) | 12 (57.14%) | 7 (38.89%) | 46 (38.66%) | 0.1467 |
| Cefepime | 27 (33.75%) | 12 (57.14%) | 7 (38.89%) | 46 (38.66%) | 0.1467 | |
| Fluoroquinolones | Ciprofloxacin | 16 (20%) | 2 (9.52%) | 1 (5.56%) | 19 (15.97%) | 0.2150 |
| Levofloxacin | 16 (20%) | 2 (9.52%) | 1 (5.56%) | 19 (15.97%) | 0.2150 | |
| Penicillin | Ticarcillin-clavulanic acid | 6 (7.5%) | 1 (4.76%) | 1 (5.56%) | 8 (6.72%) | 0.8847 |
| Piperacillin-tazobactam | 6 (7.5%) | 1 (4.76%) | 1 (5.56%) | 8 (6.72%) | 0.8847 | |
| Monobactam | Aztreonam | 23 (28.75%) | 15 (71.43%) | 2 (11.11%) | 40 (33.61%) | 0.0001 |
| Phosphonic acids | Fosfomycin | 80 (100%) | 21 (100%) | 18 (100%) | 119 (100%) | 1.00 |
| Polypeptide | Colistin | 66 (82.5%) | 9 (42.86%) | 14 (77.78%) | 89 (74.79%) | 0.0018 |
| Polymyxin B | 66 (82.5%) | 9 (42.86%) | 14 (77.78%) | 89 (74.79%) | 0.0018 | |
| MDR | - | 38 (47.5%) | 12 (57.14%) | 8 (44.44%) | 58 (48.74%) | 0.6785 |
| XDR | - | 8 (10%) | 2 (9.52%) | 1 (5.56%) | 11 (9.24%) | 0.8401 |
| PDR | - | 1 (1.25%) | 0 (0%) | 0 (0%) | 1 (0.84%) | 0.7821 |
| Compound | Retention Time (min) | Area under Peak | % |
|---|---|---|---|
| Benzyl alcohol | 8.904 | 34162727.81 | 16.67 |
| Linalyl iso-valerate | 14.991 | 5320755.75 | 2.6 |
| Cinnamaldehyde | 15.557 | 160046038.4 | 78.1 |
| Eugenol | 17.781 | 3073334.82 | 1.5 |
| β-Caryophyllene | 19.421 | 2312309.18 | 1.13 |
| Isolate No. | Source | Antimicrobial Resistant Pattern | MAR Index | MIC (µg/mL) | Checkerboard Result MIC (µg/mL) | Efflux Pump Activity | Anti-Efflux Pump Activity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | Cinnamon Oil | MC EtBr (µg/mL) | Index | MC EtBr (µg/mL) | Index | |||||
| 1 | Chicken cloacal swab | GEN, AK, NET, TOB, CAZ, FEP, PTZ, TIC, ATM, FF, PB, CT | 0.70 | 2 | 0.25 | 0.5/0.0312 | 4 | 15 | 2 | 7 |
| 2 | Chicken heart | GEN, AK, NET, TOB, CAZ, FEP, CIP, PTZ, TIC, ATM, FF, PB, CT | 0.76 | 4 | 0.25 | 4/0.25 | 2 | 7 | 1 | 3 |
| 3 | Chicken heart | GEN, AK, NET, TOB, CAZ, FEP, CIP, PTZ, TIC, ATM, FF, PB, CT | 0.76 | 4 | 0.125 | 4/0.125 | 4 | 15 | 2 | 7 |
| 4 | Chicken cecal content | GEN, AK, NET, TOB, CAZ, FEP, CIP, PTZ, TIC, ATM, FF, PB, CT | 0.76 | 16 | 0.125 | 8/0.0156 | 2 | 7 | 1 | 3 |
| 5 | Chicken cloacal swab | GEN, AK, NET, TOB, CAZ, FEP, CIP, LEV, ATM, FF, PB, CT | 0.70 | 128 | 0.125 | 2/0.0078 | 4 | 15 | 2 | 7 |
| 6 | Chicken liver | GEN, AK, NET, TOB, CAZ, FEP, CIP, LEV, ATM, FF, PB, CT | 0.70 | 128 | 0.25 | 4/0.0312 | 4 | 15 | 2.5 | 9 |
| 7 | Chicken liver | GEN, AK, NET, TOB, CAZ, FEP, CIP, LEV, ATM, FF, PB, CT | 0.70 | 256 | 0.25 | 8/0.0312 | 4 | 15 | 2 | 7 |
| 8 | Chicken cecal content | GEN, AK, NET, TOB, CAZ, FEP, CIP, IPM, MRP, DOR, PTZ, TIC, ATM, FF, PB, CT | 0.94 | 64 | 0.25 | 64/0.0156 | 2.5 | 9 | 1.5 | 5 |
| 9 | Chicken cloacal swab | GEN, AK, NET, TOB, IPM, MRP, DOR, CAZ, FEP, CIP, LEV, PTZ, TIC, ATM, FF, PB, CT | 1 | 32 | 0.125 | 32/0.0156 | 4 | 15 | 2.5 | 9 |
| 10 | Human burn | CAZ, FEP, CIP, LEV, PTZ, TIC, ATM, FF, PB, CT | 0.58 | 32 | 0.125 | 1/0.0078 | 1.5 | 5 | 1 | 3 |
| 11 | Human burn | GEN, AK, NET, TOB, IPM, MRP, DOR, CAZ, FEP, CIP, LEV, ATM, FF, PB, CT | 0.88 | 128 | 0.25 | 64/0.0312 | 4 | 15 | 2.5 | 9 |
| 12 | Mastitis milk | GEN, AK, NET, TOB, CAZ, FEP, PTZ, TIC, ATM, FF, PB, CT | 0.70 | 2 | 0.125 | 1/0.0156 | 2 | 7 | 1 | 3 |
| Mean ± SE | 66.333 ± 22.751 * | 0.187 ± 0.018 Π | 16.041 ± 6.914/ 0.048 ± 0.020 | 3.166 ± 0.303 ¶ | 11.667 ±1.214 ╥ | 1.75 ± 0.179 | 6.00 ± 0.717 | |||
| Target Gene | Primers Sequences 5 → 3′ | Specificity | Annealing Temperature (° C) | Product Size (bp) | References |
|---|---|---|---|---|---|
| 16S rRNA | F: GACGGGTGAGTAATGCCTA R: CACTGGTGTTCCTTCCTATA | Pseudomonas species | 54 | 618 | [54] |
| oprL | F: ATGGAAATGCTGAAATTCGGC R: CTTCTTCAGCTCGACGCGACG | P. aeruginosa and an internal control | 57 | 504 | [68,69] |
| mexA | F:ACCTACGAGGCCGACTACCAGA R: GTTGGTCACCAGGGCGCCTTC | Efflux pump gene | 61 | 179 | [70] |
| mexB | F: GTGTTCGGCTCGCAGTACTC R: AACCGTCGGGATTGACCTTG | Efflux pump gene | 244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelatti, M.A.I.; Abd El-Aziz, N.K.; El-Naenaeey, E.-s.Y.M.; Ammar, A.M.; Alharbi, N.K.; Alharthi, A.; Zakai, S.A.; Abdelkhalek, A. Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources. Antibiotics 2023, 12, 1514. https://doi.org/10.3390/antibiotics12101514
Abdelatti MAI, Abd El-Aziz NK, El-Naenaeey E-sYM, Ammar AM, Alharbi NK, Alharthi A, Zakai SA, Abdelkhalek A. Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources. Antibiotics. 2023; 12(10):1514. https://doi.org/10.3390/antibiotics12101514
Chicago/Turabian StyleAbdelatti, Mohamed A. I., Norhan K. Abd El-Aziz, El-sayed Y. M. El-Naenaeey, Ahmed M. Ammar, Nada K. Alharbi, Afaf Alharthi, Shadi A. Zakai, and Adel Abdelkhalek. 2023. "Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources" Antibiotics 12, no. 10: 1514. https://doi.org/10.3390/antibiotics12101514
APA StyleAbdelatti, M. A. I., Abd El-Aziz, N. K., El-Naenaeey, E.-s. Y. M., Ammar, A. M., Alharbi, N. K., Alharthi, A., Zakai, S. A., & Abdelkhalek, A. (2023). Antibacterial and Anti-Efflux Activities of Cinnamon Essential Oil against Pan and Extensive Drug-Resistant Pseudomonas aeruginosa Isolated from Human and Animal Sources. Antibiotics, 12(10), 1514. https://doi.org/10.3390/antibiotics12101514







