Antimicrobial Activity of Novel Ni(II) and Zn(II) Complexes with (E)-2-((5-Bromothiazol-2-yl)imino)methyl)phenol Ligand: Synthesis, Characterization and Molecular Docking Studies
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Schiff Base Ligand
2.2. Synthesis of Metal Complexes
2.3. Elemental Analysis
2.4. 1H NMR Analysis
2.5. 13C NMR Analysis
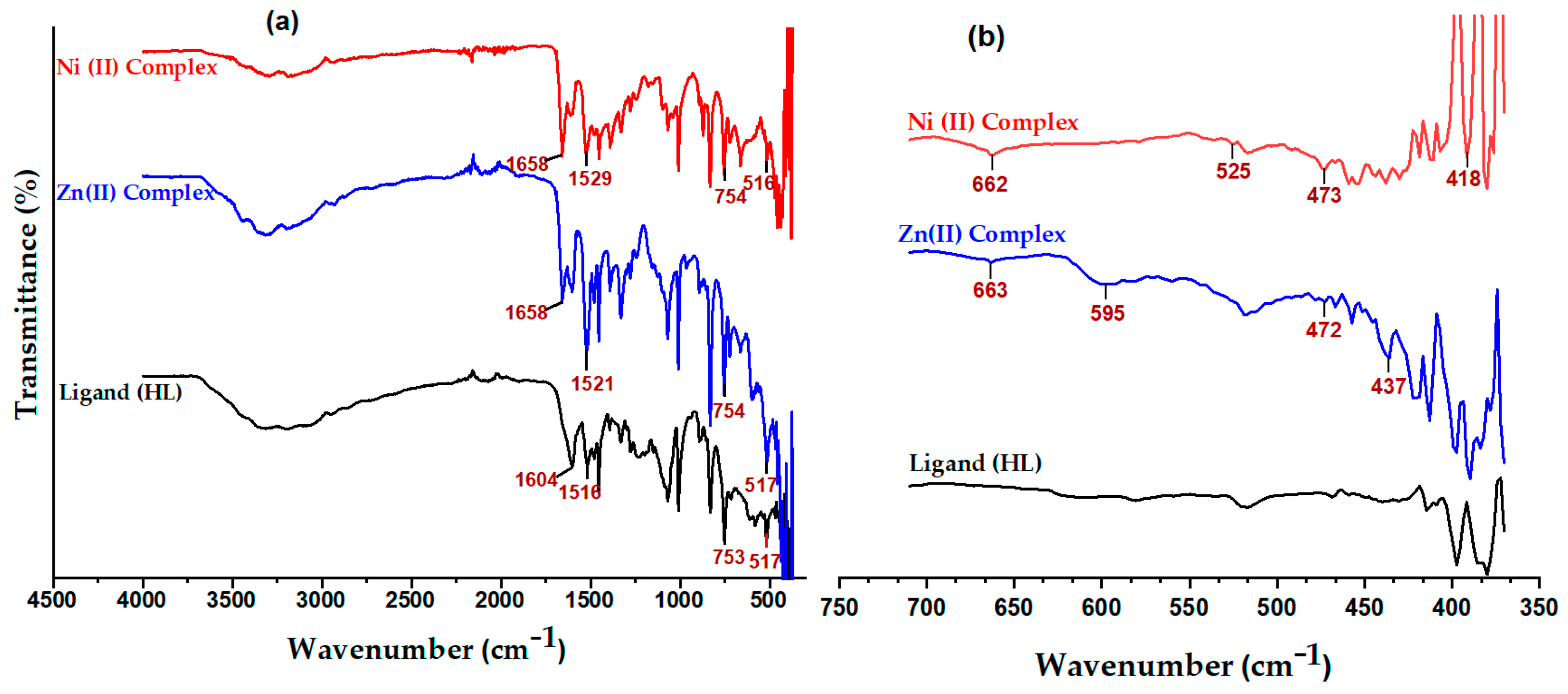
2.6. FTIR Analysis
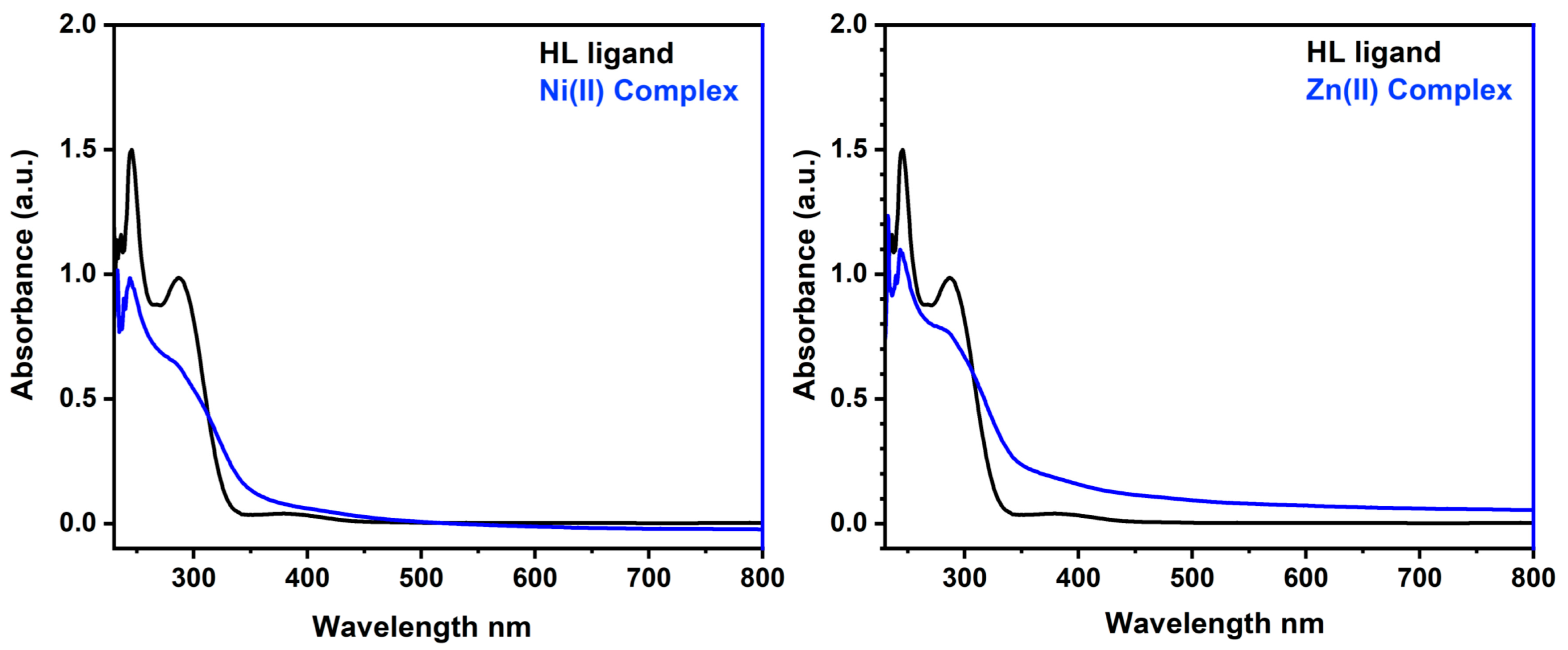
2.7. Electronic Spectra and Magnetic Moment
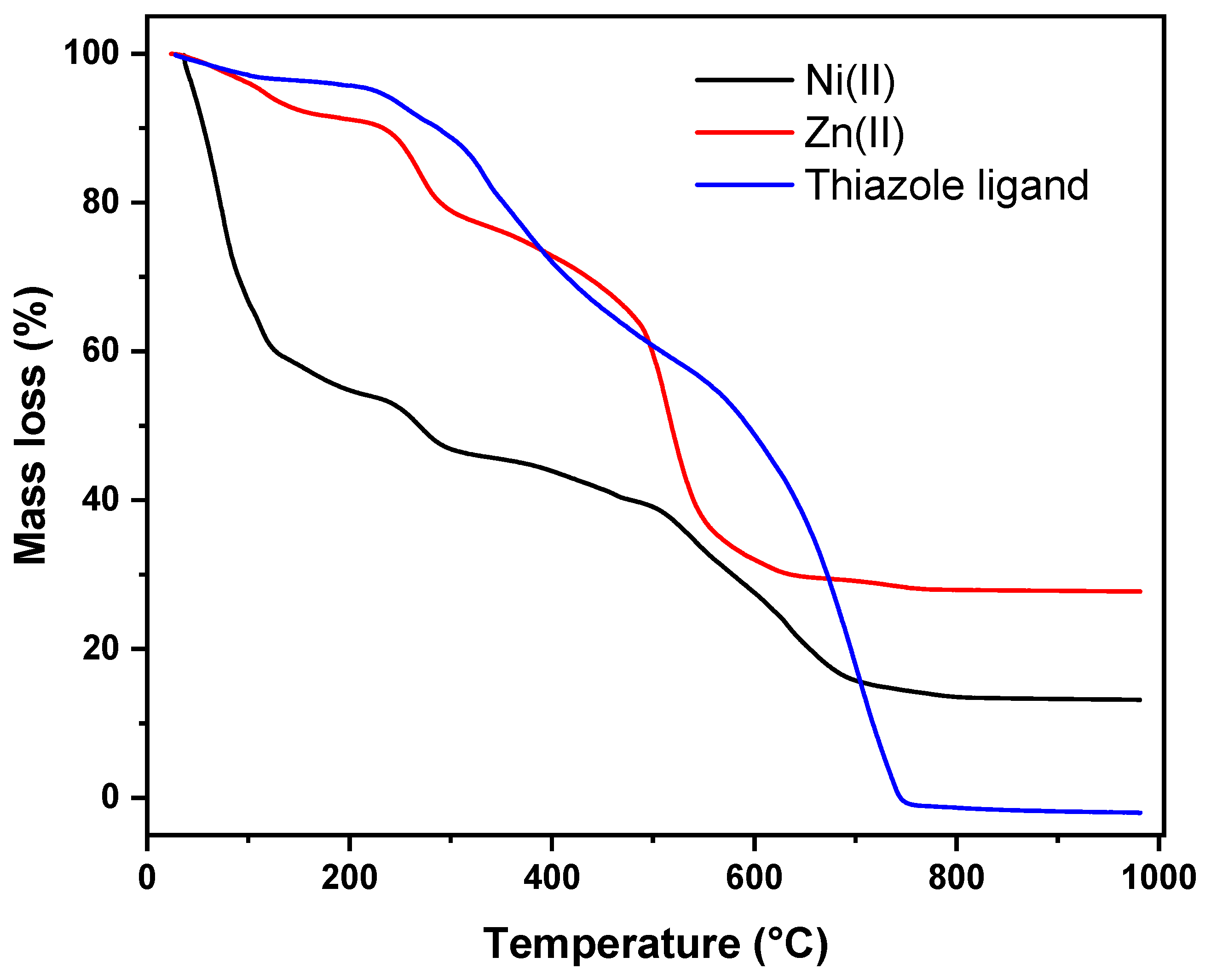
2.8. Thermal Analysis
2.9. Antibacterial Activity
2.10. Theoretical Study
Docking Study
3. Material and Methods
3.1. Chemicals
3.2. Synthesis of Thiazole Schiff Bases
3.2.1. Conventional Method(A)
3.2.2. Eco-Friendly Method(B)
3.2.3. Synthesis (E)-2-(((5-bromothiazol-2-yl)imino)methyl)phenol Complexes
3.3. Biological Tests
3.3.1. Bacterial Isolates
3.3.2. Study of Antibacterial Activity
3.4. Software Program
Molecular Docking Using MOE
3.5. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef]
- Fernández, L.; Cima-Cabal, M.D.; Duarte, A.C.; Rodriguez, A.; García, P.; García-Suárez, M.d.M. Developing diagnostic and therapeutic approaches to bacterial infections for a new era: Implications of globalization. Antibiotics 2020, 9, 916. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A. Thiazole: A versatile standalone moiety contributing to the development of various drugs and biologically active agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Borcea, A.-M.; Ionuț, I.; Crișan, O.; Oniga, O. An overview of the synthesis and antimicrobial, antiprotozoal, and antitumor activity of thiazole and bisthiazole derivatives. Molecules 2021, 26, 624. [Google Scholar] [CrossRef]
- Mohammad, H.; Reddy, P.N.; Monteleone, D.; Mayhoub, A.S.; Cushman, M.; Hammac, G.K.; Seleem, M.N. Antibacterial characterization of novel synthetic thiazole compounds against methicillin-resistant Staphylococcus pseudintermedius. PLoS ONE 2015, 10, e0130385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Xue, J.-Y.; Zhu, H.-L. Design, synthesis and antibacterial activity studies of thiazole derivatives as potent ecKAS III inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4235–4238. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Kannan, T. 2-Aminothiazole derivatives as antimycobacterial agents: Synthesis, characterization, in vitro and in silico studies. Eur. J. Med. Chem. 2014, 87, 643–656. [Google Scholar] [CrossRef]
- Bharti, S.; Nath, G.; Tilak, R.; Singh, S. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2, 4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef]
- Haroun, M.; Petrou, A.; Tratrat, C.; Kolokotroni, A.; Fesatidou, M.; Zagaliotis, P.; Gavalas, A.; Venugopala, K.N.; Sreeharsha, N.; Nair, A.B. Discovery of 5-Methylthiazole-Thiazolidinone Conjugates as Potential Anti-Inflammatory Agents: Molecular Target Identification and In Silico Studies. Molecules 2022, 27, 8137. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Altalbawy, F.M. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Int. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Chevallet, P.; Kilic, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.K.; Tripathi, R.; Katti, S.; Pannecouque, C.; De Clercq, E. Design and synthesis of 2-(2, 6-dibromophenyl)-3-heteroaryl-1, 3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem. 2008, 43, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Sever, B.; Altıntop, M.D.; Demir, Y.; Çiftçi, G.A.; Beydemir, Ş.; Özdemir, A. Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorg. Chem. 2020, 102, 104110. [Google Scholar] [CrossRef]
- Rezki, N.; Almehmadi, M.A.; Ihmaid, S.; Shehata, A.M.; Omar, A.M.; Ahmed, H.E.; Aouad, M.R. Novel scaffold hopping of potent benzothiazole and isatin analogues linked to 1, 2, 3-triazole fragment that mimic quinazoline epidermal growth factor receptor inhibitors: Synthesis, antitumor and mechanistic analyses. Bioorg. Chem. 2020, 103, 104133. [Google Scholar] [CrossRef]
- Li, M.; Zheng, K.; Chen, H.; Liu, X.; Xiao, S.; Yan, J.; Tan, X.; Zhang, N. A novel 2, 5-bis (benzo [d] thiazol-2-yl) phenol scaffold-based ratiometric fluorescent probe for sensing cysteine in aqueous solution and serum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 1–7. [Google Scholar] [CrossRef]
- GÜRSOY, A.; KARALİ, N. Synthesis, characterization and primary antituberculosis activity evaluation of 4-(3-coumarinyl)-3-benzyl-4-thiazolin-2-one benzylidenehydrazones. Turk. J. Chem. 2003, 27, 545–552. [Google Scholar]
- Poola, S.; Gundluru, M.; Nadiveedhi, M.R.; Saddala, M.S.; PTSRK, P.R.; Cirandur, S.R. Nano silver particles catalyzed synthesis, molecular docking and bioactivity of α-thiazolyl aminomethylene bisphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 409–420. [Google Scholar] [CrossRef]
- Devi, J.; Pachwania, S.; Kumar, D.; Jindal, D.K.; Jan, S.; Dash, A.K. Diorganotin (IV) complexes derived from thiazole Schiff bases: Synthesis, characterization, antimicrobial and cytotoxic studies. Res. Chem. Intermed. 2022, 48, 267–289. [Google Scholar] [CrossRef]
- Amnerkar, N.D.; Bhongade, B.A.; Bhusari, K.P. Synthesis and biological evaluation of some 4-(6-substituted-1, 3-benzothiazol-2-yl) amino-1, 3-thiazole-2-amines and their Schiff bases. Arab. J. Chem. 2015, 8, 545–552. [Google Scholar] [CrossRef]
- Saraswat, P.; Jeyabalan, G.; Hassan, M.Z.; Ahsan, M.J. Design, Synthesis and Biological Evaluation of Benzothiazole-thiophene Hybrids: A New Class of Potent Antimicrobial Agents. Anti-Infect. Agents 2018, 16, 57–63. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Narasimhan, B.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V. 4-(4-Bromophenyl)-thiazol-2-amine derivatives: Synthesis, biological activity and molecular docking study with ADME profile. BMC Chem. 2019, 13, 1–16. [Google Scholar] [CrossRef]
- Zelelew, D.; Endale, M.; Melaku, Y.; Kedir, F.; Demissie, T.B.; Ombito, J.O.; Eswaramoorthy, R. Synthesis, Antibacterial, and Antioxidant Activities of Thiazolyl-Pyrazoline Schiff Base Hybrids: A Combined Experimental and Computational Study. J. Chem. 2022, 2022, 3717826. [Google Scholar] [CrossRef]
- Shakir, M.; Hanif, S.; Sherwani, M.A.; Mohammad, O.; Azam, M.; Al-Resayes, S.I. Pharmacophore hybrid approach of new modulated bis-diimine CuII/ZnII complexes based on 5-chloro Isatin Schiff base derivatives: Synthesis, spectral studies and comparative biological assessment. J. Photochem. Photobiol. B Biol. 2016, 157, 39–56. [Google Scholar] [CrossRef]
- Al-Shemary, R.K.; Mohapatra, R.K.; Kumar, M.; Sarangi, A.K.; Azam, M.; Tuli, H.S.; Ansari, A.; Mohapatra, P.K.; Dhama, K. Synthesis, structural investigations, XRD, DFT, anticancer and molecular docking study of a series of thiazole based Schiff base metal complexes. J. Mol. Struct. 2023, 1275, 134676. [Google Scholar] [CrossRef]
- Al-Mogren, M.M.; Alaghaz, A.-N.M. Synthesis, Spectral and Quantum Chemical Calculations of Mononuclear Nickel (II), Copper (II) and Cadmium (II) Complexes of New Schiff–Base Ligand. Int. J. Electrochem. Sci. 2013, 8, 8669–8685. [Google Scholar] [CrossRef]
- Yernale, N.G.; Udayagiri, M.D.; Mruthyunjayaswam, B.H.M. Synthesis, characterization, mass spectral fragmentation, thermal study and biological evaluation of new Schiff base ligand and its metal (II) complexes derived from 4-(diethylamino) salicylaldehyde and thiazole moiety. Eur. J. Chem. 2016, 7, 56–65. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T. Antibacterial Zn (II) compounds of. Schiff bases derived from some benzothiazoles. Main Group Met. Chem. 2002, 25, 291–296. [Google Scholar]
- Al-Hiyari, B.A.; Shakya, A.K.; Naik, R.R.; Bardaweel, S. Microwave-assisted synthesis of Schiff bases of isoniazid and evaluation of their anti-proliferative and antibacterial activities. Molbank 2021, 2021, M1189. [Google Scholar] [CrossRef]
- Almalki, S.A.; Bawazeer, T.M.; Asghar, B.; Alharbi, A.; Aljohani, M.M.; Khalifa, M.E.; El-Metwaly, N. Synthesis and characterization of new thiazole-based Co (II) and Cu (II) complexes; therapeutic function of thiazole towards COVID-19 in comparing to current antivirals in treatment protocol. J. Mol. Struct. 2021, 1244, 130961. [Google Scholar] [CrossRef]
- Gavranić, M.; Kaitner, B.; Meštrović, E. Intra molecular N− H… O hydrogen bonding, quinoid effect, and partial π-electron delocalization in N-aryl Schiff bases of 2-hydroxy-1-naphthaldehyde: The crystal structures of planar N-(α-naphthyl)-and N-(β-naphthyl)-2-oxy-1-naphthaldimine. J. Chem. Crystallogr. 1996, 26, 23–28. [Google Scholar] [CrossRef]
- Etaiw, S.E.H.; Abd El-Aziz, D.M.; Abd El-Zaher, E.H.; Ali, E.A. Synthesis, spectral, antimicrobial and antitumor assessment of Schiff base derived from 2-aminobenzothiazole and its transition metal complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1331–1337. [Google Scholar] [CrossRef]
- Modrić, M.; Božičević, M.; Faraho, I.; Bosnar, M.; Škorić, I. Design, synthesis and biological evaluation of new 1, 3-thiazole derivatives as potential anti-inflammatory agents. J. Mol. Struct. 2021, 1239, 130526. [Google Scholar] [CrossRef]
- Aldelfy, Z.; Alshamkhani, Z.; Al-Assadi, M. 2-Hydroxybenzylidene-4-(4-SubstitutedPhenyl)-2-amino thiazole and their Pt (II) complexes: Synthesis, characterization and biological study. Egypt. J. Chem. 2019, 62, 1851–1867. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.M.; Labib, A.A.; Mousa, H.A.; Moustafa, S.A.; Ali, M.M.; El-Rashedy, A.A. Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 85–96. [Google Scholar] [CrossRef]
- Sunjuk, M.; Al-Najjar, L.; Shtaiwi, M.; El-Eswed, B.; Al-Noaimi, M.; Al-Essa, L.; Sweidan, K. Transition Metal Complexes of Schiff Base Ligands Prepared from Reaction of Aminobenzothiazole with Benzaldehydes. Inorganics 2022, 10, 43. [Google Scholar] [CrossRef]
- Salehi, M.; Faghani, F.; Kubicki, M.; Bayat, M. New complexes of Ni (II) and Cu (II) with tridentate ONO Schiff base ligand: Synthesis, crystal structures, electrochemical and theoretical investigation. J. Iran. Chem. Soc. 2018, 15, 2229–2240. [Google Scholar] [CrossRef]
- Gomes da Silva Dantas, F.; Araújo de Almeida-Apolonio, A.; Pires de Araújo, R.; Regiane Vizolli Favarin, L.; Fukuda de Castilho, P.; de Oliveira Galvão, F.; Inez Estivalet Svidzinski, T.; Antônio Casagrande, G.; Mari Pires de Oliveira, K. A promising copper (II) complex as antifungal and antibiofilm drug against yeast infection. Molecules 2018, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
- Alaghaz, A.-N.M.; Bayoumi, H.A. Synthesis, spectral properties and potentiometric studies on some metal Schiff base complexes derived from 4–chlorophenyl–2–aminothiazole. Int. J. Electrochem. Sci. 2013, 8, 11860–11876. [Google Scholar] [CrossRef]
- Kavitha, N.; Lakshmi, P.A. Synthesis, characterization and thermogravimetric analysis of Co (II), Ni (II), Cu (II) and Zn (II) complexes supported by ONNO tetradentate Schiff base ligand derived from hydrazino benzoxazine. J. Saudi Chem. Soc. 2017, 21, S457–S466. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Rauf, A.; Noreen, S.; Scozzafava, A.; Supuran, C.T. Antibacterial cobalt (II), nickel (II) and zinc (II) complexes of nicotinic acid-derived Schiff-bases. J. Enzym. Inhib. Med. Chem. 2002, 17, 101–106. [Google Scholar] [CrossRef]
- Abu-Yamin, A.-A.; Abduh, M.S.; Saghir, S.A.M.; Al-Gabri, N. Synthesis, characterization and biological activities of new Schiff base compound and its lanthanide complexes. Pharmaceuticals 2022, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Yernale, N.G.; Bennikallu Hire Mathada, M. Synthesis, characterization, antimicrobial, dna cleavage, and in vitro cytotoxic studies of some metal complexes of schiff base ligand derived from thiazole and quinoline moiety. Bioinorg. Chem. Appl. 2014, 2014, 314963. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, M.; Marriappan, S.; Dharmaraja, J.; Jeyakumar, T.; Muthukumaran, K. Spectral, XRD, SEM and biological activities of transition metal complexes of polydentate ligands containing thiazole moiety. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Jauch, R.; Humm, A.; Huber, R.; Wahl, M.C. Structures of Escherichia coli NAD synthetase with substrates and products reveal mechanistic rearrangements. J. Biol. Chem. 2005, 280, 15131–15140. [Google Scholar] [CrossRef]
- Taha, R.H.; Alrabie, A.; Badr, E.; Shymaa, M.; Baker, S.A. Design, synthesis, characterization, spectral, chemical analysis, biological evaluation, and molecular docking studies of novel fatty Schiff base ligands and their nickel and mercury complexes. Inorg. Chem. Commun. 2023, 158, 111382. [Google Scholar] [CrossRef]
- Mazzola, P.G.; Jozala, A.F.; Novaes, L.C.d.L.; Moriel, P.; Penna, T.C.V. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009, 45, 241–248. [Google Scholar] [CrossRef]
- Al-Qadsy, I.; Saeed, W.S.; Al-Odayni, A.-B.; Alrabie, A.; Al-Faqeeh, L.A.S.; Al-Adhreai, A.; Al-Owais, A.A.; Semlali, A.; Farooqui, M. Antidiabetic, antioxidant and cytotoxicity activities of ortho-and para-substituted Schiff bases derived from metformin hydrochloride: Validation by molecular docking and in silico ADME studies. Open Chem. 2023, 21, 20230125. [Google Scholar] [CrossRef]
- El-Shwiniy, W.H.; El-Desoky, S.I.; Alrabie, A.; Abd El-wahaab, B. Spectrophotometric determination of Zr (IV), Hg (II) and U (VI) in solution with their analytical applications: Structural characterization and molecular docking of the solid complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121400. [Google Scholar] [CrossRef]
- Arwa, A.-A.; ALSaeedy, M.; Alrabie, A.; Al-Qadsy, I.; Dawbaa, S.; Alaizeri, Z.M.; Alhadlaq, H.A.; Al-Kubati, A.; Ahamed, M.; Farooqui, M. Design and synthesis of novel enantiopure Bis (5-Isoxazolidine) derivatives: Insights into their antioxidant and antimicrobial potential via in silico drug-likeness, pharmacokinetic, medicinal chemistry properties, and molecular docking studies. Heliyon 2022, 8. [Google Scholar]
- Alrabie, A.; Al-Rabie, N.A.; Al Saeedy, M.; Al Adhreai, A.; Al-Qadsy, I.; Farooqui, M. Martynia annua safety and efficacy: Heavy metal profile, in silico and in vitro approaches on antibacterial and antidiabetic activities. Nat. Prod. Res. 2023, 37, 1016–1022. [Google Scholar] [CrossRef] [PubMed]

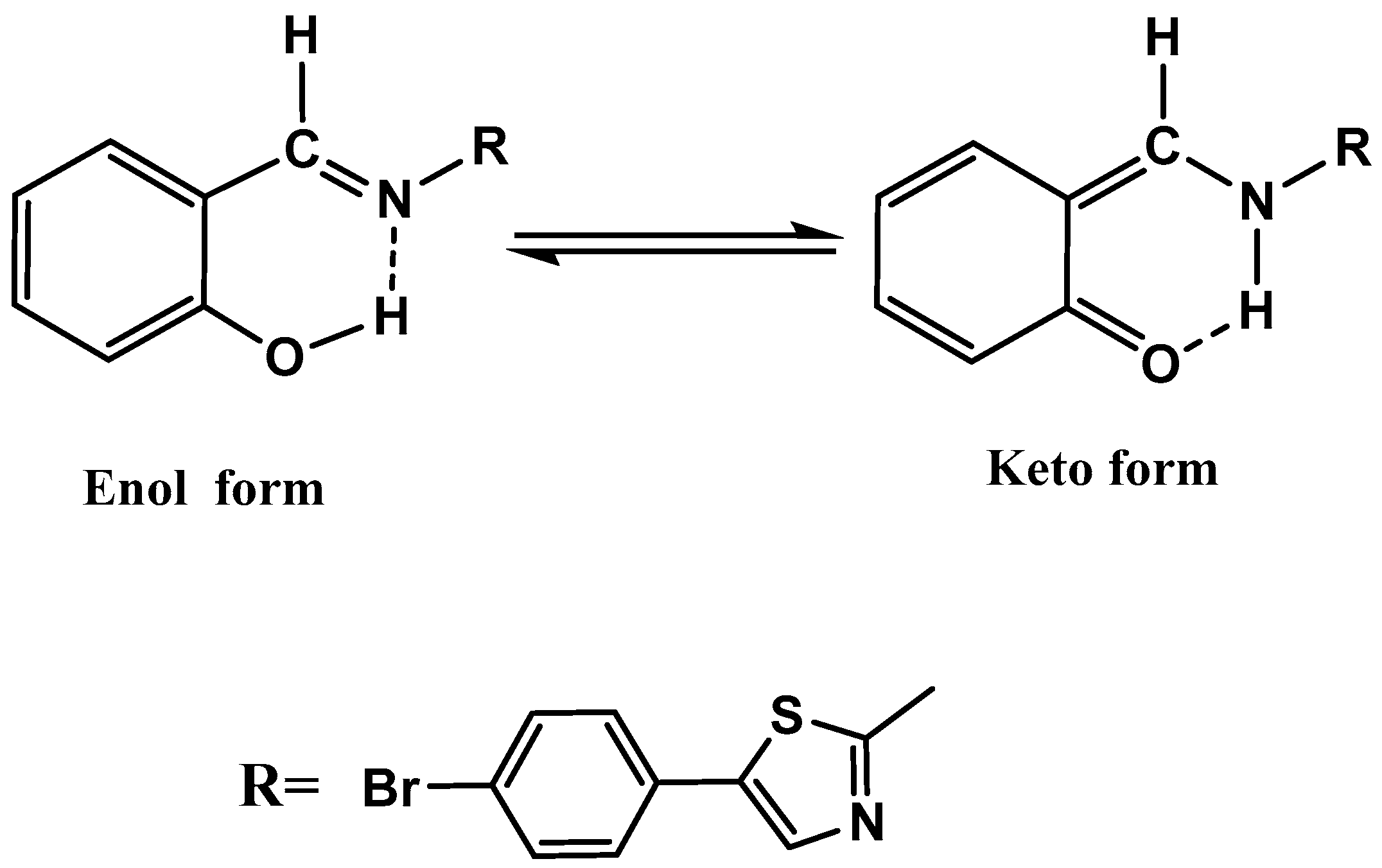

| Compound M. F. [M. wt] | Molecular Formula | Elemental Analyses % Calculated (Found) | |||
|---|---|---|---|---|---|
| C | H | N | S | ||
| HL,359.24 | C16H11BrN2OS | 53.50 (53.38) | 3.09 (3.03) | 7.80 (7.74) | 8.92 (8.87) |
| [Ni(HL)2(NO3)2]2H2O, 937.21 | C32H26Br2N6NiO10S2 | 41.01 (41.03) | 2.80 (2.78) | 8.97 (8.53) | 6.84 (6.79) |
| [Zn(HL)2(Cl)2]2H2O, 890.79 | C32H26Br2Cl2ZnN4O4S2 | 43.15 (43.09) | 2.94 (2.88) | 6.29 (6.22) | 7.19 (7.13) |
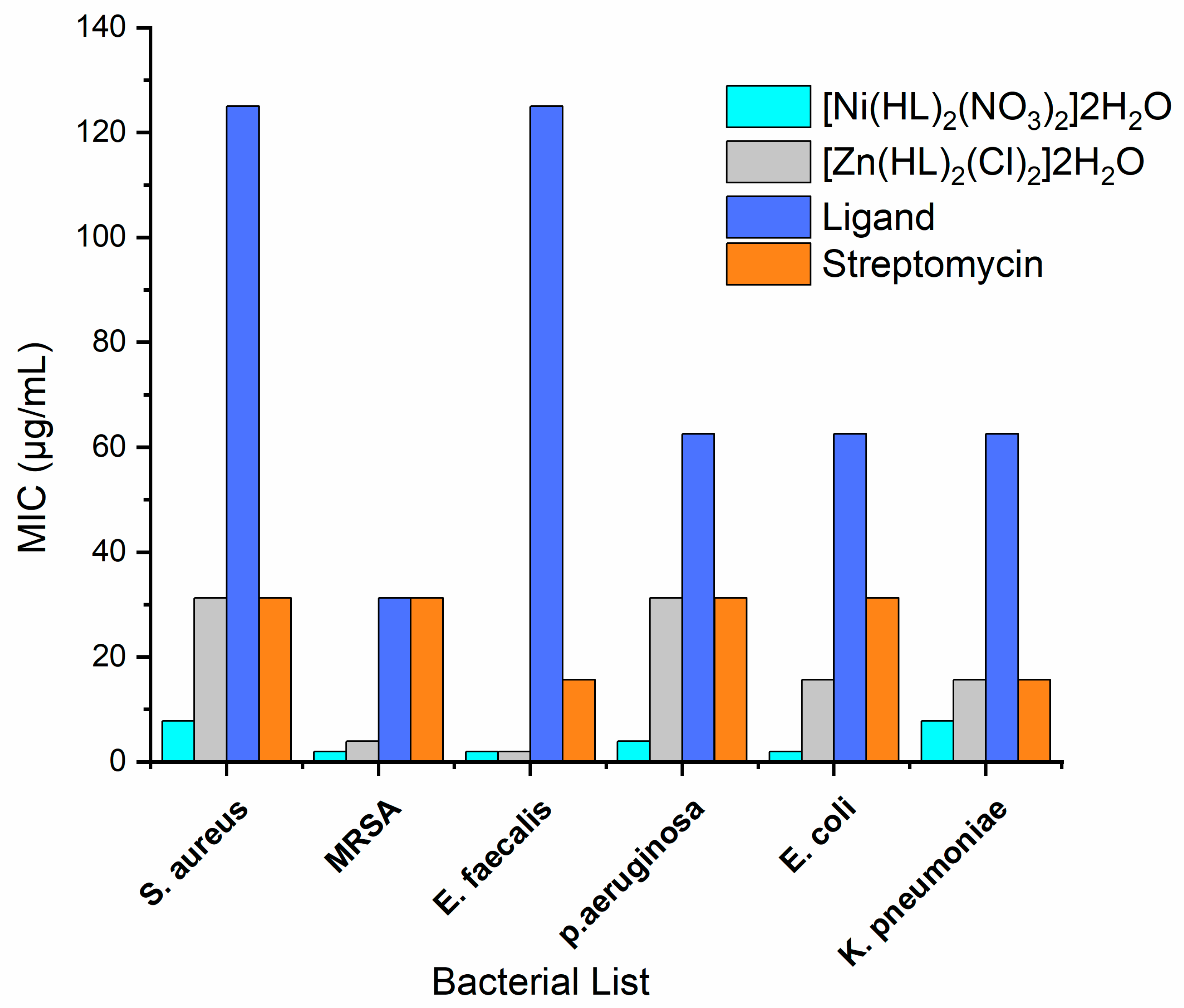
| Gram-Positive Bacteria | Gram-Negative Bacteria | |||||
|---|---|---|---|---|---|---|
| Compounds | S. aureus ATCC 25923 | MRSA ATCC 43300 | E. faecalis ATCC 29212 | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 | K. pneumoniae ATCC 700603 |
| [Ni(HL)2(NO3)2]2H2O | 7.81 | 1.95 | 1.95 | 3.91 | 1.95 | 7.81 |
| [Zn(HL)2(Cl)2]2H2O | 31.25 | 3.91 | 1.95 | 31.25 | 15.63 | 15.63 |
| Ligand(HL) | 125 | 31.25 | 125 | 62.5 | 62.5 | 62.5 |
| Streptomycin | 31.25 | 31.25 | 15.63 | 31.25 | 31.25 | 15.63 |
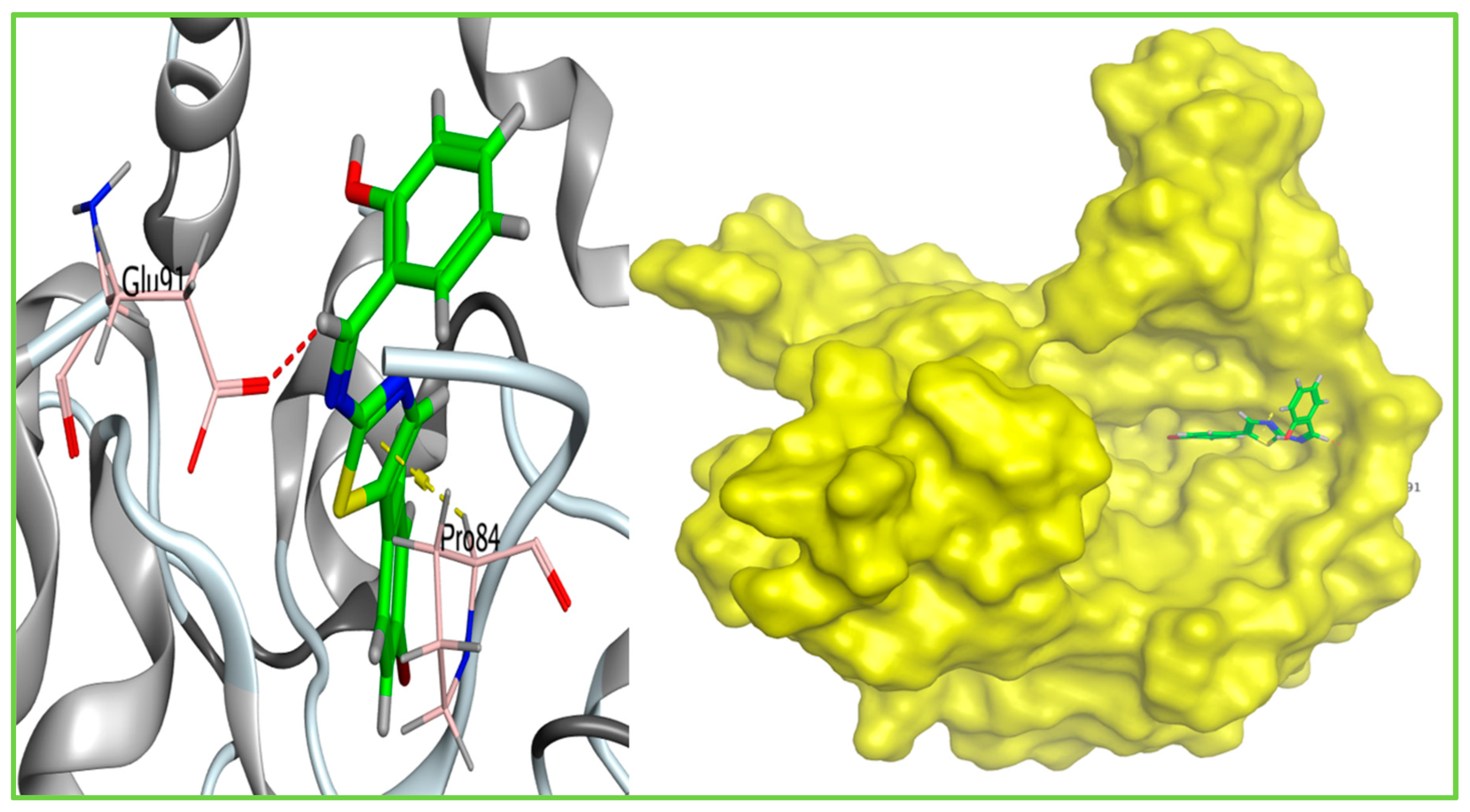
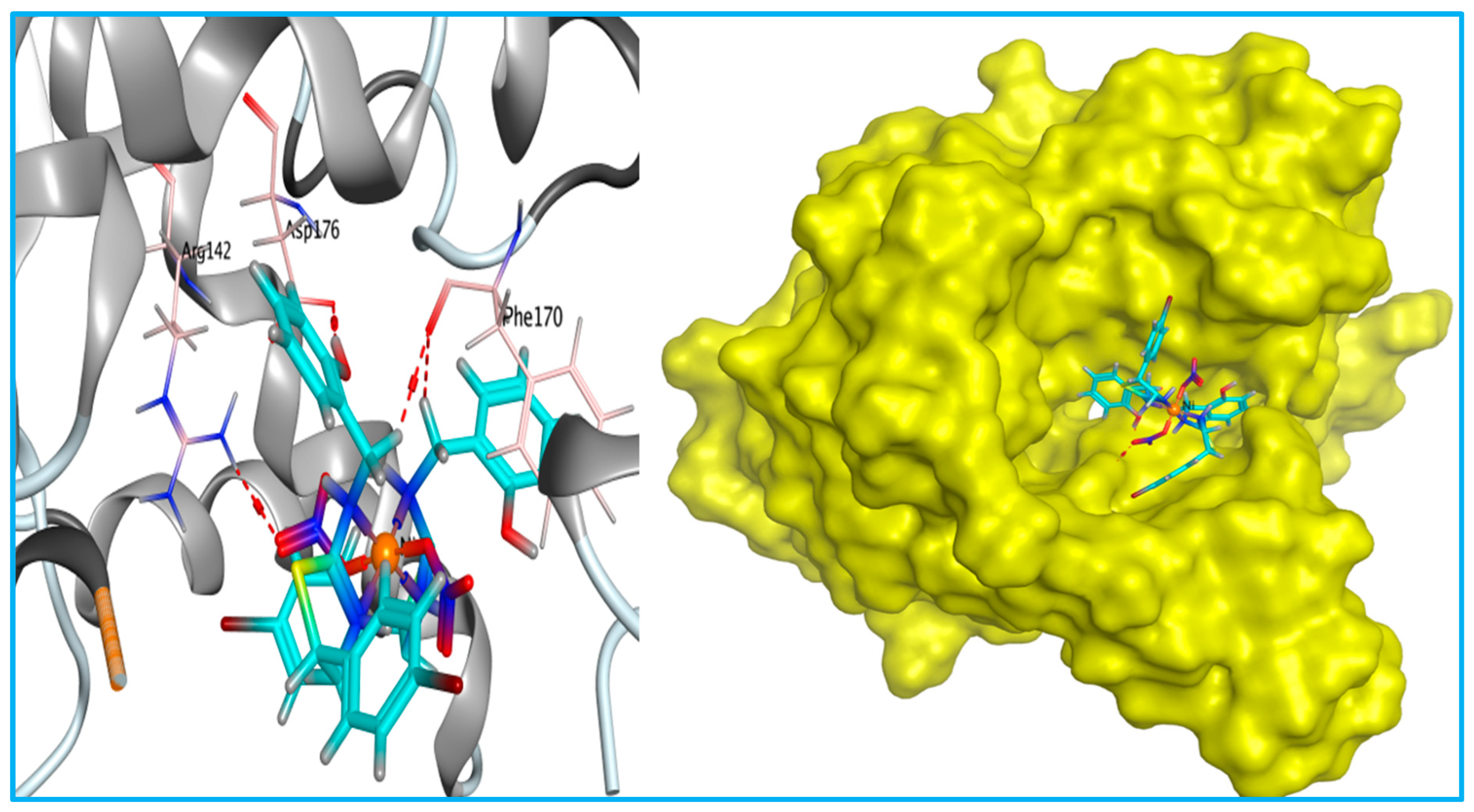
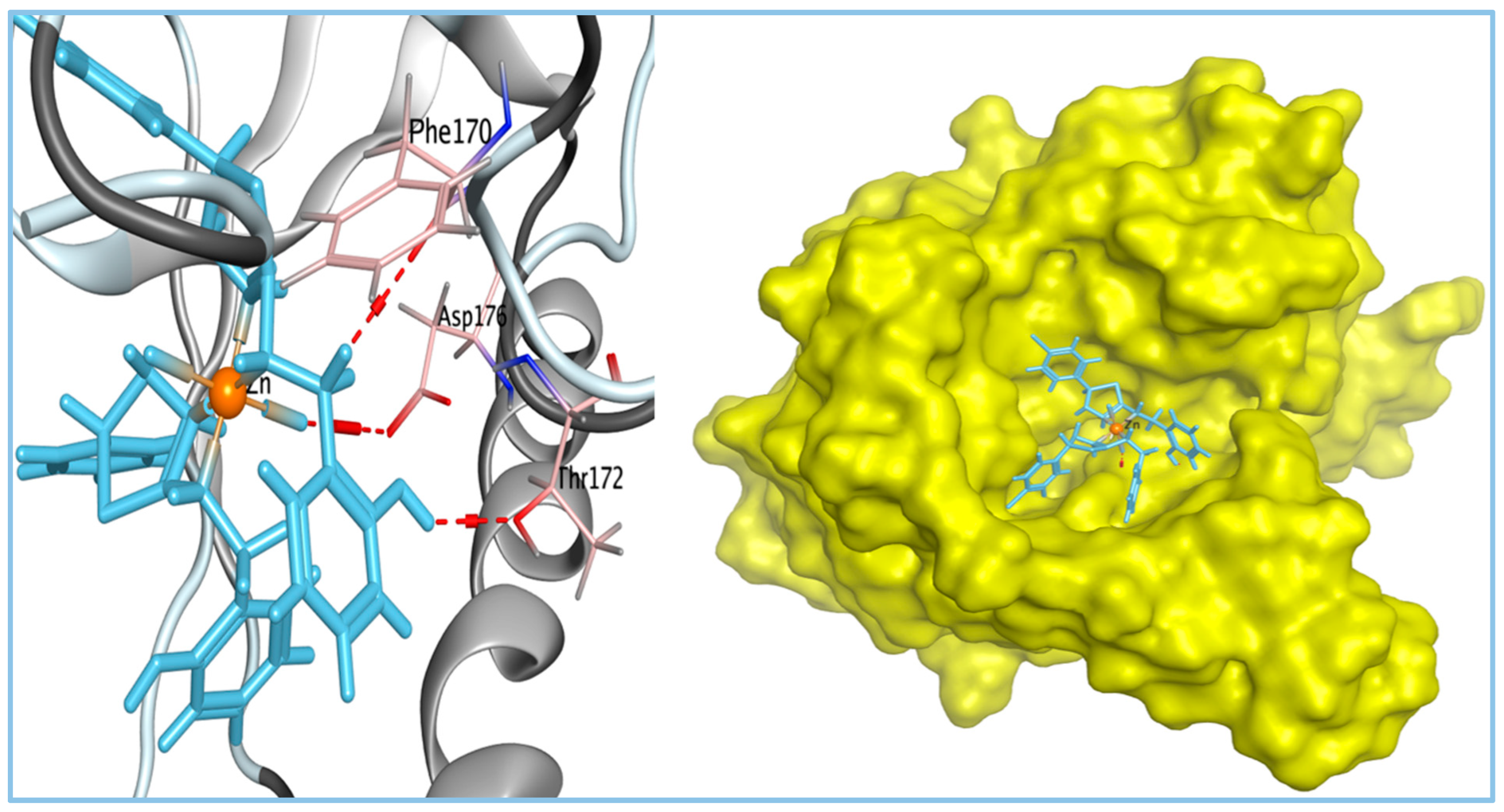
| Docking Score (Kcal/mol) | Amino Acid Residue | Type of Bond | RMSD (Kcal/mol) | Bond Length (Å) | |
|---|---|---|---|---|---|
| Ligand | −5.95 | GLU91 | H-donor | 1.04 | 3.43 |
| PRO84 | Pi-H | 3.76 | |||
| Ni complex | −7.61 | PHE170 | H-donor | 1.58 | 2.98 |
| PHE132 | H-donor | 4.36 | |||
| PHE170 | H-donor | 3.39 | |||
| ASP176 | H-donor | 3.06 | |||
| ARG142 | H-acceptor | 3.59 | |||
| Zn complex | −6.98 | PHE170 | H-donor | 1.61 | 3.08 |
| THR172 | H-donor | 2.93 | |||
| ASP176 | H-donor | 3.24 | |||
| Streptomycin | −6.29 | SER48 | H-donor | 1.86 | 2.89 |
| PHE170 | H-donor | 2.48 | |||
| ARG142 | H-acceptor | 3.02 | |||
| ASP52 | Ionic | 3.14 | |||
| ASP52 | Ionic | 3.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qadsy, I.; Saeed, W.S.; Al-Owais, A.A.; Semlali, A.; Alrabie, A.; Al-Faqeeh, L.A.S.; ALSaeedy, M.; Al-Adhreai, A.; Al-Odayni, A.-B.; Farooqui, M. Antimicrobial Activity of Novel Ni(II) and Zn(II) Complexes with (E)-2-((5-Bromothiazol-2-yl)imino)methyl)phenol Ligand: Synthesis, Characterization and Molecular Docking Studies. Antibiotics 2023, 12, 1634. https://doi.org/10.3390/antibiotics12111634
Al-Qadsy I, Saeed WS, Al-Owais AA, Semlali A, Alrabie A, Al-Faqeeh LAS, ALSaeedy M, Al-Adhreai A, Al-Odayni A-B, Farooqui M. Antimicrobial Activity of Novel Ni(II) and Zn(II) Complexes with (E)-2-((5-Bromothiazol-2-yl)imino)methyl)phenol Ligand: Synthesis, Characterization and Molecular Docking Studies. Antibiotics. 2023; 12(11):1634. https://doi.org/10.3390/antibiotics12111634
Chicago/Turabian StyleAl-Qadsy, Inas, Waseem Sharaf Saeed, Ahmad Abdulaziz Al-Owais, Abdelhabib Semlali, Ali Alrabie, Lena Ahmed Saleh Al-Faqeeh, Mohammed ALSaeedy, Arwa Al-Adhreai, Abdel-Basit Al-Odayni, and Mazahar Farooqui. 2023. "Antimicrobial Activity of Novel Ni(II) and Zn(II) Complexes with (E)-2-((5-Bromothiazol-2-yl)imino)methyl)phenol Ligand: Synthesis, Characterization and Molecular Docking Studies" Antibiotics 12, no. 11: 1634. https://doi.org/10.3390/antibiotics12111634









