Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities
Abstract
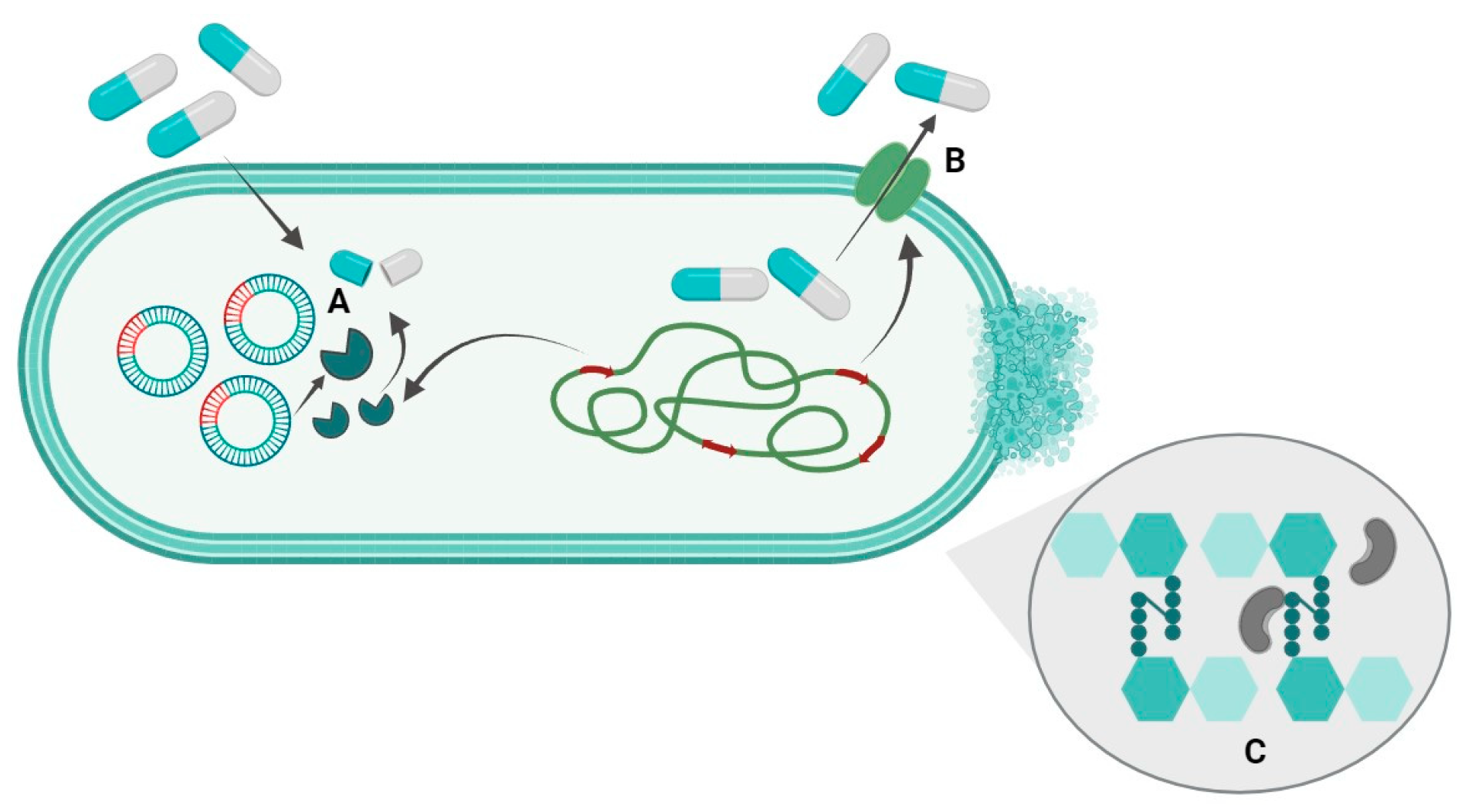
:1. Introduction
2. Results
2.1. Characteristics of the Studied Wastewater Treatment Facilities and Sewage Sludge
2.2. Antibiotic Resistance of Bacteria Isolated from Wastewater Treatment Facilities
2.3. Antibiotic Resistance of Bacteria Isolated from Sewage Sludge
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Triggiano, F.; Calia, C.; Diella, G.; Montagna, M.T.; De Giglio, O.; Caggiano, G. The Role of Urban Wastewater in the Environmental Transmission of Antimicrobial Resistance: The Current Situation in Italy (2010–2019). Microorganisms 2020, 8, 1567. [Google Scholar] [CrossRef]
- Samrot, A.V.; Wilson, S.; Preeth, R.S.S.; Prakash, P.; Sathiyasree, M.; Saigeetha, S.; Shobana, N.; Pachiyappan, S.; Rajesh, V.V. Sources of Antibiotic Contamination in Wastewater and Approaches to Their Removal—An Overview. Sustainability 2023, 15, 12639. [Google Scholar] [CrossRef]
- Lien, L.T.; Hoa, N.Q.; Chuc, N.T.; Thoa, N.T.; Phuc, H.D.; Diwan, V.; Dat, N.T.; Tamhankar, A.J.; Lundborg, C.S. Antibiotics in Wastewater of a Rural and an Urban Hospital before and after Wastewater Treatment, and the Relationship with Antibiotic Use-A One Year Study from Vietnam. Int. J. Environ. Res. Public Health 2016, 13, 588. [Google Scholar] [CrossRef]
- Jinyang, L.; Chunyan, H.; Baojiang, L.; Zhifeng, L. Dual pathway reduction of Mo4+ and photogenerated electrons restore catalytic sites to enhance heterogeneous peroxymonosulfate activation system. Chem. Eng. J. 2023, 452 Pt 3, 139246. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Perelomov, L.V.; Perelomova, I.V.; Venevtseva, U.L. The toxic effects of trace elements on male reproductive health. Hum. Physiol. 2016, 42, 454–462. [Google Scholar] [CrossRef]
- Odumbe, E.; Murunga, S.; Ndiiri, J. Heavy Metals in Wastewater Effluent: Causes, Effects, and Removal Technologies. In Trace Metals in the Environment; Joseph, D., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Feng, J.; Burke, I.T.; Chen, X.; Stewart, D.I. Assessing metal contamination and speciation in sewage sludge: Implications for soil application and environmental risk. Rev. Environ. Sci. Biotechnol. 2023, 22, 1037–1058. [Google Scholar] [CrossRef]
- Sörme, L.; Lagerkvist, R. Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, S.; Kloosterman, A.M.; van Hoesel, S.F.A.; van Wezel, G.P.; Rozen, D.E. Competition Sensing Changes Antibiotic Production in Streptomyces. mBio 2021, 12, e02729-20. [Google Scholar] [CrossRef]
- O’Brien, J.; Wright, G.D. An ecological perspective of microbial secondary metabolism. Curr. Opin. Biotechnol. 2011, 22, 552–558. [Google Scholar] [CrossRef]
- Hazra, M.; Joshi, H.; Williams, J.B.; Watts, J.E.M. Antibiotics and antibiotic resistant bacteria/genes in urban wastewater: A comparison of their fate in conventional treatment systems and constructed wetlands. Chemosphere 2022, 303 Pt 2, 135148. [Google Scholar] [CrossRef]
- Stepanauskas, R.; Glenn, T.C.; Jagoe, C.H.; Tuckfield, R.C.; Lindell, A.H.; McArthur, J.V. Elevated microbial tolerance to metals and antibiotics in metal-contaminated industrial environments. Environ. Sci. Technol. 2005, 39, 3671–3678. [Google Scholar] [CrossRef]
- Perelomov, L.; Sizova, O.; Rahman, M.M.; Perelomova, I.; Minkina, T.; Sokolov, S.; Atroshchenko, Y. Metal-Tolerant Bacteria of Wastewater Treatment Plant in a Large City. Sustainability 2022, 14, 11335. [Google Scholar] [CrossRef]
- Pais, G.M.; Chang, J.; Barreto, E.F.; Stitt, G.; Downes, K.J.; Alshaer, M.H.; Lesniki, E.; Panchal, V.; Bruzzone, M.; Bumanglag, A.V.; et al. Clinical pharmacokinetics and pharmacodynamics of cefepime. Clin. Pharmacokinet. 2022, 61, 929–953. [Google Scholar] [CrossRef]
- O’Connor, A.; Lopez, M.J.; Eranki, A.P. Cefepime; StatPearls Publishing: St. Petersburg, CA, USA, 2023. [Google Scholar]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Mantengoli, E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2005, 4, 17–32. [Google Scholar] [CrossRef]
- Daikos, G.L.; da Cunha, C.A.; Rossolini, G.M.; Stone, G.G.; Baillon-Plot, N.; Tawadrous, M.; Irani, P. Review of Ceftazidime-Avibactam for the Treatment of Infections Caused by Pseudomonas aeruginosa. Antibiotics 2021, 10, 1126. [Google Scholar] [CrossRef]
- Del Barrio-Tofiño, E.; López-Causapé, C.; Cabot, G.; Rivera, A.; Benito, N.; Segura, C.; Montero, M.M.; Sorlí, L.; Tubau, F.; Gómez-Zorrilla, S.; et al. Genomics and Susceptibility Profiles of Extensively Drug-Resistant Pseudomonas aeruginosa Isolates from Spain. Antimicrob. Agents Chemother. 2017, 61, e01589-17. [Google Scholar] [CrossRef]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of Action of Carbapenem Resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef]
- Springer, B.; Kidan, Y.G.; Prammananan, T.; Ellrott, K.; Böttger, E.C.; Sander, P. Mechanisms of streptomycin resistance: Selection of mutations in the 16S rRNA gene conferring resistance. Antimicrob. Agents Chemother. 2001, 45, 2877–2884. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef]
- Dinos, G.; Athanassopoulos, C.; Missiri, D.; Giannopoulou, P.; Vlachogiannis, I.; Papadopoulos, G.; Kalpaxis, D. Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics 2016, 5, 20. [Google Scholar] [CrossRef]
- Narendrakumar, L.; Chakraborty, M.; Kumari, S.; Paul, D.; Das, B. β-Lactam potentiators to re-sensitize resistant pathogens: Discovery, development, clinical use and the way forward. Front. Microbiol. 2023, 13, 1092556. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Arena, F.; Giani, T. Mechanisms of Antibacterial Resistance. In Infectious Diseases; Cohen, J., William, G., Steven, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1181–1196. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.J.; Huovinen, T.; Fishwick, C.W.; Chopra, I. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob. Agents Chemother. 2006, 50, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Brandis, G. Rifampicin Resistance: Fitness Costs and the Significance of Compensatory Evolution. Antibiotics 2013, 2, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.V.; Zanetti, M.O.; Pitondo-Silva, A.; Stehling, E.G. Aquatic environments polluted with antibiotics and heavy metals: A human health hazard. Environ. Sci. Pollut. Res. Int. 2014, 21, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Touahir, N.; Alouache, S.; Dehane, D. Assessment and characterization of heavy metals resistance bacteria isolated in Southwestern Mediterranean coastal waters (Bou-Ismail Bay): Impacts of anthropogenic activities. Mar. Pollut. Bull. 2023, 192, 115085. [Google Scholar] [CrossRef]
- Edet, U.O.; Bassey, I.U.; Joseph, A.P. Heavy metal co-resistance with antibiotics amongst bacteria isolates from an open dumpsite soil. Heliyon 2023, 9, e13457. [Google Scholar] [CrossRef]
- Sajjad, W.; Ali, B.; Niu, H.; Ilahi, N.; Rafiq, M.; Bahadur, A.; Banerjee, A.; Kang, S. High prevalence of antibiotic-resistant and metal-tolerant cultivable bacteria in remote glacier environment. Environ. Res. 2023, 239 Pt 2, 117444. [Google Scholar] [CrossRef]
- Fu, Y.; Ying, Z.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Kanekar, P.P.; Kanekar, S.P. Metallophilic, Metal-Resistant, and Metal-Tolerant Microorganisms. In Diversity and Biotechnology of Extremophilic Microorganisms from India; Microorganisms for Sustainability; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Sabry, S.A.; Ghozlan, H.A.; Abou-Zeid, D.M. Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. J. Appl. Microbiol. 1997, 82, 245–252. [Google Scholar] [CrossRef]
- Khaira, M.B.; Yusuf, M.B.; Khan, F. Insights to antimicrobial resistance: Heavy metals can inhibit antibiotic resistance in bacteria isolated from wastewater. Environ. Monit. Assess. 2022, 194, 252. [Google Scholar] [CrossRef]
- Perelomov, L.V. The Role of Interactions between Bacteria and Clay Minerals in Pedochemical Processes. Geochem. Int. 2023, 61, 1026–1035. [Google Scholar] [CrossRef]
- Cai, L.; Ju, F.; Zhang, T. Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2014, 98, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/18.11.2023 (accessed on 31 October 2023).
| TE | Concentration of TE in Sewage Sludge of Different Ages | |||||||
|---|---|---|---|---|---|---|---|---|
| Sewage Sludge | Treatment Facilities | |||||||
| Fresh | 1 Months | 6 Months | 1 Year | 5 Years | Secondary Sedimentation Tank (Active Sludge) | Secondary Sedimentation Tank (Water) | Digester (Suspension) | |
| Co | 28.6 | 28.8 | 27.9 | 30.4 | 309.0 | not determined | ||
| Ni | 66.8 | 33.3 | 72.3 | 50.1 | 69.0 | 0.0041 | 0.0089 | 0.1204 |
| Cu | 228.4 | 171.1 | 269.2 | 272.2 | 102.6 | 0.0037 | 0.0056 | 0.0248 |
| Zn | 1119.6 | 901.4 | 1791.2 | 1865.8 | 2360.8 | 0.00001 | 0.0002 | 0.0002 |
| Cd | 8.7 | 2.9 | 10.8 | 4.0 | 33.0 | 0.0037 | 0.0053 | 0.0186 |
| Pb | 38.0 | 27.1 | 37.4 | 27.6 | 60.0 | 0.0038 | <0.0001 | 0.0050 |
| Strain, Tolerance to the Metal(loid) and Origin | Antibiotic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group of β-Lactam Antibiotics | Group of Aminoglycosides | Group of Amphenicols | ||||||||
| Subgroup of Cephalosporins | Subgroup of Carbapenems | |||||||||
| Cefepime | Ceftazidime | Meropenem | Streptomycin | Kanamycin | Chloramphenicol | |||||
| μg/mg | ||||||||||
| 20 | 40 | 20 | 40 | 20 | 50 | 100 | 50 | 50 | 100 | |
| Serratia proteamaculans, (5 mM Ni) from methane tank | − | − | + | + | − | − | − | − | − | − |
| Pseudomonas gessardii, (3 mM Ni) from sludge of secondary sedimentation tank | − | − | + | − | − | − | − | − | + | + |
| Pseudomonas fragi, (3 mM Cd) from sludge of secondary sedimentation tank | − | − | − | − | − | − | − | − | + | + |
| Pseudomonas fragi, (3 mM Cd) from water of secondary sedimentation tank | − | − | − | − | − | − | − | − | + | + |
| Serratia proteamaculans, (5 mM Pb) from sludge of secondary sedimentation tank | − | − | + | + | − | − | − | − | − | − |
| Pseudomonas fragi, (3 mM Pb) from water of secondary sedimentation tank | − | − | − | − | − | − | − | − | + | + |
| Pseudomonas brenneri, (3 mM Pb) from water of secondary sedimentation tank | − | − | − | − | − | − | − | − | + | + |
| Pseudomonas gessardii, (5 mM Zn) from sludge of secondary sedimentation tank | + | − | − | − | − | + | + | + | + | + |
| Pseudomonas gessardii. (5 mM Zn) from water of secondary sedimentation tank | + | + | + | − | − | − | − | − | + | + |
| Klebsiella pneumonia. (3 mM Cu) from sludge of secondary sedimentation tank | − | − | − | − | − | − | − | − | − | − |
| Strain, Tolerance to the Metal(loid) | Subgroup of Penicillins | Subgroup of Cephalosporins | Subgroup of Carbapenems | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Penicillin | Carbenicillin | Cefotaxime | Ceftriaxone | Ceftazidime | Meropenem | ||||||||
| μg/mg | ||||||||||||||
| 50 | 100 | 50 | 100 | 50 | 100 | 20 | 40 | 20 | 40 | 20 | 40 | 20 | 40 | |
| Serratia fonticola SS0-1 (5 mmol Cu, 3 mmol Pb) | + | + | + | + | + | + | + | + | + | + | + | − | + | + |
| Rhodococcus qingshengii SS60-2 (5 mmol Co, 3 mmol Ni, Pb, Cu) | + | + | − | − | − | − | − | − | − | − | + | + | − | − |
| Rhodococcus qingshengii SS6-3 (5 mmol Ni, 3 mmol Pb, Cu) | + | + | − | − | − | − | − | − | − | − | + | + | − | − |
| Pseudomonas fragi SS0-4 (3 mmol Cd, Zn, Cu, Pb) | + | + | + | + | + | + | + | + | − | − | − | − | − | − |
| Stenotrophomo-nas maltophilia SS0-5 (3 mmol Zn 3 mmol Cu) | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Pseudomonas extremaustralis SS0-6 (5 mmol Zn, 3 mmol Cu, Pb) | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| Pseudomonas cedrina SS60-7 (5 mmol Zn 3 mmol Cu) | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| Serratia liquefaciens SS60-8 (5 mmol Zn, 3 mmol Cu) | + | + | + | + | + | + | + | + | + | + | + | + | − | − |
| Serratia fonticola SS0-9 (5 mmol Pb, 3 mmol Ni) | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| Stenotrophomonas maltophilia SS0-10 (5 mmol Pb, 3 mmol Zn) | + | + | + | + | + | + | + | + | + | + | − | − | + | + |
| Serratia fonticola SS12-11 (5 mmol Pb, 3 mmol Cu) | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| Citrobacter freundii SS60-12 (5 mmol Pb, 3 mmol Zn) | + | + | + | + | − | − | − | − | − | − | − | − | − | − |
| Strain, Tolerance to Metal(loids) | Antibiotics | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group of Tetracyclines | Group of Aminoglycosides | |||||||||||||||
| Tetracycline | Chlortetracycline | Streptomycin | Amikacin | Gentamicin | Neomycin | Novobiocin | Kanamycin | |||||||||
| μg/mg | ||||||||||||||||
| 20 | 40 | 20 | 40 | 50 | 100 | 20 | 40 | 20 | 40 | 20 | 40 | 20 | 40 | 20 | 40 | |
| Serratia fonticola SS0-1 5 mmol Cu, 3 mmol Pb | + | + | + | + | + | + | + | + | − | − | + | + | + | + | + | − |
| Rhodococcus qingshengii SS60-2 5 mmol Co, 3 mmol Ni, Pb, Cu | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| Rhodococcus qingshengii SS6-3 5 mmol Ni, 3 mmol Pb, Cu | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| Pseudomonas fragi SS0-4 3 mmol Cd, Zn, Cu, Pb | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Stenotrophomonas maltophilia SS0-5 3 mmol Zn, 3 mmol Cu | + | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Pseudomonas extremaustralis SS0-6 5 mmol Zn, 3 mmol Cu, Pb | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Pseudomonas cedrina SS60-7 5 mmol Zn, 3 mmol Cu | + | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Serratia liquefaciens SS60-8 5 mmol Zn, 3 mmol Cu | + | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Serratia fonticola SS0-9 5 mmol Pb, 3 mmol Ni | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Stenotrophomonas maltophilia SS0-10 5 mmol Pb, 3 mmol Zn | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Serratia fonticola SS12-11 5 mmol Pb, 3 mmol Cu | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Citrobacter freundii SS60-12 5 mmol Pb, 3 mmol Zn | + | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − |
| Strain, Tolerance to Metal(loids) | Antibiotics | |||||
|---|---|---|---|---|---|---|
| Group of Diaminopyrimidines | Group of Amphenicols | Group of Ansamycins | ||||
| Trimethoprim | Chloramphenicol | Rifampicin | ||||
| μg/mg | ||||||
| 20 | 40 | 25 | 50 | 50 | 100 | |
| Serratia fonticola SS0-1 5 mmol Cu, 3 mmol Pb | + | − | + | + | + | + |
| Rhodococcus qingshengii SS60-2 5 mmol Co, 3 mmol Ni, Pb, Cu | + | − | − | − | − | − |
| Rhodococcus qingshengii SS6-3 5 mmol Ni, 3 mmol Pb, Cu | + | − | − | − | − | − |
| Pseudomonas fragi SS0-4 3 mmol Cd, Zn, Cu, Pb | + | − | + | + | − | − |
| Stenotrophomonas maltophilia SS0-5 3 mmol Zn, 3 mmol Cu | + | + | − | − | + | − |
| Pseudomonas extremaustralis SS0-6 5 mmol Zn, 3 mmol Cu, Pb | + | + | + | + | − | − |
| Pseudomonas cedrina SS60-7 5 mmol Zn, 3 mmol Cu | + | + | + | + | − | − |
| Serratia liquefaciens SS60-8 5 mmol Zn, 3 mmol Cu | + | + | + | + | + | − |
| Serratia fonticola SS0-9 5 mmol Pb, 3 mmol Ni | − | − | − | − | − | − |
| Stenotrophomonas maltophilia SS0-10 5 mmol Pb, 3 mmol Zn | + | − | − | − | − | − |
| Serratia fonticola SS12-11 5 mmol Pb, 3 mmol Cu | − | − | − | − | + | − |
| Citrobacter freundii SS60-12 5 mmol Pb, 3 mmol Zn | + | − | + | − | + | − |
| Antibiotic | Concentration in the LB Medium µg/mL | Antibiotic | Concentration in the LB Medium µg/mL |
|---|---|---|---|
| Amikacin | 20, 40 | Kanamycin | 20, 40 |
| Ampicillin | 50, 100 | Meropenem | 20, 40 |
| Gentamicin | 20, 40 | Neomycin | 20, 40 |
| Carbenicillin | 20, 40 | Novobiocin | 20, 40 |
| Cefepime | 20,40 | Penicillin | 50, 100 |
| Cefotaxime | 20, 40 | Rifampicin | 50, 100 |
| Ceftazidime | 20, 40 | Streptomycin | 50, 100 |
| Ceftriaxone | 20, 40 | Tetracycline | 20, 40 |
| Chloramphenicol | 25, 50 | Trimethoprim | 20, 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelomov, L.; Sizova, O.; Gertsen, M.; Perelomova, I.; Arlyapov, V.; Atroshchenko, Y. Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities. Antibiotics 2023, 12, 1678. https://doi.org/10.3390/antibiotics12121678
Perelomov L, Sizova O, Gertsen M, Perelomova I, Arlyapov V, Atroshchenko Y. Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities. Antibiotics. 2023; 12(12):1678. https://doi.org/10.3390/antibiotics12121678
Chicago/Turabian StylePerelomov, Leonid, Olga Sizova, Maria Gertsen, Irina Perelomova, Vyacheslav Arlyapov, and Yury Atroshchenko. 2023. "Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities" Antibiotics 12, no. 12: 1678. https://doi.org/10.3390/antibiotics12121678
APA StylePerelomov, L., Sizova, O., Gertsen, M., Perelomova, I., Arlyapov, V., & Atroshchenko, Y. (2023). Antibiotic Resistance in Metal-Tolerant Microorganisms from Treatment Facilities. Antibiotics, 12(12), 1678. https://doi.org/10.3390/antibiotics12121678










