Activity of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety against Clinical HLAR and VRE Enterococcus spp.
Abstract
:1. Introduction
2. Results
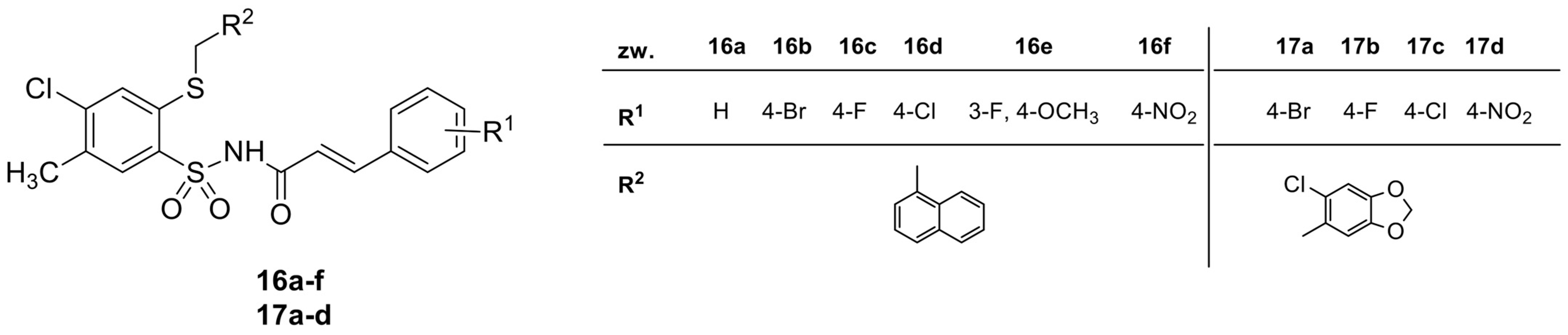
2.1. Synthesis and Chemical Characterization of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety
2.2. Antibacterial Activity of Cinnamic Acid Derivatives
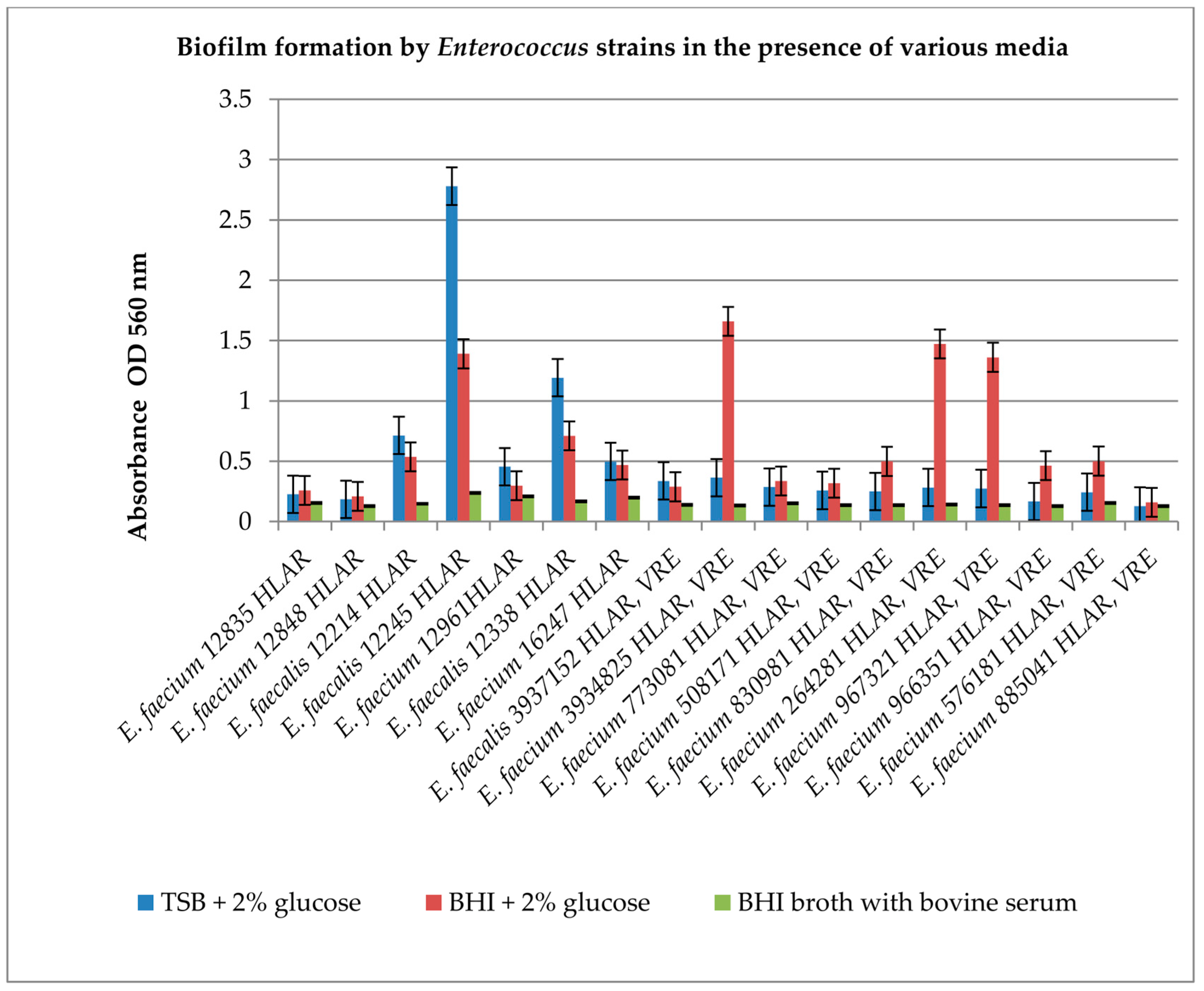
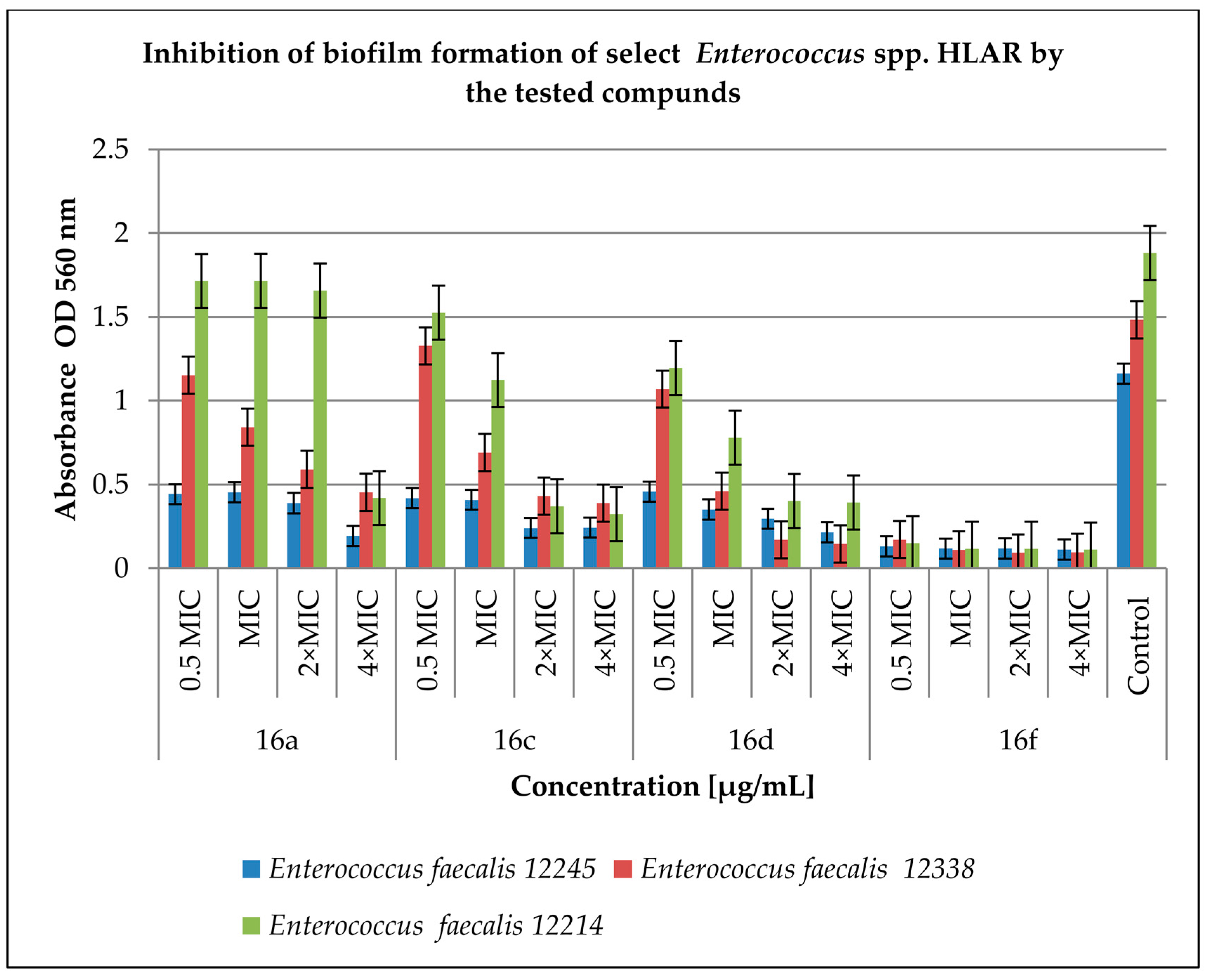
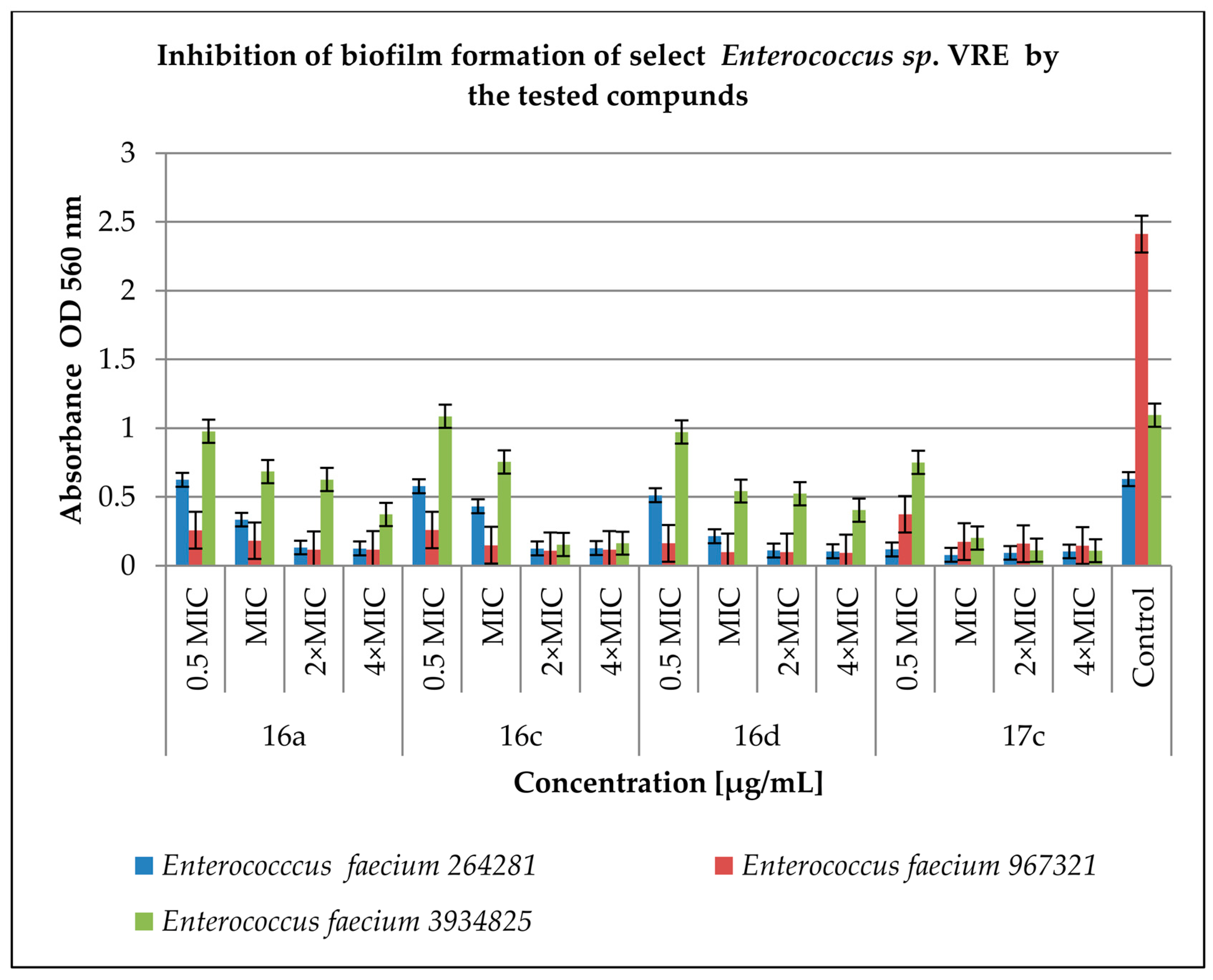
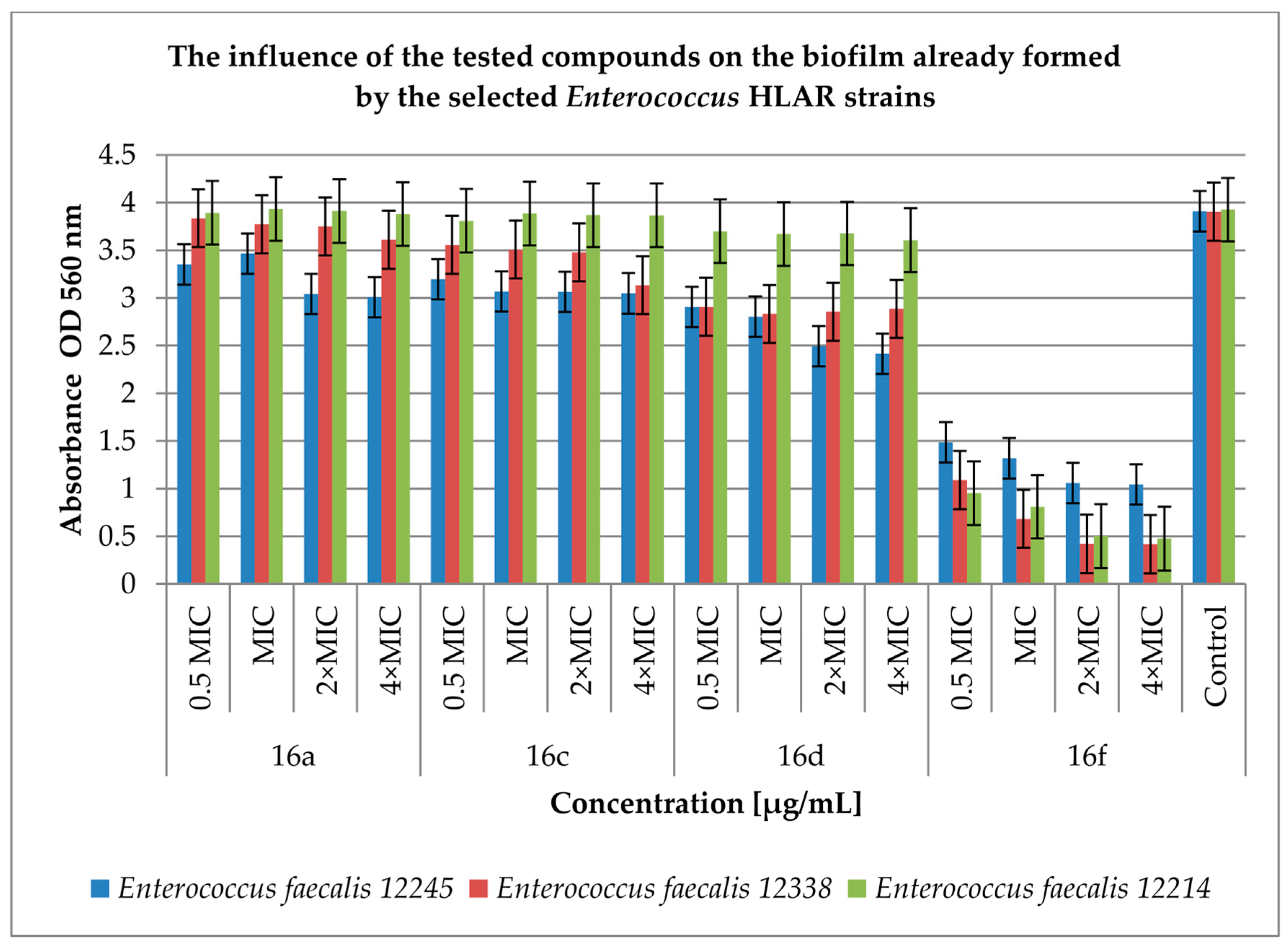
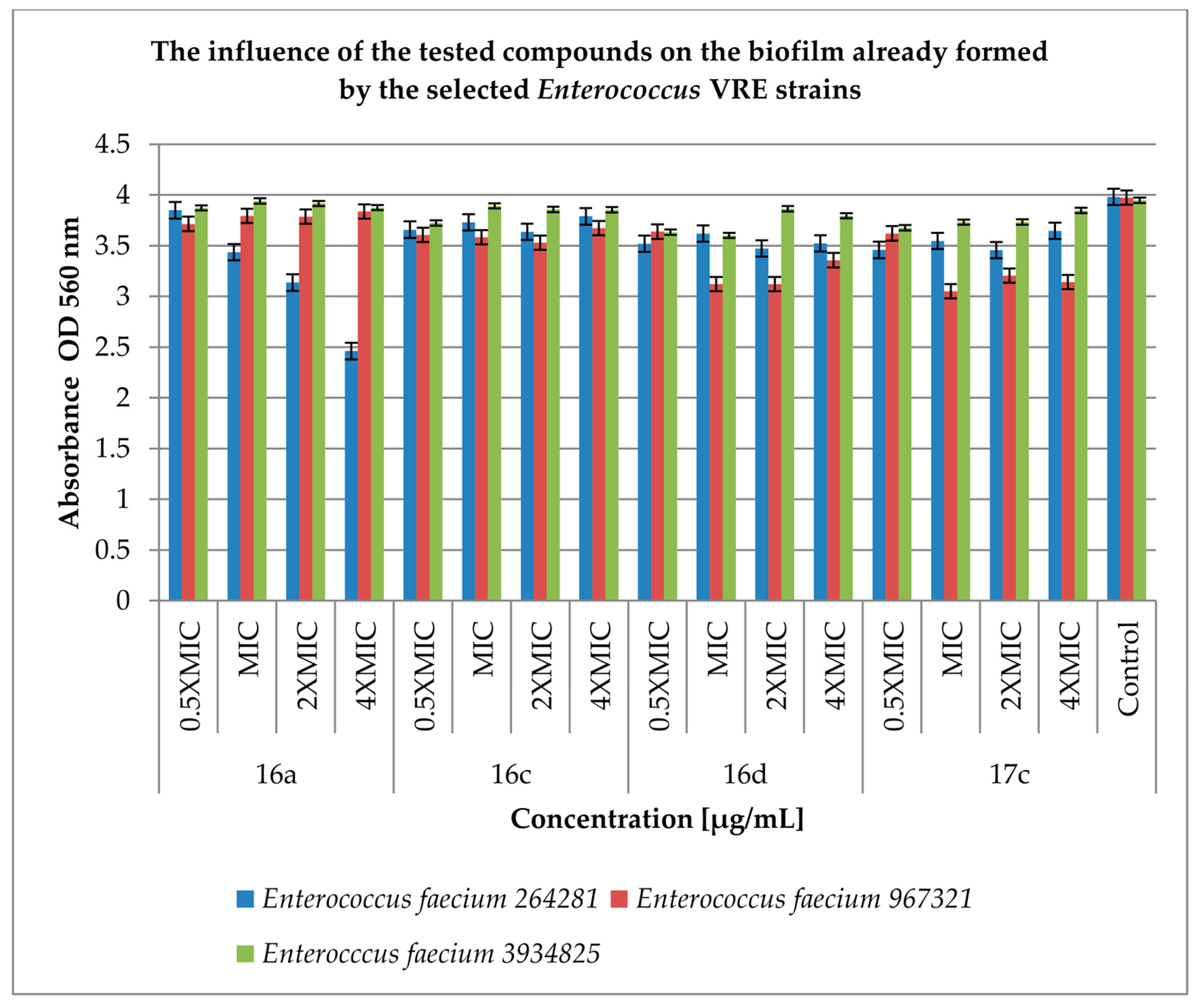
2.3. Effect of Derivatives on the Biofilm Produced by Enterococcus spp.
2.4. Blood Bacteriostatic Activity Tests
2.5. Study of Interactions of Cinnamic Acid Derivatives with Antibiotics on Resistant Strains of Enterococcus spp.
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of Susceptibility to Antibiotics by the Agar Disc Diffusion Method
4.3. Minimum Inhibitory Concentration Determination
4.4. Inhibition of Biofilm (Prior to Biofilm and Post-Biofilm) Formation by the Cinnamic Acid Derivatives
4.5. Blood Bacteriostatic Activity Tests
4.6. Checkerboard Arrays for Planktonic Bacteria (Fractional Inhibitory Concentration Index)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.A.A.; Neelakantan, P. Antibiofilm activity of phytochemicals against Enterococcus faecalis: A literature review. Phytother. Res. 2022, 36, 2824–2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Suna, Z.; Wang, F.; Liu, Y.; Zhu, Y.; Duc, L.; Wanga, D.; Xu, W. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2020, 86, 103344. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałecka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Woitschach, F.; Kloss, M.; Schlodder, K.; Borck, A.; Grabow, N.; Reisinger, E.C.; Sombetzki, M. Bacterial Adhesion and Biofilm Formation of Enterococcus faecalis on Zwitterionic Methylmethacrylat and Polysulfones. Front. Cell. Infect. Microbiol. 2022, 12, 868338. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walkera, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Codelia-Anjum, A.; Lerner, L.B.; Elterman, D.; Zorn, K.C.; Bhojani, N.; Chughtai, B. Enterococcal Urinary Tract Infections: A Review of the Pathogenicity, Epidemiology, and Treatment. Antibiotics 2023, 12, 778. [Google Scholar] [CrossRef]
- Bag, M.A.S.; Arif, M.; Riaz, S.; Khan, M.S.R.; Islam, M.S.; Punom, S.A.; Ali, M.W.; Begum, F.; Islam, M.S.; Rahman, M.T.; et al. Antimicrobial Resistance, Virulence Profiles, and Public Health Significance of Enterococcus faecalis Isolated from Clinical Mastitis of Cattle in Bangladesh. Hindawi BioMed Res. 2022, 2022, 8101866. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 20 May 2021).
- Mingoia, M.; Conte, C.; Di Rienzo, A.; Dimmito, M.P.; Marinucci, L.; Magi, G.; Turkez, H.; Cufaro, M.C.; Del Boccio, P.; Di Stefano, A.; et al. Synthesis and Biological Evaluation of Novel Cinnamic Acid-Based Antimicrobials. Pharmaceuticals 2022, 15, 228. [Google Scholar] [CrossRef]
- Meesters, K.; Alemayehu, T.; Benou, S.; Buonsenso, D.; Decloedt, E.H.; Pillay-Fuentes Lorente, V.; Downes, K.J.; Allegaert, K. Pharmacokinetics of Antimicrobials in Children with Emphasis on Challenges Faced by Low and Middle Income Countries, a Clinical Review. Antibiotics 2023, 12, 17. [Google Scholar] [CrossRef]
- Lagatolla, C.; Mehat, J.W.; La Ragione, R.M.; Luzzati, R.; Di Bella, S. In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant. Enterococcus faecium. Antibiotics 2022, 11, 1334. [Google Scholar] [CrossRef]
- Babiker, A.; Clarke, L.; Doi, Y.; Shields, R.K. Fosfomycin for Treatment of Multidrug-Resistant Pathogens Causing Urinary Tract Infection: A Real-World Perspective and Review of the Literature. Diagn. Microbiol. Infect. Dis. 2019, 95, 114856. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, J.F.; Maillard, J.-Y.; Borges, F.; Simões, M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Inter. Biodeter. Biodegrad. 2019, 141, 71–78. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives, and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Bułakowska, A.; Sławiński, J.; Hałasa, R.; Hering, A.; Gucwa, M.; Ochocka, J.R.; Stefanowicz-Hajduk, J. An In Vitro Antimicrobial, Anticancerand Antioxidant Activity of N–[(2–Arylmethylthio)phenylsulfonyl]cinnamamide Derivatives. Molecules 2023, 28, 3087. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Cheung, B.P.K.; Matinlinna, J.P.; L’evesque, C.M.; Neelakanta, P. Trans-cinnamaldehyde potently kills Enterococcus faecalis biofilm cells and prevents biofilm recovery. Microb. Pathog. 2020, 149, 104482. [Google Scholar] [CrossRef]
- Awakawa, T.; Barra, L.; Abe, I. Biosynthesis of sulfonamide and sulfamate antibiotics in actinomycete. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab001. [Google Scholar] [CrossRef]
- Gómez, C.N.; Manetsberger, J.; Benomar, N.; Castillo Gutiérrez, S.; Abriouel, H. Antibacterial and antibiofilm effects of essential oil components, EDTA and HLE disinfectant solution on Enterococcus, Pseudomonas and Staphylococcus sp. multiresistant strains isolated along the meat production chain. Front. Microbiol. 2022, 13, 1014169. [Google Scholar] [CrossRef]
- Teethaisong, Y.; Chueakwon, P.; Poolpol, K.; Ayamuang, I.; Suknasang, S.; Apinundecha, C.; Eumkeb, G. Stephania suberosa Forman extract synergistically inhibits ampicillin- and vancomycin-resistant Enterococcus faecium. Saudi J. Biol. Sci. 2023, 30, 103557. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, B.S.; Premraj, K.; Iswarya, C.; Muthusamy, S.; Ibrahim, H.-I.M.; Khalil, H.E.; Ashokkumar, V.; Vickram, S.; Kumar, V.S.; Palanisamy, S.; et al. Cinnamaldehyde inhibits Enterococcus faecalis biofilm formation and promotes clearance of its colonization by modulation of phagocytes in vitro. Microb. Pathog. 2023, 181, 106157. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R. K Phenotype–Genotype Correlations and Distribution of Key Virulence Factors in Enterococcus faecalis Isolated from Patients with Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.K.; Sakoulas, G.; Eliopoulos, G.M.; Moellering, R.C., Jr.; Murray, B.E.; Inouye, R. T Effects of Glucose on fsr-Mediated Biofilm Formation in Enterococcus faecalis. J. Infect. Dis. 2004, 190, 967–970. [Google Scholar] [CrossRef]
- Kim, M.-A.; Rosa, V.; Min, K.-S. Characterization of Enterococcus faecalis in different culture conditions. Nat. Res. Sci. Rep. 2020, 10, 21867. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2018, 139, 104405. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Test Methodology, Available online:. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology; (accessed on 2 January 2020).
- Clinical Breakpoints - Breakpoints and Guidance. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 2 January 2020).
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal Activity and Mechanism of Action of the Co(III) Coordination Complexes With Diamine Chelate Ligands Against Reference and Clinical Strains of Candida spp. Front. Microbiol. 2018, 9, 1594. [Google Scholar] [CrossRef]
| Full Strain Name, and Specific Resistance Mechanism | Ampicillin | Imipenem | Vancomicin | Teicoplanin | Co-trimoxazole | Norfloxacin | Gentamicin | Streptomycin | Doxycycline | Linezolid |
|---|---|---|---|---|---|---|---|---|---|---|
| Enterococcus faecium 12835 HLAR | 6 ± 0.00 | 6 ± 0.00 | 15 ± 0.00 | 20 ± 0.50 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 22 ± 0.0 |
| Enterococcus faecium 12848 HLAR | 6 ± 0.00 | 6 ± 0.00 | 17 ± 0.50 | 18 ± 0.50 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.00 |
| Enterococcus faecalis 12214 HLAR | 11 ± 0.50 | 21 ± 0.00 | 15 ± 0.00 | 20 ± 0.50 | 40 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 19 ± 0.00 |
| Enterococcus faecalis 12245 HLAR | 12 ± 0.50 | 22 ± 0.00 | 15 ± 0.00 | 18 ± 0.50 | 40 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.00 |
| Enterococccus faecium 12961 HLAR | 6 ± 0.00 | 6 ± 0.00 | 12 ± 0.00 | 20 ± 0.50 | 41 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 20 ± 0.00 | 26 ± 0.00 |
| Enterococcus faecalis 12338 HLAR | 12 ± 0.50 | 21 ± 0.00 | 15 ± 0.00 | 19 ± 0.00 | 35 ± 1.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.50 |
| nterococcus faecium 16247 HLAR | 6 ± 0.00 | 6 ± 0.00 | 12 ± 0.00 | 20 ± 0.50 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 26 ± 0.00 |
| Enterococcus faecalis 3937152 HLAR, VRE | 14 ± 0.50 | 22 ± 0.00 | 6 ± 0.00 | 20 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.50 |
| Enterococcus faecium 3934825 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 16 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.50 |
| Enterococcus faecium 773081 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 17 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.00 |
| Enterococcus faecium 895612 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 15 ± 0.00 | 35 ± 0.50 | 20 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 20 ± 0.00 | 26 ± 0.00 |
| Enterococcus faecium 508171 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 15 ± 0.00 | 40 ± 0.50 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 24 ± 0.00 |
| Enterococcus faecium 830981 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.50 |
| Enterococcus faecium 264281 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.00 |
| Enterococcus faecium 967321 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 15 ± 0.00 | 35 ± 0.50 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.00 |
| Enterococcus faecium 966351 HLAR, VRE | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 16 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 26 ± 0.00 |
| Enterococcus faecium 576181 HLAR, VRE | 6 ±0.00 | 6 ± 0.00 | 6 ± 0.00 | 18 ± 0.00 | 40 ± 0.50 | 18 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 25 ± 0.50 |
| Enterococcus faecium 885041 HLAR, VRE | 6 ±0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 6 ± 0.00 | 24 ± 0.00 |
| Compd | MIC [µg/mL] | |||||||
|---|---|---|---|---|---|---|---|---|
| Enterococcus sp. 773081 | Enterococcus sp. 508171 | Enterococcus sp. 830981 | Enterococcus sp. 264281 | Enterococcus sp. 967321 | Enterococcus sp. 966351 | Enterococcus sp. 576181 | Enterococcus sp. 885041 | |
| 16a | 4 ± 0.21 | 4 ± 0.18 | 4 ± 0.20 | 4 ± 0.15 | 4 ± 0.22 | 4 ± 0.30 | 4 ± 0.30 | 4 ± 0.33 |
| 16b | 8 ± 0.30 | 8 ± 0.50 | 16 ± 0.48 | 8 ± 0.18 | 8 ± 0.25 | 4 ± 0.30 | 16 ± 0.38 | 8 ± 0.42 |
| 16c | 4 ± 0.22 | 4 ± 0.38 | 4 ± 0.41 | 4 ± 0.24 | 4 ± 0.38 | 4 ± 0.31 | 4 ± 0.35 | 4 ± 0.30 |
| 16d | 2 ± 0.10 | 2 ± 0.10 | 2 ± 0.10 | 2 ± 0.05 | 2 ± 0.15 | 2 ± 0.10 | 2 ± 0.22 | 2 ± 0.10 |
| 16e | 16 ± 0.15 | 8 ± 0.50 | 16 ± 0.50 | 16 ± 0.50 | 16 ± 0.50 | 31.25 ± 0.50 | 8 ± 0.41 | 8 ± 0.50 |
| 16f | 8 ± 0.45 | 4 ± 0.25 | 4 ± 0.18 | 8 ± 0.50 | 4 ± 0.25 | 4 ± 0.28 | 4 ± 0.28 | 4 ± 0.35 |
| 17a | 8 ± 0.45 | 16 ± 0.50 | 16 ± 0.52 | 8 ± 0.50 | 16 ± 0.56 | 16 ± 0.50 | 16 ± 0.50 | 4 ± 0.20 |
| 17b | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 17c | 2 ± 0.18 | 4 ± 0.25 | 4 ± 0.25 | 4 ± 0.19 | 2 ± 0.05 | 4 ± 0.25 | 2 ± 0.25 | 4 ± 0.25 |
| 17d | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| co-trimoxazole | >2000:400 | 2000:400 ± 0.50 | >2000:400 | >2000: 400 | >2000:400 | 1250:250 ± 0.50 | >2000:400 | >2000:400 |
| Concentration (μg/mL) | 0.5 | 1 | 2 | 4 | 8 | 16 |
|---|---|---|---|---|---|---|
| 16a | [1.08 ± 0.65] × 108 | [9.00 ± 0.35] × 107 | [7.00 ± 0.14] × 107 | [6.50 ± 0.10] × 107 | [5.00 ± 0.21] × 107 | [5.10 ± 0.35] × 107 |
| 16c | [2.00 ± 0.35] × 108 | [9.30 ±0.14] × 107 | [8.30 ± 0.14] × 107 | [5.90 ± 0.21] × 107 | [5.90 ± 0.24] × 107 | [4.10 ± 0.35] × 107 |
| 16d | [1.80 ± 0.10] × 108 | [8.20 ± 0.10] × 107 | [8.16 ± 0.13] × 107 | [4.20 ± 0.14] × 107 | [3.90 ± 0.42] × 107 | [3.50 ± 0.77] × 107 |
| 16f | [1.59 ± 0.17] × 108 | [1.27 ± 0.30] × 108 | [5.70 ± 0.14] × 107 | [4.40 ± 0.21] × 107 | [2.60 ± 0.20] × 107 | [2.00 ± 0.45] × 107 |
| Concentration (µg/mL) | 0.5 | 1 | 2 | 4 | 8 | 16 |
|---|---|---|---|---|---|---|
| 16a | [1.80 ± 0.28] × 108 | [1.30 ± 0.85] × 107 | [1.30 ± 0.41] × 107 | [1.30 ± 0.70] × 107 | [1.20 ± 0.70]× 107 | [3.07 ± 0.64] × 107 |
| 16c | [1.90 ± 0.23] × 108 | [3.00 ± 0.35] × 107 | [3.00 ± 0.71] × 107 | [3.10 ± 084] × 107 | [8.00 ± 0.21] × 106 | [7.00 ± 0.71] × 106 |
| 16d | [1.50 ± 0.56] × 108 | [1.10 ± 0.60] × 107 | [7.00 ± 0.77] × 106 | [6.00 ± 0.71] × 106 | [5.40 ± 0.42] × 106 | [1.20 ± 0.55] × 106 |
| 17c | [2.27 ± 0.16] × 108 | [1.59 ± 0.43] × 108 | [1.15 ± 0.58] × 108 | [4.40 ± 0.41] × 107 | [2.60 ± 0.35] × 107 | [2.00 ± 0.62] × 107 |
| Strains | Antibiotic | MIC Antibiotic (mg/mL) | Cinnamic Acid Derivative | MIC Cinnamic Acid Derivative (mg/mL) | FICI | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Alone | Comb. | Alone | Comb. | |||||
| Enterococcus faecalis 12214 | ampicillin | 10 | 0.001 | 16a | 0.004 | 0.0005 | 0.126 | synergy |
| 0.001 | 16c | 0.004 | 0.0005 | 0.126 | synergy | |||
| 0.002 | 16d | 0.001 | 0.001 | 1 | additivity | |||
| 0.001 | 16f | 0.004 | 0.0005 | 0.126 | synergy | |||
| gentamycin | 2 | 0.001 | 16a | 0.004 | 0.002 | 0.5 | synergy | |
| 0.001 | 16c | 0.004 | 0.0005 | 0.125 | synergy | |||
| 0.001 | 16d | 0.0005 | 0.0005 | 1 | additivity | |||
| 0.001 | 16f | 0.002 | 0.0005 | 0.25 | synergy | |||
| streptomycin | 10 | 0.001 | 16a | 0.004 | 0.002 | 0.25 | synergy | |
| 0.001 | 16c | 0.004 | 0.0005 | 0.126 | synergy | |||
| 0.001 | 16d | 0.002 | 0.001 | 0.5 | synergy | |||
| 0.001 | 16f | 0.002 | 0.0005 | 0.25 | synergy | |||
| Enterococcus faecalis 12338 | ampicillin | 0.8 | 0.001 | 16a | 0.004 | 0.0005 | 0.126 | synergy |
| 0.001 | 16c | 0.004 | 0.0005 | 0.126 | synergy | |||
| 0.001 | 16d | 0.002 | 0.0005 | 0.375 | synergy | |||
| 0.001 | 16f | 0.004 | 0.0005 | 0.126 | synergy | |||
| gentamycin | 2 | 0.001 | 16a | 0.008 | 0.002 | 0.25 | synergy | |
| 0.001 | 16c | 0.008 | 0.0005 | 0.062 | synergy | |||
| 0.001 | 16d | 0.001 | 0.001 | 1 | additivity | |||
| 0.001 | 16f | 0.004 | 0.001 | 0.25 | synergy | |||
| streptomycin | 10 | 0.001 | 16a | 0.004 | 0.001 | 0.25 | synergy | |
| 0.001 | 16c | 0.002 | 0.001 | 0.5 | synergy | |||
| 0.001 | 16d | 0.001 | 0.0005 | 0.5 | synergy | |||
| 0.002 | 16f | 0.002 | 0.0005 | 0.25 | synergy | |||
| Strains | Antibiotic | MIC Antibiotic (mg/mL) | Cinnamic Acid Derivative | MIC Cinnamic Acid Derivative (mg/mL) | FICI | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Alone | Comb. | Alone | Comb. | |||||
| E. faecium 966351 VRE | Ampicillin | 0.8 | 0.016 | 16a | 0.008 | 0.0005 | 0.082 | synergy |
| 0.008 | 16c | 0.016 | 0.0005 | 0.041 | synergy | |||
| 0.002 | 16d | 0.002 | 0.0005 | 0.252 | synergy | |||
| 0.016 | 17c | 0.016 | 0.0005 | 0.082 | synergy | |||
| Gentamycin | 2 | 0.001 | 16a | 0.004 | 0.001 | 0.25 | synergy | |
| 0.001 | 16c | 0.002 | 0.001 | 0.5 | synergy | |||
| 0.001 | 16d | 0.001 | 0.001 | 1 | additivity | |||
| 0.032 | 17c | 0.008 | 0.008 | 1.016 | additivity | |||
| Streptomycin | 10 | 0.001 | 16a | 0.004 | 0.002 | 0.5 | synergy | |
| 0.032 | 16c | 0.008 | 0.008 | 1.003 | additivity | |||
| 0.001 | 16d | 0.001 | 0.0005 | 0.5 | synergy | |||
| 0.032 | 17c | 0.008 | 0.008 | 1.003 | additivity | |||
| Vancomycin | 0.008 | 0.001 | 16a | 0.004 | 0.0005 | 0.25 | synergy | |
| 0.001 | 16c | 0.002 | 0.001 | 0.625 | partial synergism | |||
| 0.001 | 16d | 0.001 | 0.001 | 1.125 | additivity | |||
| 0.001 | 17c | 0.008 | 0.0005 | 0.188 | synergy | |||
| E. faecium 830981 VRE | Ampicillin | 0.5 | 0.016 | 16a | 0.008 | 0.0005 | 0.082 | synergy |
| 0.008 | 16c | 0.016 | 0.0005 | 0.041 | synergy | |||
| 0.002 | 16d | 0.002 | 0.0005 | 0.252 | synergy | |||
| 0.016 | 17c | 0.016 | 0.0005 | 0.082 | synergy | |||
| Gentamycin | 2 | 0.016 | 16a | 0.002 | 0.0005 | 0.33 | synergy | |
| 0.016 | 16c | 0.001 | 0.0005 | 0.58 | partial synergism | |||
| 0.008 | 16d | 0.001 | 0.001 | 1 | additivity | |||
| 0.001 | 17c | 0.008 | 0.0005 | 0.063 | synergy | |||
| Streptomycin | 5 | 0.001 | 16a | 0.002 | 0.002 | 1 | additivity | |
| 0.001 | 16c | 0.002 | 0.001 | 0.5 | synergy | |||
| 0.001 | 16d | 0.001 | 0.0005 | 0.5 | synergy | |||
| 0.002 | 17c | 0.008 | 0.001 | 0.125 | synergy | |||
| Vancomycin | 1 | 0.002 | 16a | 0.004 | 0.0005 | 0.126 | synergy | |
| 0.002 | 16c | 0.004 | 0.0005 | 0.126 | synergy | |||
| 0.001 | 16d | 0.001 | 0.0005 | 0.5 | synergy | |||
| 0.001 | 17c | 0.016 | 0.004 | 0.251 | synergy | |||
| (a) | |
| List of Strains Listed in the Publication [18] | Full Strain Name and Specific Resistance Mechanism |
| Enterococcus hirae ATCC 10541 | |
| Enterococcus faecalis ATCC 51299 | Enterococcus faecalis ATCC 51299 VRE |
| Enterococcus sp. 12835 | Enterococcus faecium 12835 HLAR |
| Enterococcus sp. 12848 | Enterococcus faecium 12848 HLAR |
| Enterococcus sp. 12214 | Enterococcus faecalis 12214 HLAR |
| Enterococcus sp. 12245 | Enterococcus faecalis 12245 HLAR |
| Enterococcus sp.12961 | Enterococccus faecium 12961HLAR |
| Enterococcus sp. 12338 | Enterococcus faecalis 12338 HLAR |
| Enterococcus sp. 16247 | Enterococcus faecium 16247 HLAR |
| Enterococcus faecalis 3937152 | Enterococcus faecalis 3937152 HLAR, VRE |
| Enterococcus faecium 3934825 | Enterococcus faecium 3934825 HLAR, VRE |
| (b) | |
| List of Strains Listed Only in This Publication | Full Strain Name, and Specific Resistance Mechanism |
| Enterococcus sp. 773081 | Enterococcus faecium 773081 HLAR, VRE |
| Enterococcus sp. 508171 | Enterococcus faecium 508171 HLAR, VRE |
| Enterococcus sp. 830981 | Enterococcus faecium 830981 HLAR, VRE |
| Enterococcus sp. 264281 | Enterococcus faecium 264281 HLAR, VRE |
| Enterococcus sp. 967321 | Enterococcus faecium 967321 HLAR, VRE |
| Enterococcus sp. 966351 | Enterococcus faecium 966351 HLAR, VRE |
| Enterococcus sp. 576181 | Enterococcus faecium 576181 HLAR, VRE |
| Enterococcus sp. 885041 | Enterococcus faecium 885041 HLAR, VRE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hałasa, R.; Bułakowska, A.; Sławiński, J.; Smoktunowicz, M.; Rapacka-Zdończyk, A.; Mizerska, U. Activity of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety against Clinical HLAR and VRE Enterococcus spp. Antibiotics 2023, 12, 1691. https://doi.org/10.3390/antibiotics12121691
Hałasa R, Bułakowska A, Sławiński J, Smoktunowicz M, Rapacka-Zdończyk A, Mizerska U. Activity of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety against Clinical HLAR and VRE Enterococcus spp. Antibiotics. 2023; 12(12):1691. https://doi.org/10.3390/antibiotics12121691
Chicago/Turabian StyleHałasa, Rafał, Anita Bułakowska, Jarosław Sławiński, Magdalena Smoktunowicz, Aleksandra Rapacka-Zdończyk, and Urszula Mizerska. 2023. "Activity of Cinnamic Acid Derivatives with 4-Chloro-2-mercaptobenzenesulfonamide Moiety against Clinical HLAR and VRE Enterococcus spp." Antibiotics 12, no. 12: 1691. https://doi.org/10.3390/antibiotics12121691