A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates
Abstract
1. Introduction
2. Patients and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Preparation of Stock Solutions, Calibration Standards, and Quality Control Samples
2.4. Sample Preparation
3. Method Validation
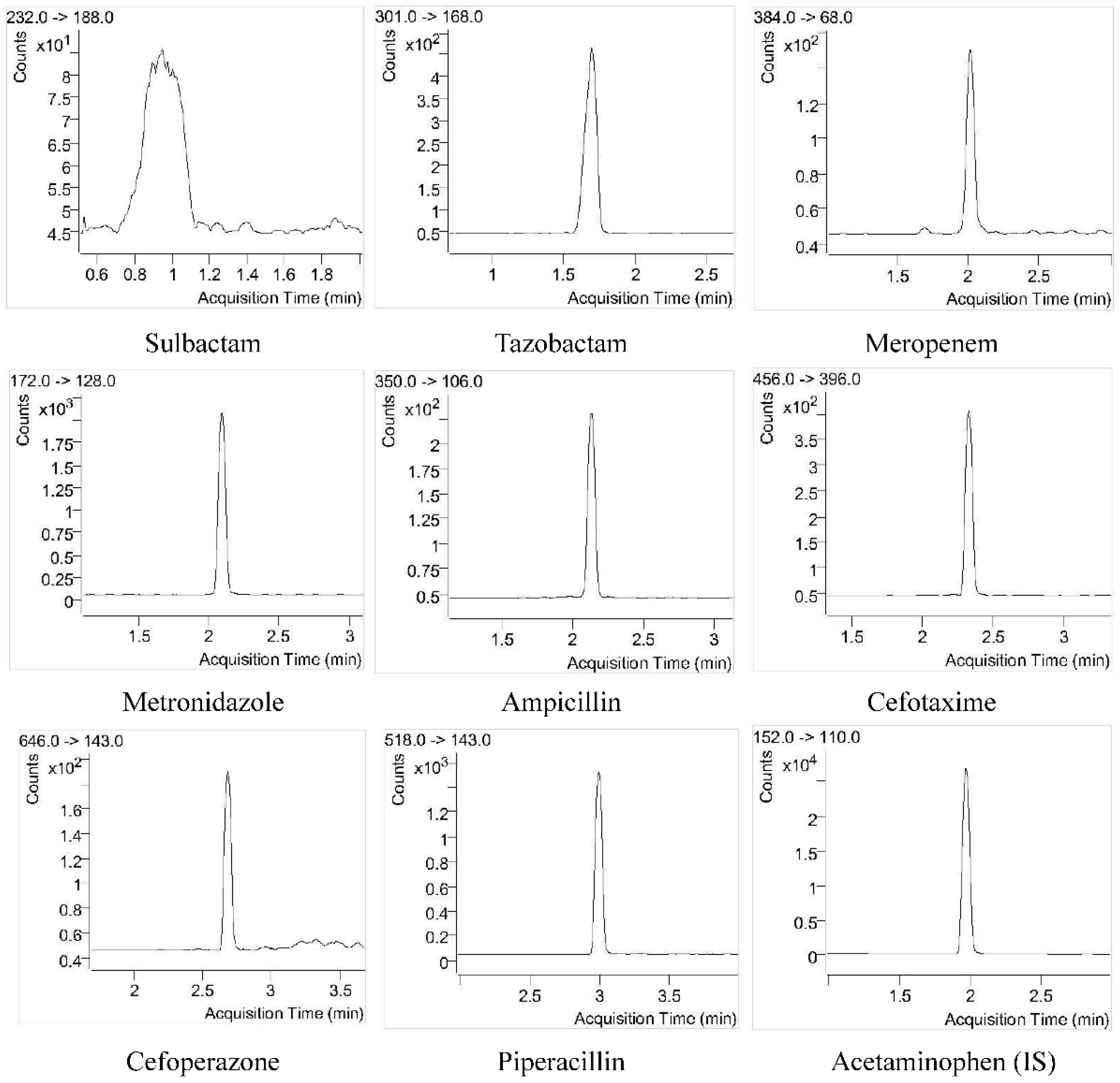
3.1. Selectivity
3.2. Carryover
3.3. Calibration Curve
3.4. Accuracy and Precision
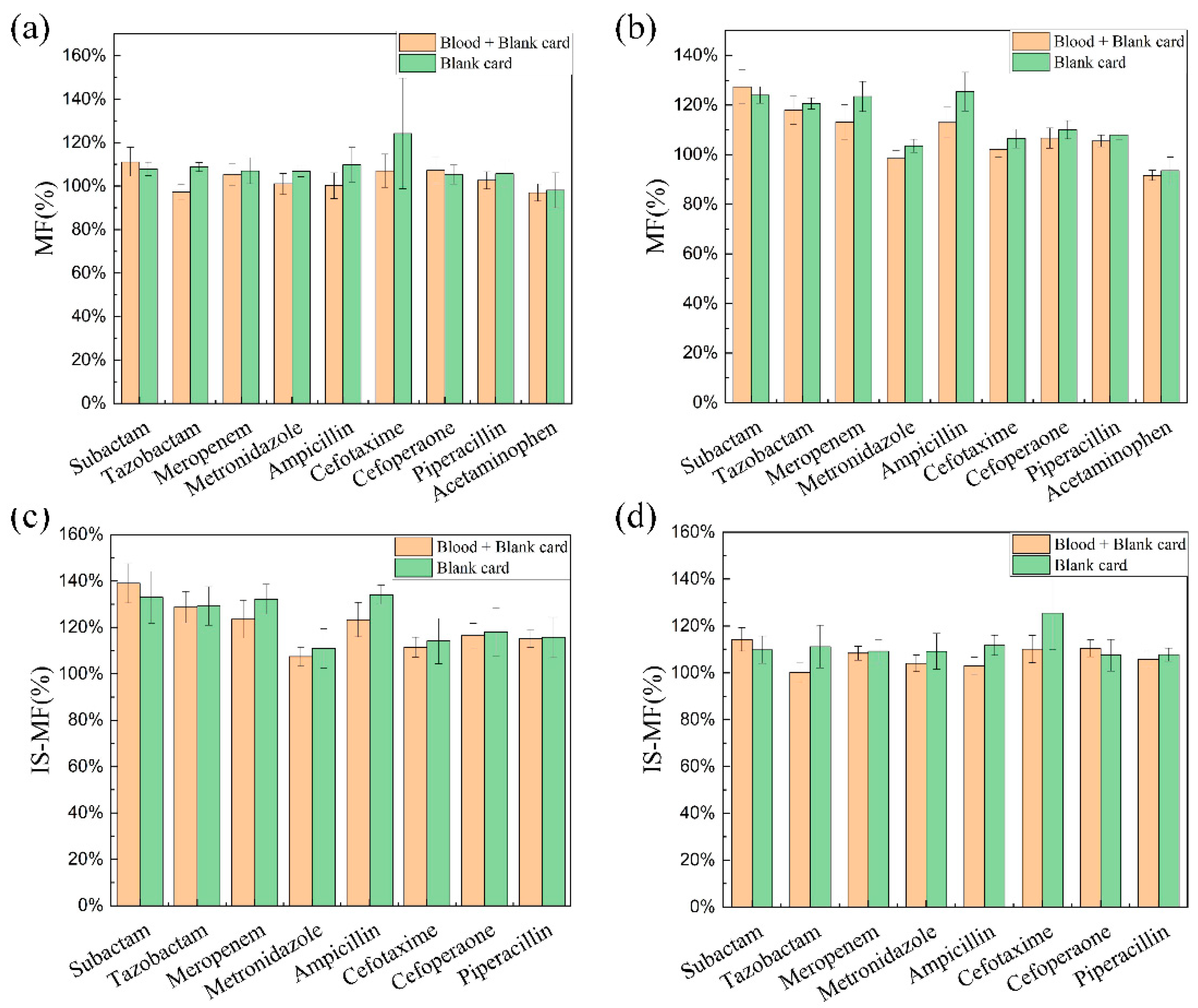
3.5. Matrix Effect
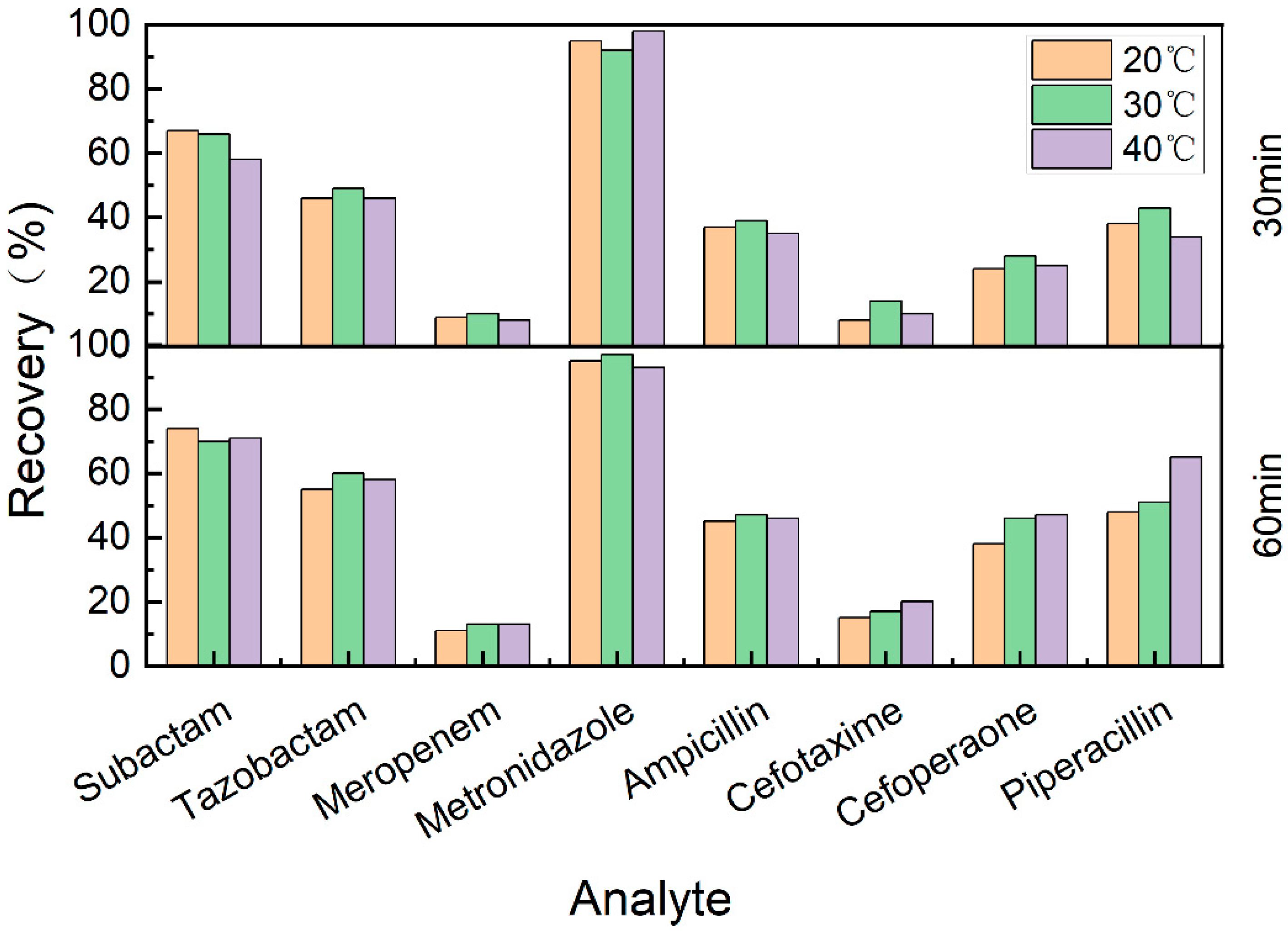
3.6. Recovery
3.7. Stability
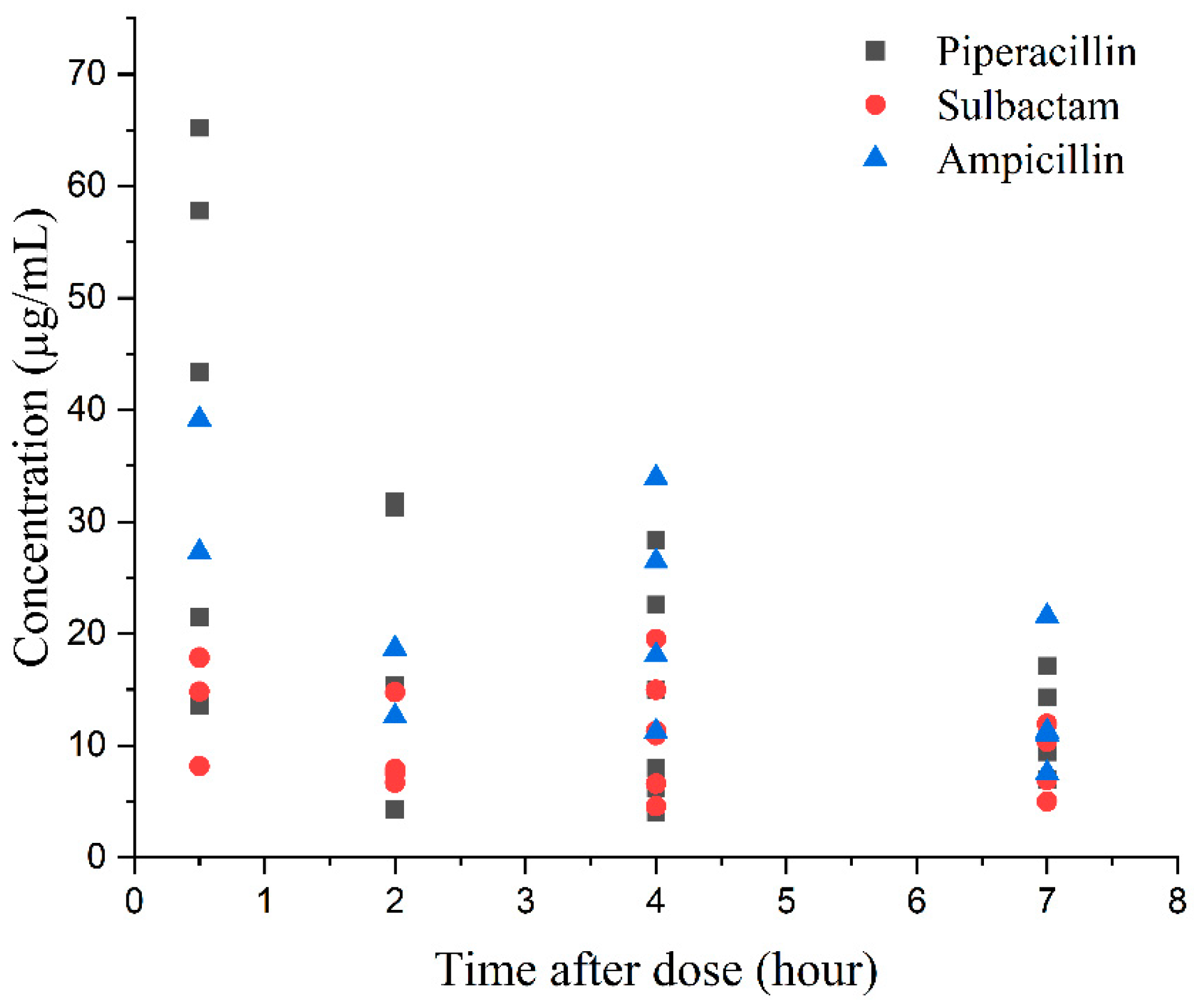
3.8. Application to Clinical Samples
4. Results and Discussion
4.1. Method Development
4.1.1. Chromatography and Mass Spectrometric Detection
4.1.2. Extraction Conditions
4.2. Method Validation
4.2.1. Selectivity
4.2.2. Carryover
4.2.3. Calibration Curve
4.2.4. Accuracy and Precision
4.2.5. Matrix Effect
4.2.6. Recovery
4.2.7. Stability
4.2.8. Method Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gastine, S.; Hsia, Y.; Clements, M.; Barker, C.I.S.; Bielicki, J.; Hartmann, C.; Sharland, M.; Standing, J.F. Variation in Target Attainment of Beta-Lactam Antibiotic Dosing Between International Pediatric Formularies. Clin. Pharmacol. Ther. 2021, 109, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A. Population pharmacokinetic models of first choice beta-lactam antibiotics for severe infections treatment: What antibiotic regimen to prescribe in children? J. Pharm. Pharm. Sci. 2020, 23, 470–485. [Google Scholar] [CrossRef]
- Gotfred-Rasmussen, H.; Stensvold, C.R.; Ingham, A.C.; Johannesen, T.B.; Andersen, L.O.; Röser, D.; Nielsen, H.V. Impact of Metronidazole Treatment and Dientamoeba Fragilis Colonization on Gut Microbiota Diversity. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Commander, S.J.; Gao, J.; Zinkhan, E.K.; Heresi, G.; Courtney, S.E.; Lavery, A.P.; Delmore, P.; Sokol, G.M.; Moya, F.; Benjamin, D.; et al. Safety of Metronidazole in Late Pre-term and Term Infants with Complicated Intra-abdominal Infections. Pediatr. Infect. Dis. J. 2020, 39, e245–e248. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar] [CrossRef]
- Sridharan, K.; Al Jufairi, M.; Al Ansari, E. Off-label drug use and the risk of medication errors in critically ill neonates: A conceptual pilot study. Int. J. Risk Saf. Med. 2021, 32, 279–293. [Google Scholar] [CrossRef]
- De Cock, R.F.; Piana, C.; Krekels, E.H.; Danhof, M.; Allegaert, K.; Knibbe, C.A. The role of population PK-PD modelling in paediatric clinical research. Eur. J. Clin. Pharmacol. 2011, 67 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Enderle, Y.; Foerster, K.; Burhenne, J. Clinical feasibility of dried blood spots: Analytics, validation, and applications. J. Pharm. Biomed. Anal. 2016, 130, 231–243. [Google Scholar] [CrossRef]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom. Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef]
- Delahaye, L.; Veenhof, H.; Koch, B.C.P.; Alffenaar, J.C.; Linden, R.; Stove, C. Alternative Sampling Devices to Collect Dried Blood Microsamples: State-of-the-Art. Ther. Drug Monit. 2021, 43, 310–321. [Google Scholar] [CrossRef]
- McClendon-Weary, B.; Putnick, D.L.; Robinson, S.; Yeung, E. Little to Give, Much to Gain-What Can You Do With a Dried Blood Spot? Curr. Environ. Health Rep. 2020, 7, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; Delahaye, L.; Stove, C.P. Is the hematocrit still an issue in quantitative dried blood spot analysis? J. Pharm. Biomed. Anal. 2019, 163, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Spooner, N.; Denniff, P.; Michielsen, L.; De Vries, R.; Ji, Q.C.; Arnold, M.E.; Woods, K.; Woolf, E.J.; Xu, Y.; Boutet, V.; et al. A device for dried blood microsampling in quantitative bioanalysis: Overcoming the issues associated blood hematocrit. Bioanalysis 2015, 7, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Spooner, N.; Olatunji, A.; Webbley, K. Investigation of the effect of blood hematocrit and lipid content on the blood volume deposited by a disposable dried blood spot collection device. J. Pharm. Biomed. Anal. 2018, 149, 419–424. [Google Scholar] [CrossRef]
- Neto, R.; Gooley, A.; Breadmore, M.C.; Hilder, E.F.; Lapierre, F. Precise, accurate and user-independent blood collection system for dried blood spot sample preparation. Anal. Bioanal. Chem. 2018, 410, 3315–3323. [Google Scholar] [CrossRef]
- Vu, D.H.; Koster, R.A.; Alffenaar, J.W.; Brouwers, J.R.; Uges, D.R. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J. Chromatogr. B 2011, 879, 1063–1070. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Monastyrski, D.; Tröger, U.; Bode-Böger, S.M. Stability of meropenem in plasma versus dried blood spots (DBS). J. Pharm. Biomed. Anal. 2019, 170, 279–284. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Angermair, S.; Bode-Böger, S.M. Quantification of ceftazidime/avibactam in human plasma and dried blood spots: Implications on stability and sample transport. J. Chromatogr. B 2022, 1193, 123164. [Google Scholar] [CrossRef]
- Cohen-Wolkowiez, M.; Watt, K.M.; Zhou, C.; Bloom, B.T.; Poindexter, B.; Castro, L.; Gao, J.; Capparelli, E.V.; Benjamin, D.K., Jr.; Smith, P.B. Developmental pharmacokinetics of piperacillin and tazobactam using plasma and dried blood spots from infants. Antimicrob. Agents Chemother. 2014, 58, 2856–2865. [Google Scholar] [CrossRef]
- la Marca, G.; Giocaliere, E.; Villanelli, F.; Malvagia, S.; Funghini, S.; Ombrone, D.; Filippi, L.; De Gaudio, M.; De Martino, M.; Galli, L. Development of an UPLC-MS/MS method for the determination of antibiotic ertapenem on dried blood spots. J. Pharm. Biomed. Anal. 2012, 61, 108–113. [Google Scholar] [CrossRef]
- Anibaletto Dos Santos, A.L.; Cezimbra da Silva, A.C.; Feltraco Lizot, L.L.; Schneider, A.; Meireles, Y.F.; Hahn, R.Z.; Pagnussat, L.R.; Nonnenmacher, J.L.; Hahn, S.R.; Linden, R. Development and validation of an assay for the measurement of gentamicin concentrations in dried blood spots using UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2022, 208, 114448. [Google Scholar] [CrossRef] [PubMed]
- Suyagh, M.F.; Iheagwaram, G.; Kole, P.L.; Millership, J.; Collier, P.; Halliday, H.; McElnay, J.C. Development and validation of a dried blood spot-HPLC assay for the determination of metronidazole in neonatal whole blood samples. Anal. Bioanal. Chem. 2010, 397, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Poindexter, B.; Sullivan, J.E.; Laughon, M.; Delmore, P.; Blackford, M.; Yogev, R.; James, L.P.; Melloni, C.; Harper, B.; et al. Comparative Analysis of Ampicillin Plasma and Dried Blood Spot Pharmacokinetics in Neonates. Ther. Drug Monit. 2018, 40, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kole, P.L.; Majithia, R.; Singh, T.R.; Garland, M.J.; Migalska, K.; Donnelly, R.F.; McElnay, J. Dried blood spot assay for estimation of metronidazole concentrations in rats and its application in single animal drug pharmacokinetic study. J. Chromatogr. B 2011, 879, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, V.; Carlucci, M.; Cotellese, R.; Raimondi, P.; Cichella, A.; Di Marco, L.; Genovese, S.; Carlucci, G. Development of a dried blood spot HPLC-PDA method for the analysis of linezolid and ciprofloxacin in hospital-acquired pneumonia patients. Drug Test. Anal. 2017, 9, 1611–1619. [Google Scholar] [CrossRef]
- Chaudhari, B.B.; Sridhar, P.; Moorkoth, S.; Lewis, L.E.; Mallayasamy, S. Validation of an HPLC method for estimation of cefotaxime from dried blood spot: Alternative to plasma-based PK evaluation in neonates. Bioanalysis 2021, 13, 1245–1258. [Google Scholar] [CrossRef]
- Barco, S.; Castagnola, E.; Moscatelli, A.; Rudge, J.; Tripodi, G.; Cangemi, G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot. J. Pharm. Biomed. Anal. 2017, 145, 704–710. [Google Scholar] [CrossRef]
- Colin, P.; De Bock, L.; T’Jollyn, H.; Boussery, K.; Van Bocxlaer, J. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2013, 103, 285–293. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.; Wang, Y.; Peng, Y.; Li, S. An UPLC-PDA assay for simultaneous determination of seven antibiotics in human plasma. J. Pharm. Biomed. Anal. 2022, 210, 114558. [Google Scholar] [CrossRef]
- Timmerman, P.; White, S.; Globig, S.; Lüdtke, S.; Brunet, L.; Smeraglia, J. EBF recommendation on the validation of bioanalytical methods for dried blood spots. Bioanalysis 2011, 3, 1567–1575. [Google Scholar] [CrossRef]
- Timmerman, P.; White, S.; Cobb, Z.; de Vries, R.; Thomas, E.; van Baar, B. Update of the EBF recommendation for the use of DBS in regulated bioanalysis integrating the conclusions from the EBF DBS-microsampling consortium. Bioanalysis 2013, 5, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (ms) | Fragmentor Voltage (V) | Collision Energy (eV) | ESI Polarity |
|---|---|---|---|---|---|---|
| Sulbactam | 232 | 188 | 50 | 135 | 8 | Negative |
| Tazobactam | 301 | 168 | 50 | 135 | 15 | Positive |
| Meropenem | 384 | 68 | 50 | 135 | 30 | Positive |
| Metronidazole | 172 | 128 | 50 | 135 | 30 | Positive |
| Ampicillin | 350 | 106 | 50 | 135 | 25 | Positive |
| Cefotaxime | 456 | 396 | 50 | 135 | 10 | Positive |
| Cefoperazone | 646 | 143 | 50 | 135 | 25 | Positive |
| Piperacillin | 518 | 143 | 50 | 135 | 25 | Positive |
| Acetaminophen | 152 | 110 | 50 | 135 | 15 | Positive |
| Analyte | Calibration Standards (μg/mL) | QC Samples (μg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STD8 | STD7 | STD6 | STD5 | STD4 | STD3 | STD2 | STD1 | QC-LLOQ | QC-L | QC-M | QC-H | ||
| Sulbactam | 1.00 | 2.00 | 4.00 | 8.00 | 20.00 | 40.00 | 100.00 | 200.00 | 1.00 | 2.00 | 8.00 | 160.00 | |
| Tazobactam | 1.00 | 2.00 | 4.00 | 8.00 | 20.00 | 40.00 | 100.00 | 200.00 | 1.00 | 2.00 | 8.00 | 160.00 | |
| Meropenem | 0.375 | 0.75 | 1.50 | 3.00 | 7.50 | 15.00 | 37.50 | 75.00 | 0.375 | 0.75 | 3.00 | 60.00 | |
| Metronidazole | 0.25 | 0.50 | 1.00 | 2.00 | 5.00 | 10.00 | 25.00 | 50.00 | 0.25 | 0.50 | 2.00 | 40.00 | |
| Ampicillin | 0.25 | 0.50 | 1.00 | 2.00 | 5.00 | 10.00 | 25.00 | 50.00 | 0.25 | 0.50 | 2.00 | 160.00 | |
| Cefotaxime | 1.00 | 2.00 | 4.00 | 8.00 | 20.00 | 40.00 | 100.00 | 200.00 | 1.00 | 2.00 | 8.00 | 240.00 | |
| Cefoperazone | 1.50 | 3.00 | 6.00 | 12.00 | 30.00 | 60.00 | 150.00 | 300.00 | 1.50 | 3.00 | 12.00 | 240.00 | |
| Piperacillin | 2.00 | 4.00 | 8.00 | 16.00 | 40.00 | 80.00 | 200.00 | 400.00 | 2.00 | 4.00 | 16.00 | 320.00 | |
| Analyte | Nominal Concentration (μg/mL) | Within-Run (n = 6) | Between-Run (n = 18) | |||
|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD%) | Accuracy (%) | Precision (RSD%) | |||
| Sulbactam | 1–2–8–160 | 91.9–98.0–105.2–103.9 | 8.9–9.5–7.2–11.0 | 103.8–100.7–107.7–98.7 | 10.6–7.9–9.2–8.4 | |
| Tazobactam | 1–2–8–160 | 103.0–100.5–105.3–99.6 | 2.3–8.0–7.8–8.2 | 103.9–102.2–113.6–100.4 | 5.6–7.3–10.7–5.4 | |
| Meropenem | 0.375–0.75–3–60 | 96.9–92.8–94.8–89.9 | 8.7–8.0–9.3–4.0 | 101.4–88.6–94.4–88.5 | 9.2–9.3–8.6–6.0 | |
| Metronidazole | 0.25–0.5–2–40 | 87.2–95.8–104.5–96.8 | 3.4–4.2–3.6–9.3 | 93.9–94.8–108.4–101.6 | 15.0–9.2–9.7–14.0 | |
| Ampicillin | 0.25–0.5–2–40 | 99.1–95.4–100.4–92.8 | 1.7–10.4–8.9–8.8 | 101.4–94.8–99.0–92.5 | 8.1–9.0–10.1–6.0 | |
| Cefotaxime | 1–2–8–160 | 102.8–83.3–102.5–105.5 | 5.2–10.8–15.6–10.9 | 103.7–83.7–97.4–97.5 | 15.5–7.7–11.2–11.3 | |
| Cefoperazone | 1.5–3–12–240 | 98.3–93.6–92.4–97.9 | 6.3–8.8–9.1–9.3 | 100.0–93.2–95.1–96.4 | 8.1–8.0–12.2–2.5 | |
| Piperacillin | 2–4–16–320 | 106.6–92.1–92.2–96.7 | 1.5–10.3–9.2–9.7 | 106.1–89.1–94.7–93.4 | 4.1–8.2–11.9–6.5 | |
| Analyte | Nominal Concentration (μg/mL) | Mean Percent Accuracy (%, n = 6) | ||||||
|---|---|---|---|---|---|---|---|---|
| Autosampler Stability (10 h) | Freeze and Thaw Stability | Storage Stability | ||||||
| 25 °C 24 h | 14 °C 72 h | −80 °C 30 Days | ||||||
| Sulbactam | 2–160 | 102.1–101.3 | 112.0–103.5 | 86.5–98.2 | 94.1–105.3 | 87.9–96.0 | ||
| Tazobactam | 2–160 | 104.6–105.7 | 107.2–99.2 | 90.5–93.6 | 94.4–104.7 | 97.5–90.2 | ||
| Meropenem | 0.75–60 | 90.2–86.9 | 90.5–96.8 | 79.6–78.6 | 88.1–89.0 | 91.0–90.4 | ||
| Metronidazole | 0.5–40 | 92.0–103.6 | 108.0–98.0 | 103.6–111.1 | 90.9–104.0 | 89.4–100.4 | ||
| Ampicillin | 0.5–40 | 94.8–90.5 | 92.4–96.1 | 79.6–86.1 | 86.1–92.0 | 99.8–98.5 | ||
| Cefotaxime | 2–160 | 85.6–102.5 | 92.8–104.3 | 77.5–76.6 | 97.5–100.9 | 90.8–89.3 | ||
| Cefoperazone | 3–240 | 90.8–91.4 | 102.6–98.2 | 106.8–113.4 | 88.8–103.1 | 100.5–88.6 | ||
| Piperacillin | 4–320 | 90.9–86.2 | 105.4–98.6 | 86.0–96.1 | 89.6–98.6 | 96.8–92.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, L.; Yuan, Y.; Xie, F. A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates. Antibiotics 2023, 12, 199. https://doi.org/10.3390/antibiotics12020199
Liu Q, Liu L, Yuan Y, Xie F. A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates. Antibiotics. 2023; 12(2):199. https://doi.org/10.3390/antibiotics12020199
Chicago/Turabian StyleLiu, Qian, Lanyu Liu, Yu Yuan, and Feifan Xie. 2023. "A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates" Antibiotics 12, no. 2: 199. https://doi.org/10.3390/antibiotics12020199
APA StyleLiu, Q., Liu, L., Yuan, Y., & Xie, F. (2023). A Validated UHPLC–MS/MS Method to Quantify Eight Antibiotics in Quantitative Dried Blood Spots in Support of Pharmacokinetic Studies in Neonates. Antibiotics, 12(2), 199. https://doi.org/10.3390/antibiotics12020199





