Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-lactamase Producing E. coli Isolated from Healthy and Diseased Cats
Abstract
1. Introduction
2. Results
2.1. Cat Population
2.2. Prevalence of E. coli Isolates
2.3. Antimicrobial Susceptibility Testing
2.4. MDR and ESBL-Producing E. coli
2.5. Risk Factors for MDR and ESBL-Producing E. coli
3. Discussion
4. Materials and Methods
4.1. Study Design and Sampling
4.2. E. coli Isolation and Identification
4.3. Antimicrobial Susceptibility Test
4.4. ESBL-Producing E. coli Identification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; Van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Antimicrobial resistance in companion animals. Anim. Health Res. Rev. 2008, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of antibiotic resistance genes (ARGs) via integrons in Escherichia coli: A risk to human health. Environ. Pollut. 2020, 266, 115260. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Weese, J.; Giguère, S.; Guardabassi, L.; Morley, P.; Papich, M.; Ricciuto, D.; Sykes, J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015, 29, 487–498. [Google Scholar] [CrossRef]
- Zhang, P.L.; Shen, X.; Chalmers, G.; Reid-Smith, R.J.; Slavic, D.; Dick, H.; Boerlin, P. Prevalence and mechanisms of extended-spectrum cephalosporin resistance in clinical and fecal Enterobacteriaceae isolates from dogs in Ontario, Canada. Vet. Microbiol. 2018, 213, 82–88. [Google Scholar] [CrossRef]
- Bortolami, A.; Zendri, F.; Maciuca, E.I.; Wattret, A.; Ellis, C.; Schmidt, V.; Pinchbeck, G.; Timofte, D. Diversity, virulence, and clinical significance of extended-spectrum β-lactamase-and pAmpC-producing Escherichia coli from companion animals. Front. Microbiol. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended spectrum β-lactamase (ESBL) production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Salinas, L.; Loayza, F.; Cárdenas, P.; Saraiva, C.; Johnson, T.J.; Amato, H.; Graham, J.P.; Trueba, G. Environmental spread of extended spectrum beta-lactamase (ESBL) producing Escherichia coli and ESBL genes among children and domestic animals in Ecuador. Environ. Health Perspect. 2021, 129, 027007. [Google Scholar] [CrossRef]
- Alrukban, M.O.; Alekrish, Y.A.; Alshehri, M.H.; Bajeaifer, Y.A.; Alhamad, M.H.; Sambas, F.A.; Alsouan, A.A. Awareness of pet owners in Riyadh regarding pet-related health risks and their associated preventative measures. Vector-Borne Zoonotic Dis. 2022, 22, 419–424. [Google Scholar] [CrossRef]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D. Bacterial zoonoses transmitted by household pets: State-of-the-art and future perspectives for targeted research and policy actions. J. Comp. Pathol. 2016, 155, S27–S40. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats–A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef]
- Johnson, J.R.; Miller, S.; Johnston, B.; Clabots, C.; DebRoy, C. Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and urovirulent E. coli strains among dogs and cats within a household. J. Clin. Microbiol. 2009, 47, 3721–3725. [Google Scholar] [CrossRef]
- Carvalho, A.; Barbosa, A.; Arais, L.; Ribeiro, P.; Carneiro, V.; Cerqueira, A. Resistance patterns, ESBL genes, and genetic relatedness of Escherichia coli from dogs and owners. Braz. J. Microbiol. 2016, 47, 150–158. [Google Scholar] [CrossRef]
- Sidjabat, H.E.; Townsend, K.M.; Lorentzen, M.; Gobius, K.S.; Fegan, N.; Chin, J.J.-C.; Bettelheim, K.A.; Hanson, N.D.; Bensink, J.C.; Trott, D.J. Emergence and spread of two distinct clonal groups of multidrug-resistant Escherichia coli in a veterinary teaching hospital in Australia. J. Med. Microbiol. 2006, 55, 1125–1134. [Google Scholar] [CrossRef]
- Shaheen, B.; Boothe, D.; Oyarzabal, O.; Smaha, T. Antimicrobial resistance profiles and clonal relatedness of canine and feline Escherichia coli pathogens expressing multidrug resistance in the United States. J. Vet. Intern. Med. 2010, 24, 323–330. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Li, R.; Han, Y.; Hao, G.; Wang, G.; Sun, S. Companion Animals as Potential Reservoirs of Antibiotic Resistant Diarrheagenic Escherichia coli in Shandong, China. Antibiotics 2022, 11, 828. [Google Scholar] [CrossRef]
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Féria, C.; Machado, J.; Correia, J.D.; Gonçalves, J.; Gaastra, W. Virulence genes and P fimbriae PapA subunit diversity in canine and feline uropathogenic Escherichia coli. Vet. Microbiol. 2001, 82, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Awosile, B.B.; McClure, J.T.; Saab, M.E.; Heider, L.C. Antimicrobial resistance in bacteria isolated from cats and dogs from the Atlantic Provinces, Canada from 1994–2013. Can. Vet. J. 2018, 59, 885. [Google Scholar] [PubMed]
- Chan, O.S.; Baranger-Ete, M.; Lam, W.W.; Wu, P.; Yeung, M.; Lee, E.; Bond, H.; Swan, O.; Tun, H.M. A retrospective study of antimicrobial resistant bacteria associated with feline and canine urinary tract infection in Hong Kong SAR, China—A case study on implication of first-line antibiotics use. Antibiotics 2022, 11, 1140. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-I.; Seo, K.-W.; Kim, D.-H.; Cheon, D.-S. Prevalence, co-infection and seasonality of fecal enteropathogens from diarrheic cats in the Republic of Korea (2016–2019): A retrospective study. BMC Vet. Res. 2021, 17, 1–13. [Google Scholar] [CrossRef]
- Piccolo, F.L.; Belas, A.; Foti, M.; Fisichella, V.; Marques, C.; Pomba, C. Detection of multidrug resistance and extended-spectrum/plasmid-mediated AmpC beta-lactamase genes in Enterobacteriaceae isolates from diseased cats in Italy. J. Feline Med. Surg. 2020, 22, 613–622. [Google Scholar] [CrossRef]
- Saputra, S.; Jordan, D.; Mitchell, T.; San Wong, H.; Abraham, R.J.; Kidsley, A.; Turnidge, J.; Trott, D.J.; Abraham, S. Antimicrobial resistance in clinical Escherichia coli isolated from companion animals in Australia. Vet. Microbiol. 2017, 211, 43–50. [Google Scholar] [CrossRef]
- Jung, W.K.; Shin, S.; Park, Y.K.; Lim, S.-K.; Moon, D.-C.; Park, K.T.; Park, Y.H. Distribution and antimicrobial resistance profiles of bacterial species in stray cats, hospital-admitted cats, and veterinary staff in South Korea. BMC Vet. Res. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Sáenz, Y.; Coelho, A.C.; Matos, M.; Vinué, L.; Rodrigues, J.; Torres, C. Prevalence of antimicrobial resistance and resistance genes in faecal Escherichia coli isolates recovered from healthy pets. Vet. Microbiol. 2008, 127, 97–105. [Google Scholar] [CrossRef]
- Van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- Rzewuska, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Chrobak, D.; Błaszczak, B.; Binek, M. Multidrug resistance in Escherichia coli strains isolated from infections in dogs and cats in Poland (2007–2013). Sci. World J. 2015, 2015, 408205. [Google Scholar] [CrossRef]
- Harada, K.; Nakai, Y.; Kataoka, Y. Mechanisms of resistance to cephalosporin and emergence of O25b-ST131 clone harboring CTX-M-27 β-lactamase in extraintestinal pathogenic Escherichia coli from dogs and cats in Japan. Microbiol. Immunol. 2012, 56, 480–485. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Shaheen, B.W.; Nayak, R.; Foley, S.L.; Kweon, O.; Deck, J.; Park, M.; Rafii, F.; Boothe, D.M. Molecular characterization of resistance to extended-spectrum cephalosporins in clinical Escherichia coli isolates from companion animals in the United States. Antimicrob. Agents Chemother. 2011, 55, 5666–5675. [Google Scholar] [CrossRef]
- Huber, H.; Zweifel, C.; Wittenbrink, M.M.; Stephan, R. ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 2013, 162, 992–996. [Google Scholar] [CrossRef]
- Jackson, C.; Davis, J.; Frye, J.; Barrett, J.; Hiott, L. Diversity of Plasmids and Antimicrobial Resistance Genes in Multidrug-Resistant Escherichia coli Isolated from Healthy Companion Animals. Zoonoses Public Health 2015, 62, 479–488. [Google Scholar] [CrossRef]
- Timofte, D.; Maciuca, I.E.; Williams, N.J.; Wattret, A.; Schmidt, V. Veterinary hospital dissemination of CTX-M-15 extended-spectrum beta-lactamase–producing Escherichia coli ST410 in the United Kingdom. Microb. Drug Resist. 2016, 22, 609–615. [Google Scholar] [CrossRef]
- Bogaerts, P.; Huang, T.-D.; Bouchahrouf, W.; Bauraing, C.; Berhin, C.; El Garch, F.; Glupczynski, Y.; Group, C.S. Characterization of ESBL-and AmpC-producing Enterobacteriaceae from diseased companion animals in Europe. Microb. Drug Resist. 2015, 21, 643–650. [Google Scholar] [CrossRef]
- Dahmen, S.; Haenni, M.; Châtre, P.; Madec, J.-Y. Characterization of bla CTX-M IncFII plasmids and clones of Escherichia coli from pets in France. J. Antimicrob. Chemother. 2013, 68, 2797–2801. [Google Scholar] [CrossRef]
- Dierikx, C.; van Duijkeren, E.; Schoormans, A.; van Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Huijsdens, X.; van der Zwaluw, K.; Wagenaar, J.; Mevius, D. Occurrence and characteristics of extended-spectrum-β-lactamase-and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2012, 67, 1368–1374. [Google Scholar] [CrossRef]
- Murphy, C.; Reid-Smith, R.J.; Prescott, J.F.; Bonnett, B.N.; Poppe, C.; Boerlin, P.; Weese, J.S.; Janecko, N.; McEwen, S.A. Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: A preliminary study. Can. Vet. J. 2009, 50, 1047. [Google Scholar] [PubMed]
- Sun, Y.; Zeng, Z.; Chen, S.; Ma, J.; He, L.; Liu, Y.; Deng, Y.; Lei, T.; Zhao, J.; Liu, J.-H. High prevalence of blaCTX-M extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 2010, 16, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, J.; Schoormans, A.; Kwakernaak, M.; Duim, B.; Broens, E.; Dierikx, C.; Mevius, D.; Wagenaar, J.A. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front. Microbiol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Mairi, A.; Touati, A.; Hassissene, L.; Brasme, L.; Guillard, T.; De Champs, C. Extended spectrum β-lactamase and plasmid mediated quinolone resistance in Escherichia coli fecal isolates from healthy companion animals in Algeria. J. Infect. Chemother. 2016, 22, 431–435. [Google Scholar] [CrossRef]
- Yasir, M.; Ajlan, A.M.; Shakil, S.; Jiman-Fatani, A.A.; Almasaudi, S.B.; Farman, M.; Baazeem, Z.M.; Baabdullah, R.; Alawi, M.; Al-Abdullah, N. Molecular characterization, antimicrobial resistance and clinico-bioinformatics approaches to address the problem of extended-spectrum β-lactamase-producing Escherichia coli in western Saudi Arabia. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Fadlelmula, A.; Al-Hamam, N.A.; Al-Dughaym, A.M. A potential camel reservoir for extended-spectrum β-lactamase-producing Escherichia coli causing human infection in Saudi Arabia. Trop. Anim. Health Prod. 2016, 48, 427–433. [Google Scholar] [CrossRef]
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High prevalence of extended-spectrum β-lactamase producing Enterobacteriaceae among clinical isolates from cats and dogs admitted to a veterinary hospital in Switzerland. Front. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Briñas, L.; Sáenz, Y.; Rodrigues, J.; Torres, C. Detection of CTX-M-1 and TEM-52 β-lactamases in Escherichia coli strains from healthy pets in Portugal. J. Antimicrob. Chemother. 2004, 54, 960–961. [Google Scholar] [CrossRef]
- Rao, L.; Lv, L.; Zeng, Z.; Chen, S.; He, D.; Chen, X.; Wu, C.; Wang, Y.; Yang, T.; Wu, P. Increasing prevalence of extended-spectrum cephalosporin-resistant Escherichia coli in food animals and the diversity of CTX-M genotypes during 2003–2012. Vet. Microbiol. 2014, 172, 534–541. [Google Scholar] [CrossRef]
- Puño-Sarmiento, J.; Medeiros, L.; Chiconi, C.; Martins, F.; Pelayo, J.; Rocha, S.; Blanco, J.; Blanco, M.; Zanutto, M.; Kobayashi, R. Detection of diarrheagenic Escherichia coli strains isolated from dogs and cats in Brazil. Vet. Microbiol. 2013, 166, 676–680. [Google Scholar] [CrossRef]
- Watson, V.E.; Jacob, M.E.; Flowers, J.R.; Strong, S.J.; DebRoy, C.; Gookin, J.L. Association of atypical enteropathogenic Escherichia coli with diarrhea and related mortality in kittens. J. Clin. Microbiol. 2017, 55, 2719–2735. [Google Scholar] [CrossRef]
- Meyer, E.; Gastmeier, P.; Kola, A.; Schwab, F. Pet animals and foreign travel are risk factors for colonisation with extended-spectrum β-lactamase-producing Escherichia coli. Infection 2012, 40, 685–687. [Google Scholar] [CrossRef]
- Van den Bunt, G.; Fluit, A.; Spaninks, M.; Timmerman, A.; Geurts, Y.; Kant, A.; Scharringa, J.; Mevius, D.; Wagenaar, J.; Bonten, M. Faecal carriage, risk factors, acquisition and persistence of ESBL-producing Enterobacteriaceae in dogs and cats and co-carriage with humans belonging to the same household. J. Antimicrob. Chemother. 2020, 75, 342–350. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Zhang, J.; Zhang, Y.-L.; Wang, Y.-C.; Xiao, S.-Z.; Gu, F.-F.; Guo, X.-K.; Ni, Y.-X.; Han, L.-Z. Epidemiology and risk factors for faecal extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) carriage derived from residents of seven nursing homes in western Shanghai, China. Epidemiol. Infect. 2016, 144, 695–702. [Google Scholar] [CrossRef]
- Karkaba, A.; Hill, K.; Benschop, J.; Pleydell, E.; Grinberg, A. Carriage and population genetics of extended spectrum β-lactamase-producing Escherichia coli in cats and dogs in New Zealand. Vet. Microbiol. 2019, 233, 61–67. [Google Scholar] [CrossRef]
- Hernandez, J.; Bota, D.; Farbos, M.; Bernardin, F.; Ragetly, G.; Médaille, C. Risk factors for urinary tract infection with multiple drug-resistant Escherichia coli in cats. J. Feline Med. Surg. 2014, 16, 75–81. [Google Scholar] [CrossRef]
- Baede, V.O.; Broens, E.M.; Spaninks, M.P.; Timmerman, A.J.; Graveland, H.; Wagenaar, J.A.; Duim, B.; Hordijk, J. Raw pet food as a risk factor for shedding of extended-spectrum beta-lactamase-producing Enterobacteriaceae in household cats. PLoS One 2017, 12, e0187239. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Thirtieth CLSI Supplement M100-S30; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Davis, R.; Brown, P.D. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 2016, 65, 261–271. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Brian, M.; Frosolono, M.; Murray, B.; Miranda, A.; Lopez, E.; Gomez, H.; Cleary, T. Polymerase chain reaction for diagnosis of enterohemorrhagic Escherichia coli infection and hemolytic-uremic syndrome. J. Clin. Microbiol. 1992, 30, 1801–1806. [Google Scholar] [CrossRef]
- Pitout, J.; Thomson, K.; Hanson, N.; Ehrhardt, A.; Moland, E.; Sanders, C. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 1998, 42, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Gallien, P. Detection and Subtyping of ShigaToxin-Producing Escherichia coli (STEC). In PCR Detection of Microbial Pathogens; Springer: Berlin/Heidelberg, Germany, 2003; pp. 163–184. [Google Scholar]
- Pitout, J.D.; Hossain, A.; Hanson, N.D. Phenotypic and molecular detection of CTX-M-β-lactamases produced by Escherichia coli and Klebsiella spp. J. Clin. Microbiol. 2004, 42, 5715–5721. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Martin, W.; Stryhn, H.E. Veterinary Epidemiologic Research; University of Prince Edward Island: Charlottetown, PE, Canada, 2003. [Google Scholar]
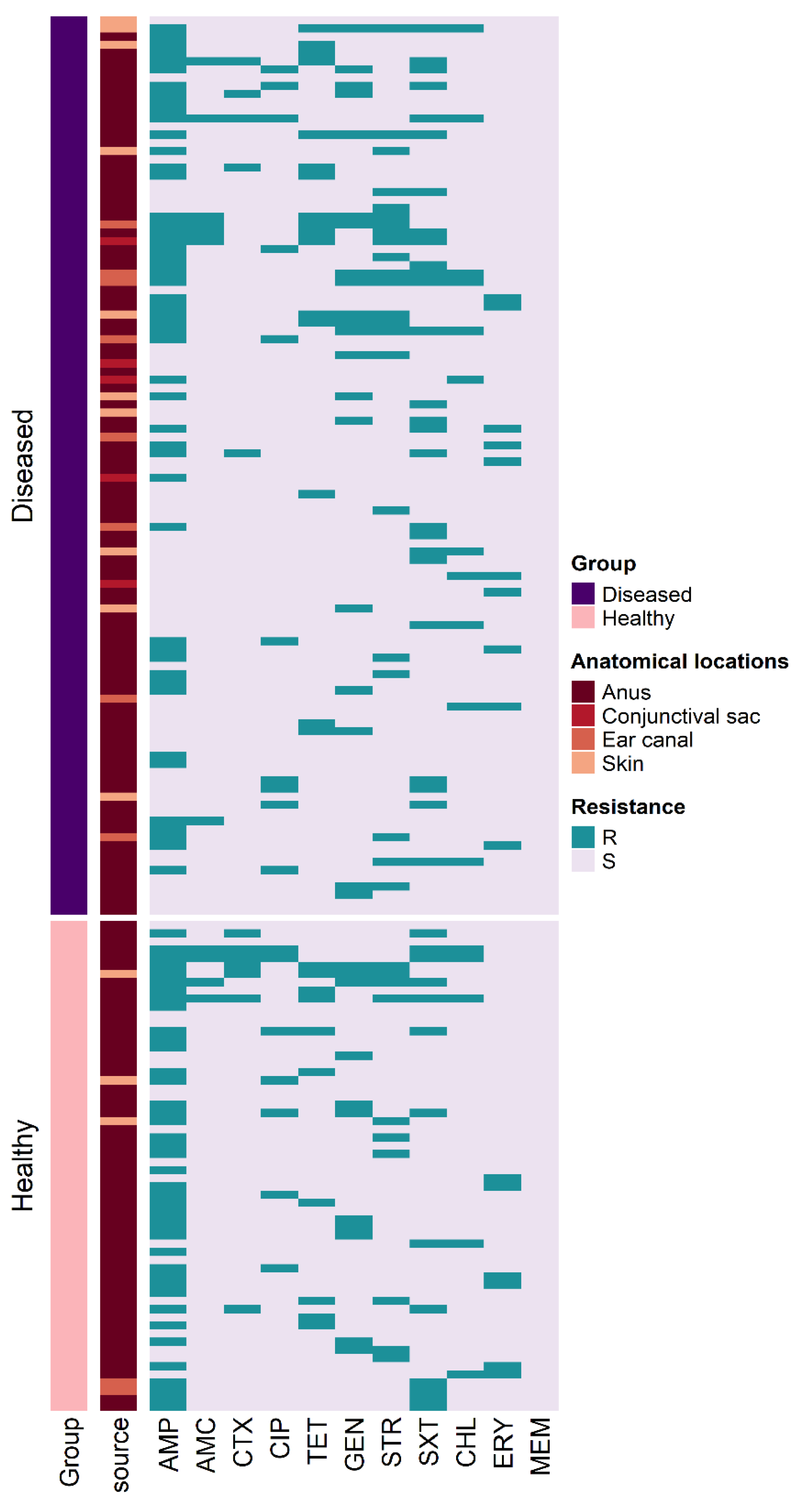
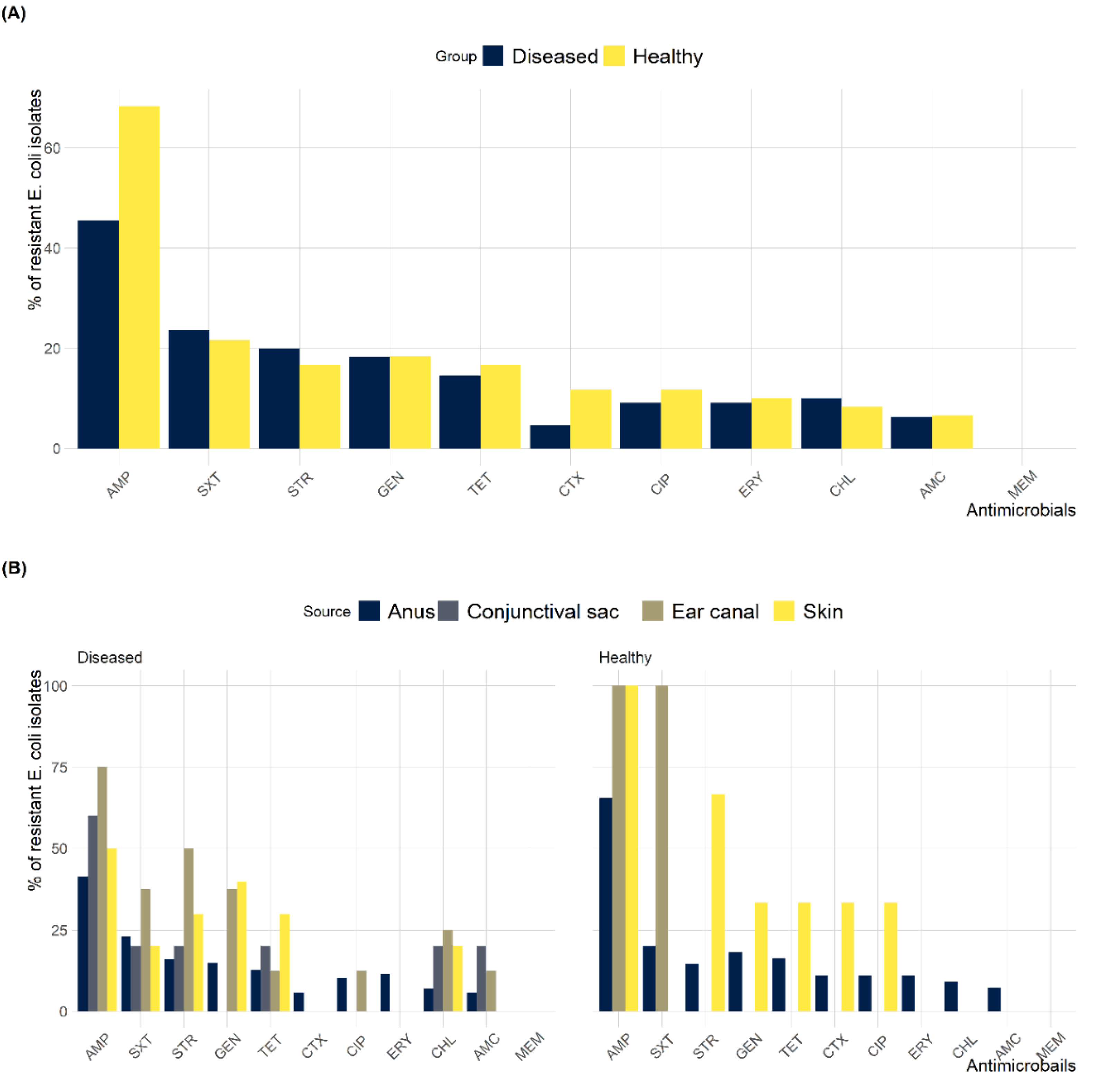
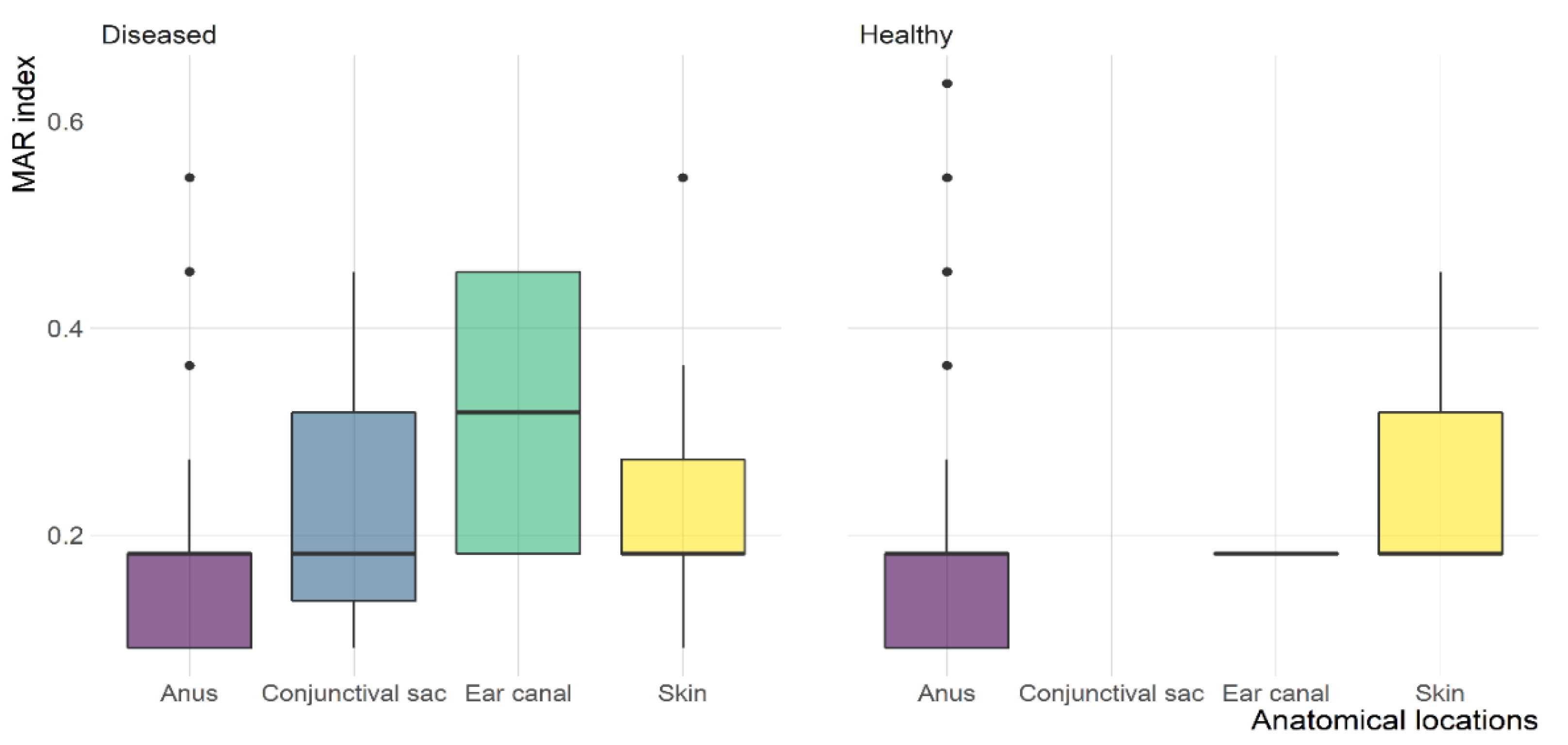
| Anatomical Location | Number (%) of E. coli Isolates | Total (n = 400) | |
|---|---|---|---|
| Healthy Cats (n = 209) | Diseased Cats (n = 191) | ||
| Anus | 55 (26.3) | 87 (45.5) | 142 (35.5) |
| Skin | 3 (1.4) | 10 (5.2) | 13 (3.3) |
| Ear canal | 2 (1.0) | 8 (4.2) | 10 (2.5) |
| Conjunctival sac | 0 (0.0) | 5 (2.6) | 5 (1.3) |
| Nares | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 60 (28.7) | 110 (57.6) | 170 (42.5) |
| Anatomical Location | N | No. (%) of MDR E. coli | Total | No. (%) ESBL E. coli | Total | ||
|---|---|---|---|---|---|---|---|
| Healthy | Diseased | Healthy | Diseased | ||||
| Anus | 142 | 9 (6.3) | 14 (9.9) | 23 (16.2) | 6 (4.2) | 5 (3.5) | 11 (7.7) |
| Skin | 13 | 1 (7.7) | 2 (15.4) | 3 (23.1) | 1 (7.7) | 0 (0.0) | 1 (7.7) |
| Ear canal | 10 | 0 (0.0) | 3 (30.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Conjunctival sac | 5 | 0 (0.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nares | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 170 | 10 (5.9) | 20 (11.8) | 30 (17.6) | 7 (4.1) | 5 (2.9) | 12 (7.1) |
| Cat No. | Group | Anatomical Location | Resistance Genes 1 | Virulence Genes 1 | Antimicrobial Resistance Patterns | MAR 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| blaCTX-M | blaTEM | blaSHV | eaeA | stx1 | stx2 | hlyA | |||||
| 3 | Healthy | Anus | CTX-M-1 | – | – | + | – | – | – | AMP, CTX, SXT | 0.27 |
| 8 | Healthy | Anus | CTX-M-1 | + | – | – | – | – | – | AMP, AMC, CTX, CIP, SXT, CHL | 0.55 |
| 19 | Healthy | Anus | CTX-M-15 | + | – | – | – | – | – | AMP, AMC, CTX, CIP, SXT, CHL | 0.55 |
| 25 | Diseased | Anus | CTX-M-15 | + | – | + | – | + | + | AMP, AMC, CTX, TCY, SXT | 0.45 |
| 29 | Healthy | Anus | CTX-M-15 | + | – | – | – | – | – | AMP, CTX, TCY, GEN, STR | 0.45 |
| 29 | Healthy | Skin | CTX-M-15 | + | – | – | – | – | – | AMP, CTX, TCY, GEN, STR | 0.45 |
| 41 | Diseased | Anus | CTX-M-15 | – | – | + | – | – | – | AMP, CTX, GEN, | 0.27 |
| 44 | Healthy | Anus | CTX-M-15 | + | – | – | – | – | – | AMP, AMC, CTX, TCY, STR, SXT, CHL | 0.64 |
| 53 | Diseased | Anus | CTX-M-1 | – | + | – | – | – | – | AMP, AMC, CTX, CIP, SXT, CHL | 0.55 |
| 82 | Diseased | Anus | CTX-M-1 | – | – | – | – | – | – | AMP, CTX, TCY | 0.27 |
| 216 | Diseased | Anus | CTX-M-15 | – | – | + | – | + | + | AMP, CTX, SXT | 0.27 |
| 267 | Healthy | Anus | CTX-M-15 | – | – | + | – | – | – | AMP, CTX, SXT | 0.27 |
| Factors | MDR E. coli | ESBL E. coli | ||
|---|---|---|---|---|
| OR 1 | p-Value | OR 1 | p-Value | |
| Family use antimicrobials | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 13.1 | 0.000 | 9.7 | 0.001 |
| Family member with acne | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 3.3 | 0.004 | 0.89 | 0.870 |
| Hospitalization | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 3.1 | 0.009 | 1.9 | 0.337 |
| Previous antimicrobials use for cat | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 3.4 | 0.006 | 4.1 | 0.040 |
| Current antimicrobials use for cat | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 8.3 | 0.000 | - | - |
| Child at home | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 2.4 | 0.052 | 2.9 | 0.122 |
| Cat Living | ||||
| Indoor | 1.00 (ref.) | 1.00 (ref.) | ||
| Indoors–outdoors | 0.7 | 0.461 | 2.3 | 0.295 |
| Reason being at clinic | ||||
| Vaccination and/or grooming | 1.00 (ref.) | 1.00 (ref.) | ||
| Treatment | 2.03 | 0.099 | 0.91 | 0.877 |
| Cat care | ||||
| Adult male | 1.00 (ref.) | 0.000 | 1.00 (ref.) | 0.016 |
| Adult female | 0.15 | 0.000 | 0.21 | 0.025 |
| Child | - | - | - | - |
| All family | 0.15 | 0.013 | - | - |
| Food type | ||||
| Dry | 1.00 (ref.) | 0.003 | 1.00 (ref.) | 0.006 |
| Wet | 0.99 | 0.992 | 2.5 | 0.456 |
| Raw | 4.2 | 0.013 | 16.8 | 0.001 |
| Home available | 5.2 | 0.001 | 12.1 | 0.005 |
| Factors | MDR E. coli | ESBL E. coli | ||
|---|---|---|---|---|
| OR (95% CI) 1 | p-Value | OR (95% CI) 1 | p-Value | |
| Family use antimicrobials | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 20.0 (6.29–63.69) | 0.000 | 16.6 (3.29–84.08) | 0.001 |
| Previous antimicrobials use for cat | ||||
| No | 1.00 (ref.) | 1.00 (ref.) | ||
| Yes | 5.5 (1.83–16.36) | 0.002 | 7.8 (1.66–36.34) | 0.009 |
| Child at home | ||||
| No | 1.00 (ref.) | |||
| Yes | 3.4 (1.14–10.30) | 0.027 | - | - |
| Cat care | ||||
| Adult male | 1.00 (ref.) | 0.019 | - | - |
| Adult female | 0.20 (0.06–0.69) | 0.011 | - | - |
| Child | - | - | - | - |
| All family | 0.21 (0.03–1.29) | 0.092 | - | - |
| Food type | ||||
| Dry | 1.00 (ref.) | 0.006 | 1.00 (ref.) | 0.001 |
| Wet | 0.93 (0.15–5.64) | 0.939 | 2.7 (0.22–33.50) | 0.431 |
| Raw | 8.5 (1.74–41.70) | 0.008 | 59.7 (7.16–497.54) | 0.000 |
| Home available | 6.3 (1.76–22.80) | 0.005 | 12.4 (1.94–79.63) | 0.008 |
| _cons | 0.004 (0.001–0.020) | 0.000 | 0.001 (0.0001–0.005) | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayez, M.; Elmoslemany, A.; Al Romaihi, A.A.; Azzawi, A.Y.; Almubarak, A.; Elsohaby, I. Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-lactamase Producing E. coli Isolated from Healthy and Diseased Cats. Antibiotics 2023, 12, 229. https://doi.org/10.3390/antibiotics12020229
Fayez M, Elmoslemany A, Al Romaihi AA, Azzawi AY, Almubarak A, Elsohaby I. Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-lactamase Producing E. coli Isolated from Healthy and Diseased Cats. Antibiotics. 2023; 12(2):229. https://doi.org/10.3390/antibiotics12020229
Chicago/Turabian StyleFayez, Mahmoud, Ahmed Elmoslemany, Ahmad A. Al Romaihi, Abdulfattah Y. Azzawi, Abdullah Almubarak, and Ibrahim Elsohaby. 2023. "Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-lactamase Producing E. coli Isolated from Healthy and Diseased Cats" Antibiotics 12, no. 2: 229. https://doi.org/10.3390/antibiotics12020229
APA StyleFayez, M., Elmoslemany, A., Al Romaihi, A. A., Azzawi, A. Y., Almubarak, A., & Elsohaby, I. (2023). Prevalence and Risk Factors Associated with Multidrug Resistance and Extended-Spectrum β-lactamase Producing E. coli Isolated from Healthy and Diseased Cats. Antibiotics, 12(2), 229. https://doi.org/10.3390/antibiotics12020229








