Targeting the Impossible: A Review of New Strategies against Endospores
Abstract
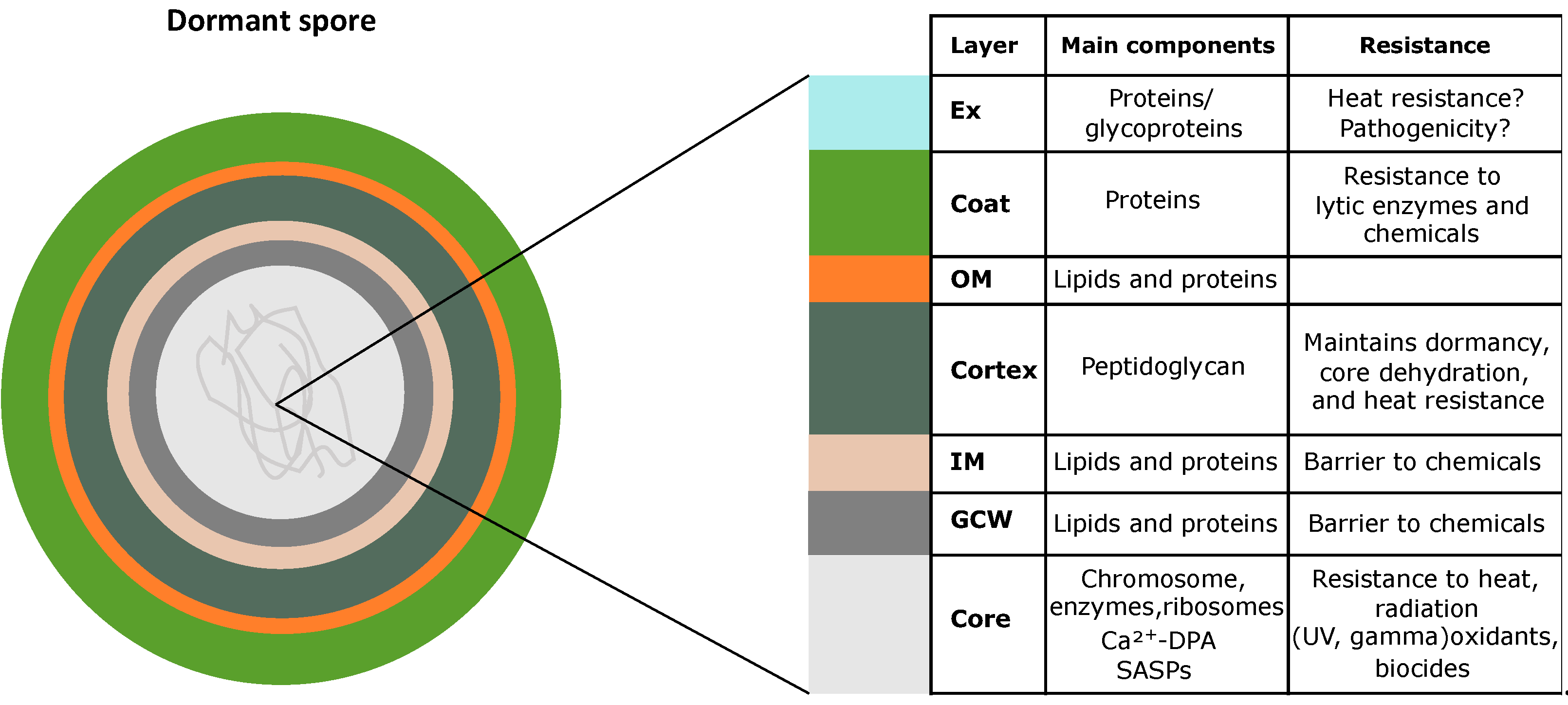
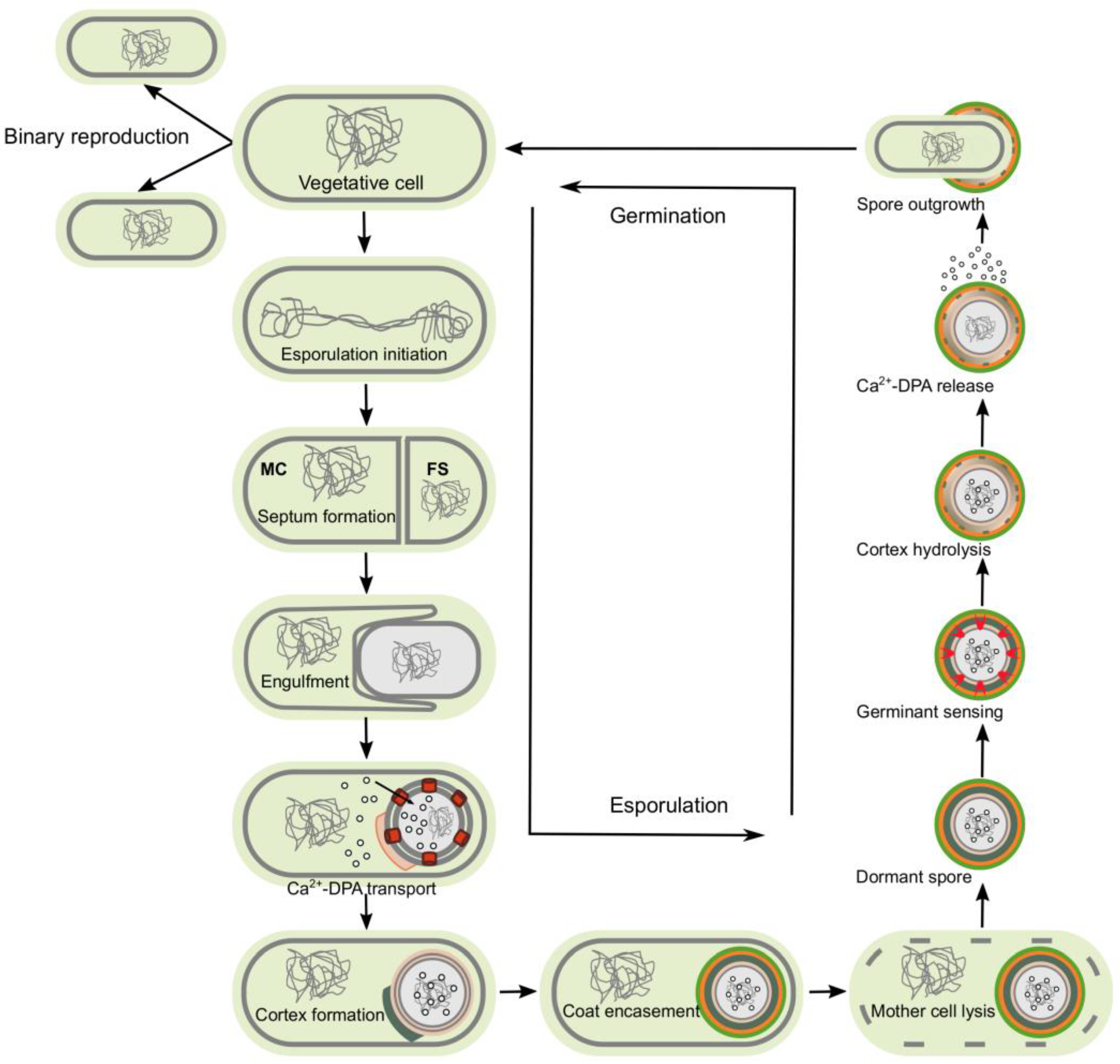
:1. Endospore
2. Spore Resistance and Killing Procedures
3. Pathogen Spore-Forming Bacteria
| Disease | SFP | Target | Toxins | Remarks | References |
|---|---|---|---|---|---|
| Anthrax | B. anthracis | Cutaneous, gastrointestinal, and pulmonary infection | Tripartite anthrax-toxin | Anthrax is endemic in several regions around the world, and its epidemiology mainly depends on its dynamics in wildlife, local agriculture, community education on transmission routes, and access to health and vaccination. | [94,95,96,97] |
| Food poisoning | B. cereus | Gastrointestinal system: diarrheal and emetic syndrome | Hbl, Nhe, CytK, and cyclic peptide c cereulide | Both syndromes, gastrointestinal and emetic, are generally mild and self-limiting. However, severe, and even lethal cases of emetic foodborne B. cereus disease have been reported. | [8,98] |
| Botulism | C. botulinum, C. baratii, and C. butyricum | Gastrointestinal, nervous, and muscular systems | Botulinum neurotoxin (BoNT) | Metabolic and biochemical tests have divided the C. botulinum strains into four groups, while antibody neutralization has separated the neurotoxins into seven serotypes. | [99,100,101,102,103,104] |
| Food poisoning, myonecrosis (Gas gangrene), fatal infections in postdelivery women, and necrotizing colitis | C. perfringens | Gastrointestinal system, and wound and extremities | α-, β-, ε-, and ι toxins are the most common toxins | Currently, 23 virulence genes that encode toxins and virulent enzymes have been identified in C. perfringens, making it the most prolific toxin-producing pathogen presently known. | [105,106,107] |
| C. tetani | Tetanus | Nervous and muscular systems: local, cephalic, and neonatal tetanus | Tetanospasmin (also called tetanus neurotoxin; TeNT) | People who have not been vaccinated are more likely to have cases of generalized tetanus, while people who are poorly immunized are more likely to have local cases. With this disease, the most enduring challenge has been the prevention measure of free vaccination campaigns. | [108,109,110,111] |
| Pseudomem-braneous colitis | C. difficile | Gastrointestinal system (colon) | Toxin A (TcdA) and toxin B (TcdB) | The fecal–oral route transmits spores. These bacteria colonize the large intestine when there is dysbiosis in the gut microbiota caused by antibiotic treatments. It is associated with multiple relapses and recurrence. | [112,113,114] |
| Septic shock and necrotizing fasciitis | C. sordelii | Mostly associated with gynecological complications in women | Pathogenic strains of C. sordellii generate up to 7 identified exotoxins; among them, the lethal toxin (LT) and hemorrhagic toxin (HT) are regarded as the major virulence factors | The infection progresses rapidly. Thus, therapeutic interventions are rarely successful. At present, there is no antitoxin available. | [89,115,116] |
4. Approaches in the Food Industry to Control of Spore-Forming Bacteria
5. Natural Products against the Spore
| Bacteriocin | Producer | Spore Tested | Remarks |
|---|---|---|---|
| Haloduracin | Bacillus halodurans | B. anthracis | It inhibited spore outgrowth [133] |
| Nisin | Lactococcus lactis | C. perfringens | It arrested outgrowth of germinated spores in rich medium, but it did not affect a meat model system [126] |
| C. sporogenes | Effective when spores germinated but not on spores themselves [134] | ||
| C. difficile | At high concentrations, nisin appeared to cause a statistically significant decrease in the viability of non-germinated spores [124] | ||
| C. beijerinckii | Active with previous high temperature [124] | ||
| C. botulinum | Heated spores were very sensitive to nisin, and nisin-treated spores became more heat sensitive; nisin acted as a pro-germinant [135] | ||
| B. subtilis | Sporicidal on germinated spores [123] | ||
| A. acidoterrestris | At high concentrations ranging from 0.1 to 1.5 mg liter−1, nisin had an inhibitory effect without the application of any previous thermal treatment [136]. | ||
| Enterocin ASK48 | Enterococcus faecalis | A. acidoterrestris | Active on resting spores [137] |
| Enterocin EJ9 | Enterococcus faecalis EJ97 | Geobacillus stearothermophilus | Heat-activated endospores became sensitive [138] |
| Bificin C6165 | Bifidobacterium animalis subsp. animalis CICC 6165 | A. acidoterrestris | In commercial diluted apple juice, no significant activity of bificin was observed against the endospores, but its addition contributed to the reduction in thermal resistance [139] |
| Lacticin | L. lactis IFPL 3593 two-peptide lantibiotic | C. tyrobutyricum | It inhibited the germination of clostridia spores and decreased the number of clostridia spores [140] |
| Plantaricin | Lactobacillus plantarum TF711 | C. sporogenes | Clostridia spore count was significantly lower in the experimental cheese model [141] |
| Plantaricin JY22 | Lactobacillus plantarum JY22 | B. cereus | It affected spore integrity; the exosporium was peeled out, and leaking spores were hollow [129] |
| Plantaricin YKX | L. plantarum | Alicyclobacillus spp. | Bacteriocins could induce the germination of A. acidoterrestris spores [142]; in endospore suspensions, cell viability decreased in proportion to the bacteriocin concentration added [130] |
| Thurincin H | Bacillus thuringiensis | B. cereus | Decrease in viable counts only when germination was induced by BHI (p < 0.05) [143] |
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dworkin, J.; Losick, R. Linking nutritional status to gene activation and development. Genes Dev. 2001, 15, 1051–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. MMBR 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, E.M.; Boekhorst, J.; Kuipers, O.P.; Wells-Bennik, M.H. A mobile genetic element profoundly increases heat resistance of bacterial spores. ISME J. 2016, 10, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, D.R.; Nicholson, W.L. Experimental evolution of Bacillus subtilis. Environ. Microbiol. 2017, 19, 3415–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tocheva, E.I.; Ortega, D.R.; Jensen, G.J. Sporulation, bacterial cell envelopes and the origin of life. Nat. Rev. Microbiol. 2016, 14, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.O.; Carlin, F.; Broussolle, V. Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Henriques, A.O.; Moran, C.P., Jr. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [Green Version]
- Traag, B.A.; Driks, A.; Stragier, P.; Bitter, W.; Broussard, G.; Hatfull, G.; Chu, F.; Adams, K.N.; Ramakrishnan, L.; Losick, R. Do mycobacteria produce endospores? Proc. Natl. Acad. Sci. USA 2010, 107, 878–881. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, E.A.; Miller, D.A.; Angert, E.R. Sporulation in Bacteria: Beyond the Standard Model. Microbiol. Spectr. 2014, 2, 87–102. [Google Scholar] [CrossRef]
- Higgins, D.; Dworkin, J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 2012, 36, 131–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, T.L.; Reis, A.; Kent, C.A.; Kosseva, M.; Roseiro, J.C.; Hewitt, C.J. Stress-induced physiological responses to starvation periods as well as glucose and lactose pulses in Bacillus licheniformis CCMI 1034 continuous aerobic fermentation processes as measured by multi-parameter flow cytometry. Biochem. Eng. J. 2005, 24, 31–41. [Google Scholar] [CrossRef]
- Makarova, K.S.; Anantharaman, V.; Aravind, L.; Koonin, E.V. Live virus-free or die: Coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 2012, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Mearls, E.B.; Izquierdo, J.A.; Lynd, L.R. Formation and characterization of non-growth states in Clostridium thermocellum: Spores and L-forms. BMC Microbiol. 2012, 12, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, A.N.; Nawrocki, K.L.; McBride, S.M. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect. Immun. 2014, 82, 4276–4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef] [Green Version]
- Underwood, S.; Guan, S.; Vijayasubhash, V.; Baines, S.D.; Graham, L.; Lewis, R.J.; Wilcox, M.H.; Stephenson, K. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J. Bacteriol. 2009, 191, 7296–7305. [Google Scholar] [CrossRef] [Green Version]
- Pettit, L.J.; Browne, H.P.; Yu, L.; Smits, W.K.; Fagan, R.P.; Barquist, L.; Martin, M.J.; Goulding, D.; Duncan, S.H.; Flint, H.J.; et al. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genom. 2014, 15, 160. [Google Scholar] [CrossRef] [Green Version]
- Meeske, A.J.; Rodrigues, C.D.; Brady, J.; Lim, H.C.; Bernhardt, T.G.; Rudner, D.Z. High-Throughput Genetic Screens Identify a Large and Diverse Collection of New Sporulation Genes in Bacillus subtilis. PLoS Biol. 2016, 14, e1002341. [Google Scholar] [CrossRef] [Green Version]
- Boone, T.J.; Mallozzi, M.; Nelson, A.; Thompson, B.; Khemmani, M.; Lehmann, D.; Dunkle, A.; Hoeprich, P.; Rasley, A.; Stewart, G.; et al. Coordinated Assembly of the Bacillus anthracis Coat and Exosporium during Bacterial Spore Outer Layer Formation. mBio 2018, 9, e01166-18. [Google Scholar] [CrossRef]
- Swick, M.C.; Koehler, T.M.; Driks, A. Surviving Between Hosts: Sporulation and Transmission. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, I.S.; Ramamurthi, K.S. Spore formation in Bacillus subtilis. Environ. Microbiol. Rep. 2014, 6, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Carrera, M.; Zandomeni, R.O.; Fitzgibbon, J.; Sagripanti, J.L. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2007, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007, 15, 172–180. [Google Scholar] [CrossRef]
- Setlow, P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. Soc. Appl. Bacteriol. Symp. Ser. 1994, 23, 49S–60S. [Google Scholar]
- Atrih, A.; Foster, S.J. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek 1999, 75, 299–307. [Google Scholar] [CrossRef]
- Westphal, A.J.; Price, P.B.; Leighton, T.J.; Wheeler, K.E. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 2003, 100, 3461–3466. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P.; Wang, S.; Li, Y.Q. Germination of Spores of the Orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 2017, 71, 459–477. [Google Scholar] [CrossRef]
- Zheng, L.; Abhyankar, W.; Ouwerling, N.; Dekker, H.L.; van Veen, H.; van der Wel, N.N.; Roseboom, W.; de Koning, L.J.; Brul, S.; de Koster, C.G. Bacillus subtilis Spore Inner Membrane Proteome. J. Proteome Res. 2016, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Abel-Santos, E. The Ger receptor family from sporulating bacteria. Curr. Issues Mol. Biol. 2010, 12, 147–158. [Google Scholar]
- Alabdali, Y.A.J.; Oatley, P.; Kirk, J.A.; Fagan, R.P. A cortex-specific penicillin-binding protein contributes to heat resistance in Clostridioides difficile spores. Anaerobe 2021, 70, 102379. [Google Scholar] [CrossRef]
- Rao, L.; Liao, X.; Setlow, P. Bacillus spore wet heat resistance and evidence for the role of an expanded osmoregulatory spore cortex. Lett. Appl. Microbiol. 2016, 63, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Butzin, X.Y.; Troiano, A.J.; Coleman, W.H.; Griffiths, K.K.; Doona, C.J.; Feeherry, F.E.; Wang, G.; Li, Y.Q.; Setlow, P. Analysis of the effects of a gerP mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 2012, 194, 5749–5758. [Google Scholar] [CrossRef] [Green Version]
- Behravan, J.; Chirakkal, H.; Masson, A.; Moir, A. Mutations in the gerP locus of Bacillus subtilis and Bacillus cereus affect access of germinants to their targets in spores. J. Bacteriol. 2000, 182, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Aronson, A. Regulation of expression of a select group of Bacillus anthracis spore coat proteins. FEMS Microbiol. Lett. 2018, 365, fny063. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Driks, A.; Eichenberger, P. The Spore Coat. Microbiol. Spectr. 2016, 4, 179–200. [Google Scholar] [CrossRef]
- de Hoon, M.J.; Eichenberger, P.; Vitkup, D. Hierarchical evolution of the bacterial sporulation network. Curr. Biol. CB 2010, 20, R735–R745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.J.; Todd, S.J.; Ball, D.A.; Shepherd, A.M.; Sylvestre, P.; Moir, A. ExsY and CotY Are Required for the Correct Assembly of the Exosporium and Spore Coat of Bacillus cereus. J. Bacteriol. 2006, 188, 7905–7913. [Google Scholar] [CrossRef]
- Stewart, G.C. The Exosporium Layer of Bacterial Spores: A Connection to the Environment and the Infected Host. Microbiol. Mol. Biol. Rev. MMBR 2015, 79, 437–457. [Google Scholar] [CrossRef] [Green Version]
- Castro-Cordova, P.; Mora-Uribe, P.; Reyes-Ramirez, R.; Cofre-Araneda, G.; Orozco-Aguilar, J.; Brito-Silva, C.; Mendoza-Leon, M.J.; Kuehne, S.A.; Minton, N.P.; Pizarro-Guajardo, M.; et al. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat. Commun. 2021, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Fimlaid, K.A.; Bond, J.P.; Schutz, K.C.; Putnam, E.E.; Leung, J.M.; Lawley, T.D.; Shen, A. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 2013, 9, e1003660. [Google Scholar] [CrossRef]
- Fimlaid, K.A.; Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 2015, 24, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.C.; Saujet, L.; Tome, A.R.; Serrano, M.; Monot, M.; Couture-Tosi, E.; Martin-Verstraete, I.; Dupuy, B.; Henriques, A.O. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet. 2013, 9, e1003782. [Google Scholar] [CrossRef] [Green Version]
- Setlow, P. Dynamics of the assembly of a complex macromolecular structure the coat of spores of the bacterium Bacillus subtilis. Mol. Microbiol. 2012, 83, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Imamura, D.; Kuwana, R.; Takamatsu, H.; Watabe, K. Proteins involved in formation of the outermost layer of Bacillus subtilis spores. J. Bacteriol. 2011, 193, 4075–4080. [Google Scholar] [CrossRef] [Green Version]
- Imamura, D.; Kuwana, R.; Takamatsu, H.; Watabe, K. Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J. Bacteriol. 2010, 192, 518–524. [Google Scholar] [CrossRef] [Green Version]
- McKenney, P.T.; Eichenberger, P. Dynamics of spore coat morphogenesis in Bacillus subtilis. Mol. Microbiol. 2012, 83, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Niu, S.; Yankova, M.; Mecklenburg, M.; King, S.M.; Ravichandran, J.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Setlow, P. Analysis of killing of growing cells and dormant and germinated spores of Bacillus species by black silicon nanopillars. Sci. Rep. 2017, 7, 17768. [Google Scholar] [CrossRef] [PubMed]
- Kochan, T.J.; Foley, M.H.; Shoshiev, M.S.; Somers, M.J.; Carlson, P.E.; Hanna, P.C. Updates to Clostridium difficile Spore Germination. J. Bacteriol. 2018, 200, e00218-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moir, A.; Cooper, G. Spore Germination. Microbiol. Spectr. 2015, 3, 217–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, D.; McAllister, K.N.; Sorg, J.A. Germinants and Their Receptors in Clostridia. J. Bacteriol. 2016, 198, 2767–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, A. Clostridioides difficile Spore Formation and Germination: New Insights and Opportunities for Intervention. Annu. Rev. Micro. 2020, 74, 545–566. [Google Scholar] [CrossRef]
- Wood, J.P.; Adrion, A.C. Review of Decontamination Techniques for the Inactivation of Bacillus anthracis and Other Spore-Forming Bacteria Associated with Building or Outdoor Materials. Environ. Sci. Technol. 2019, 53, 4045–4062. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.L.; Liu, D.H.; Zhou, J.W. Bacterial spore inactivation by non-thermal technologies: Resistance and inactivation mechanisms. Curr. Opin. Food Sci. 2021, 42, 31–36. [Google Scholar] [CrossRef]
- Taylor, W.; Camilleri, E.; Craft, D.L.; Korza, G.; Granados, M.R.; Peterson, J.; Szczpaniak, R.; Weller, S.K.; Moeller, R.; Douki, T.; et al. DNA Damage Kills Bacterial Spores and Cells Exposed to 222-Nanometer UV Radiation. Appl. Environ. Microbiol. 2020, 86, e03039-19. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore Resistance Properties. Microbiol Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Clair, G.; Esbelin, J.; Mallea, S.; Bornard, I.; Carlin, F. The spore coat is essential for Bacillus subtilis spore resistance to pulsed light, and pulsed light treatment eliminates some spore coat proteins. Int. J. Food Microbiol. 2020, 323, 108592. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mosher, W.; Wiertzema, J.; Peng, P.; Min, M.; Cheng, Y.; An, J.; Ma, Y.; Fan, X.; Niemira, B.A.; et al. Effects of intense pulsed light and gamma irradiation on Bacillus cereus spores in mesquite pod flour. Food Chem. 2021, 344, 128675. [Google Scholar] [CrossRef]
- Ortatatli, M.; Canitez, K.; Sezigen, S.; Eyison, R.K.; Kenar, L. Evaluation of Gamma-Radiation Inactivation of a Bioterrorism Agent, Bacillus anthracis Spores, on Different Materials. Indian J. Microbiol. 2018, 58, 76–80. [Google Scholar] [CrossRef]
- Cote, C.K.; Buhr, T.; Bernhards, C.B.; Bohmke, M.D.; Calm, A.M.; Esteban-Trexler, J.S.; Hunter, M.; Katoski, S.E.; Kennihan, N.; Klimko, C.P.; et al. A Standard Method To Inactivate Bacillus anthracis Spores to Sterility via Gamma Irradiation. Appl. Environ. Microbiol. 2018, 84, e00106-18. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Chen, Z.; Wang, S.; Wu, M.; Setlow, P.; Li, Y.Q. Germination, Outgrowth, and Vegetative-Growth Kinetics of Dry-Heat-Treated Individual Spores of Bacillus Species. Appl. Environ. Microbiol. 2018, 84, e02618-17. [Google Scholar] [CrossRef] [Green Version]
- Shirey, B.T.; Schubert, W.; Benardini, J. An Overview of Surface Heat Microbial Reduction as a Viable Microbial Reduction Modality for Spacecraft Surfaces. In Proceedings of the 47th International Conference on Environmental Systems (ICES), Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Joslyn, L. Sterilization by Heat in Disinfection, Sterlization, and Preservation; Block, S.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Sandle, T.S. Sterility, Sterilisation and Sterility Assurance for Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 93–109. [Google Scholar]
- Soni, A.; Parlane, N.A.; Khan, F.; Derraik, J.G.B.; Wild, C.E.K.; Anderson, Y.C.; Brightwell, G. Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators. Pathogens 2022, 11, 871. [Google Scholar] [CrossRef]
- Huesca-Espitia, L.C.; Suvira, M.; Rosenbeck, K.; Korza, G.; Setlow, B.; Li, W.; Wang, S.; Li, Y.Q.; Setlow, P. Effects of steam autoclave treatment on Geobacillus stearothermophilus spores. J. Appl. Microbiol. 2016, 121, 1300–1311. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Smelt, J.; Vischer, N.O.E.; de Vos, A.L.; Setlow, P.; Brul, S. Heat Activation and Inactivation of Bacterial Spores: Is There an Overlap? Appl. Environ. Microbiol. 2022, 88, e0232421. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, D.; Dahlberg, T.; Wiklund, K.; Andersson, P.O.; Henriksson, S.; Andersson, M. Mode of Action of Disinfection Chemicals on the Bacterial Spore Structure and Their Raman Spectra. Anal. Chem. 2021, 93, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Kenters, N.; Huijskens, E.G.W.; de Wit, S.C.J.; Sanders, I.; van Rosmalen, J.; Kuijper, E.J.; Voss, A. Effectiveness of various cleaning and disinfectant products on Clostridium difficile spores of PCR ribotypes 010, 014 and 027. Antimicrob. Resist. Infect. Control 2017, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, M.C.; Massicotte, R.; Hurtubise, Y.; Gagne-Bourque, F.; Mafu, A.A.; Yahia, L. Evaluating the Sporicidal Activity of Disinfectants against Clostridium difficile and Bacillus amyloliquefaciens Spores by Using the Improved Methods Based on ASTM E2197-11. Front. Public Health 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stier, P.; Kulozik, U. Effect of Sporulation Conditions Following Submerged Cultivation on the Resistance of Bacillus atrophaeus Spores against Inactivation by H2O2. Molecules 2020, 25, 2985. [Google Scholar] [CrossRef]
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial spore inactivation induced by cold plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Miri, T.; Nwabor, O.F.; Hart, A.; Al-Sharify, Z.T.; Al-Najjar, S.; Anumudu, C. Recent advances in radio frequency, pulsed light, and cold plasma technologies for food safety. J. Food Process. Eng. 2022, 45, e14138. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Ayub, Z.; Muhammad Aadil, R.; Abid, M.; Zhang, J.; Liqing, Z. Recent Advances in Plasma Technology: Influence of Atmospheric Cold Plasma on Spore Inactivation. Food Rev. Int. 2021, 38, 789–811. [Google Scholar] [CrossRef]
- Buszewski, B.; Wrona, O.; Mayya, R.P.; Zakharenko, A.M.; Kalenik, T.K.; Golokhvast, K.S.; Piekoszewski, W.; Rafinska, K. The potential application of supercritical CO(2) in microbial inactivation of food raw materials and products. Crit. Rev. Food Sci. Nutr. 2022, 62, 6535–6548. [Google Scholar] [CrossRef]
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: A review. J. Food Sci. Technol. 2022, 59, 417–428. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2020, 13, 570–591. [Google Scholar] [CrossRef]
- Delbruck, A.I.; Tritten, Y.; Nanni, P.; Heydenreich, R.; Mathys, A. Moderate High-Pressure Superdormancy in Bacillus Spores: Properties of Superdormant Spores and Proteins Potentially Influencing Moderate High-Pressure Germination. Appl. Environ. Microbiol. 2022, 88, e0240621. [Google Scholar] [CrossRef]
- Lay, C.L.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I.L. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mathys, A. Superdormant Spores as a Hurdle for Gentle Germination-Inactivation Based Spore Control Strategies. Front. Microbiol. 2018, 9, 3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetz, G.; Tetz, V. Introducing the sporobiota and sporobiome. Gut Pathog. 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippidou, S.; Junier, T.; Wunderlin, T.; Lo, C.C.; Li, P.E.; Chain, P.S.; Junier, P. Under-detection of endospore-forming Firmicutes in metagenomic data. Comput. Struct. Biotechnol. J. 2015, 13, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, D.L.; Aldape, M.J.; Bryant, A.E. Life-threatening clostridial infections. Anaerobe 2012, 18, 254–259. [Google Scholar] [CrossRef]
- Milton, A.A.P.; Sanjukta, R.; Gogoi, A.P.; Momin, K.M.; Priya, G.B.; Das, S.; Ghatak, S.; Sen, A.; Kandpal, B.K. Prevalence, molecular typing and antibiotic resistance of Clostridium perfringens in free range ducks in Northeast India. Anaerobe 2020, 64, 102242. [Google Scholar] [CrossRef]
- Kiersnowska, Z.M.; Lemiech-Mirowska, E.; Michalkiewicz, M.; Marczak, M. Hand hygiene as the basic method of reducing Clostridium difficile infections (CDI) in a hospital environment. Ann. Agric. Environ. Med. 2021, 28, 535–540. [Google Scholar] [CrossRef]
- Sasahara, T.; Hayashi, S.; Hosoda, K.; Morisawa, Y.; Hirai, Y. Comparison of hand hygiene procedures for removing Bacillus cereus spores. Biocontrol Sci. 2014, 19, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.A.; Anderson, D.J. Hospital Infection Control: Clostridioides difficile. Clin. Colon Rectal Surg. 2020, 33, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Pilo, P.; Frey, J. Pathogenicity, population genetics and dissemination of Bacillus anthracis. Infect. Genet. Evol. 2018, 64, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Anthrax. 2017. Available online: https://www.cdc.gov/anthrax/index.html (accessed on 1 November 2022).
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Getz, W.M.; Kausrud, K.L.; Cizauskas, C.A.; Blackburn, J.K.; Bustos Carrillo, F.A.; Colwell, R.; Easterday, W.R.; Ganz, H.H.; Kamath, P.L.; et al. Spores and soil from six sides: Interdisciplinarity and the environmental biology of anthrax (Bacillus anthracis). Biol. Rev. 2018, 93, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Warda, A.K.; Siezen, R.J.; Boekhorst, J.; Wells-Bennik, M.H.; de Jong, A.; Kuipers, O.P.; Nierop Groot, M.N.; Abee, T. Linking Bacillus cereus Genotypes and Carbohydrate Utilization Capacity. PLoS ONE 2016, 11, e0156796. [Google Scholar] [CrossRef]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef]
- Schaumann, R.; Dallacker-Losensky, K.; Rosenkranz, C.; Genzel, G.H.; Stingu, C.S.; Schellenberger, W.; Schulz-Stubner, S.; Rodloff, A.C.; Eschrich, K. Discrimination of Human Pathogen Clostridium Species Especially of the Heterogeneous C. sporogenes and C. botulinum by MALDI-TOF Mass Spectrometry. Curr. Microbiol. 2018, 75, 1506–1515. [Google Scholar] [CrossRef]
- Connan, C.; Popoff, M.R. Two-component systems and toxinogenesis regulation in Clostridium botulinum. Res. Microbiol. 2015, 166, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Nagalli, S. Clostridium botulinum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Antonucci, L.; Locci, C.; Schettini, L.; Clemente, M.G.; Antonucci, R. Infant botulism: An underestimated threat. Infect. Dis. 2021, 53, 647–660. [Google Scholar] [CrossRef]
- Edmunds, S.; Vugia, D.J.; Rosen, H.E.; Wong, K.K.; Dykes, J.K.; Griffin, P.M.; Chatham-Stephens, K. Inadequate Refrigeration of Some Commercial Foods Is a Continued Cause of Foodborne Botulism in the United States, 1994–2021. Foodborne Pathog. Dis. 2022, 19, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Shimizu, T. Regulation of Toxin Production in Clostridium perfringens. Toxins 2016, 8, 207. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Paredes-Sabja, D.; Sarker, M.R.; McClane, B.A. Clostridium perfringens Sporulation and Sporulation-Associated Toxin Production. Microbiol. Spectr. 2016, 4, 3. [Google Scholar] [CrossRef]
- George, E.K.; De Jesus, O.; Vivekanandan, R. Clostridium tetani. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Yen, L.M.; Thwaites, C.L. Tetanus. Lancet 2019, 393, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Rood, J.I.; Lyras, D. Clostridial Genetics: Genetic Manipulation of the Pathogenic Clostridia. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Bruggemann, H.; Brzuszkiewicz, E.; Chapeton-Montes, D.; Plourde, L.; Speck, D.; Popoff, M.R. Genomics of Clostridium tetani. Res. Microbiol. 2015, 166, 326–331. [Google Scholar] [CrossRef]
- Hall, A.J.; Curns, A.T.; McDonald, L.C.; Parashar, U.D.; Lopman, B.A. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin. Infect. Dis. 2012, 55, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Burke, K.E.; Lamont, J.T. Clostridium difficile infection: A worldwide disease. Gut Liver 2014, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.; Auchtung, J.M. Control of Clostridium difficile Infection by Defined Microbial Communities. Microbiol. Spectr. 2017, 5, 267–289. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, J.; Deleon-Carnes, M.; Kellar, K.L.; Bandyopadhyay, K.; Antoniadou, Z.A.; Shieh, W.J.; Paddock, C.D.; Zaki, S.R. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: Diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect. Dis. Obstet. Gynecol. 2012, 2012, 972845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.L.; Bhatnagar, J.; Reagan, S.; Zane, S.B.; D’Angeli, M.A.; Fischer, M.; Killgore, G.; Kwan-Gett, T.S.; Blossom, D.B.; Shieh, W.J.; et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet. Gynecol. 2007, 110, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- McHugh, A.J.; Feehily, C.; Hill, C.; Cotter, P.D. Detection and Enumeration of Spore-Forming Bacteria in Powdered Dairy Products. Front. Microbiol. 2017, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Wells-Bennik, M.H.; Eijlander, R.T.; den Besten, H.M.; Berendsen, E.M.; Warda, A.K.; Krawczyk, A.O.; Nierop Groot, M.N.; Xiao, Y.; Zwietering, M.H.; Kuipers, O.P.; et al. Bacterial Spores in Food: Survival, Emergence, and Outgrowth. Annu. Rev. Food Sci. Technol. 2016, 7, 457–482. [Google Scholar] [CrossRef]
- Doll, E.V.; Scherer, S.; Wenning, M. Spoilage of Microfiltered and Pasteurized Extended Shelf Life Milk Is Mainly Induced by Psychrotolerant Spore-Forming Bacteria that often Originate from Recontamination. Front. Microbiol. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Gut, I.M.; Blanke, S.R.; van der Donk, W.A. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem. Biol. 2011, 6, 744–752. [Google Scholar] [CrossRef]
- Omardien, S.; Drijfhout, J.W.; Zaat, S.A.; Brul, S. Cationic Amphipathic Antimicrobial Peptides Perturb the Inner Membrane of Germinated Spores Thus Inhibiting Their Outgrowth. Front. Microbiol. 2018, 9, 2277. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, S.; Gebhardt, D.; Ho, L.; Ganzle, M.; McMullen, L.M. Effects of nisin and reutericyclin on resistance of endospores of Clostridium spp. to heat and high pressure. Food Microbiol. 2013, 34, 46–51. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.; O’Connor, P.M.; O’Sullivan, O.; Rea, M.C.; Hill, C.; Ross, R.P. Impact of nisin on Clostridioides difficile and microbiota composition in a faecal fermentation model of the human colon. J. Appl. Microbiol. 2022, 132, 1397–1408. [Google Scholar] [CrossRef]
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 2012, 77, M51–M56. [Google Scholar] [CrossRef] [PubMed]
- Portinha, I.M.; Douillard, F.P.; Korkeala, H.; Lindström, M. Sporulation Strategies and Potential Role of the Exosporium in Survival and Persistence of Clostridium botulinum. In Int. J. Mol. Sci. 2022, 23, 754. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, J.; Lu, X.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lu, F. Purification, Characterization, and Mode of Action of Plantaricin GZ1-27, a Novel Bacteriocin against Bacillus cereus. J. Agric. Food Chem. 2018, 66, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Miao, L.; Ma, H.; Bai, F.; Lin, Y.; Sun, M.; Li, J. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci. Biotechnol. 2018, 27, 695–703. [Google Scholar] [CrossRef]
- Yue, T.; Pei, J.; Yuan, Y. Purification and characterization of anti-Alicyclobacillus bacteriocin produced by Lactobacillus rhamnosus. J. Food Prot. 2013, 76, 1575–1581. [Google Scholar] [CrossRef]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. Lantibiotic resistance. Microbiol. Mol. Biol. Rev. MMBR 2015, 79, 171–191. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, A.S.; Crandall, A.D.; Montville, T.J. Nisin Resistance in Clostridium botulinum Spores and Vegetative Cells. Appl. Environ. Microbiol. 1997, 63, 2654–2659. [Google Scholar] [CrossRef] [Green Version]
- Oman, T.J.; van der Donk, W.A. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 2009, 4, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Ros-Chumillas, M.; Esteban, M.D.; Huertas, J.P.; Palop, A. Effect of Nisin and Thermal Treatments on the Heat Resistance of Clostridium sporogenes Spores. J. Food Prot. 2015, 78, 2019–2023. [Google Scholar] [CrossRef]
- Mazzotta, A.S.; Montville, T.J. Characterization of fatty acid composition, spore germination, and thermal resistance in a nisin-resistant mutant of Clostridium botulinum 169B and in the wild-type strain. Appl. Environ. Microbiol. 1999, 65, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Huertas, J.P.; Esteban, M.D.; Antolinos, V.; Palop, A. Combined effect of natural antimicrobials and thermal treatments on Alicyclobacillus acidoterrestris spores. Food Control 2014, 35, 73–78. [Google Scholar] [CrossRef]
- Grande, M.J.; Lucas, R.; Abriouel, H.; Omar, N.B.; Maqueda, M.; Martinez-Bueno, M.; Martinez-Canamero, M.; Valdivia, E.; Galvez, A. Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int. J. Food Microbiol. 2005, 104, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Martínez Viedma, P.; Abriouel, H.; Ben Omar, N.; Lucas López, R.; Gálvez, A. Effect of enterocin EJ97 against Geobacillus stearothermophilus vegetative cells and endospores in canned foods and beverages. Eur. Food Res. Technol. 2009, 230, 513–519. [Google Scholar] [CrossRef]
- Pei, J.; Yue, T.; Yuan, Y. Control of Alicyclobacillus acidoterrestris in fruit juices by a newly discovered bacteriocin. World J. Microbiol. Biotechnol. 2014, 30, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Carmen Martínez-Cuesta, M.; Bengoechea, J.; Bustos, I.; Rodríguez, B.; Requena, T.; Peláez, C. Control of late blowing in cheese by adding lacticin 3147-producing Lactococcus lactis IFPL 3593 to the starter. Int. Dairy J. 2010, 20, 18–24. [Google Scholar] [CrossRef]
- Gonzalez, L.; Zarate, V. Inhibitory activity of Lactobacillus plantarum TF711 against Clostridium sporogenes when used as adjunct culture in cheese manufacture. J. Dairy Res. 2015, 82, 236–241. [Google Scholar] [CrossRef]
- Pei, J.; Jin, W.; Wang, J.; Huang, Y.; Li, X.; Zhang, H.; Zhang, Y.; Ramadan, A.; Abd El-Aty, A.M. Purification and Characterization of Plantaricin YKX and Assessment of Its Inhibitory Activity Against Alicyclobacillus spp. Front. Microbiol. 2021, 12, 783266. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Manns, D.C.; Guron, G.K.; Churey, J.J.; Worobo, R.W. Large-Scale Purification, Characterization, and Spore Outgrowth Inhibitory Effect of Thurincin H, a Bacteriocin Produced by Bacillus thuringiensis SF361. Probiotics Antimicrob Proteins 2014, 6, 105–113. [Google Scholar] [CrossRef]
| Agent | Remarks | Target | Resistance Mechanism | Application | Disadvantages | References |
|---|---|---|---|---|---|---|
| UV radiation | UV 254 nm radiation is the most effective in killing spores; potentially, UV 222 nm | DNA | SASPs, DNA repair enzymes, and coat | Surface decontamination | Mutagenic effects of UV 254; few dosage/efficacy data | [57,58,60,61,62,63] |
| γ-Radiation | Cobalt-60 (Co60) and/or cesium-137 are generally used for gamma-radiation procedures, and 3 kGy was the lowest and 30 kGy the highest doses adequate for the inactivation of 106–108 spores | DNA | SASPs and DNA repair enzymes | Food processing applications, medical devices and pharmaceutical products, and maybe powdered foods | Specialized and expensive equipment: concern about biosecurity of γ-irradiated foods | [62,64,65,66] |
| Dry heat | Can be conducted with hot air using a forced convection oven; standard treatments are 160 °C for 2 h or 170 °C for 1 h | DNA | SASPs | Glass and metal medical devices, and materials of spacecraft hardware | Incompatible for several materials (plastic and rubber): spores of some bacteria are resistant to dry heat; cost of electricity | [58,67,68,69,70,71] |
| Wet heat | Refers to environments at elevated temperatures and saturated with moisture (100% RH) or boiling water | Inactivation of core enzymes | Low water content and SASPs | Medical/surgical supplies, microbiological growth media, and hospital waste | Material incompatibility Incorrect heat treatment could also generate heterogeneous spore germination; spores of some species are resistant to ≥100 °C | [34,72,73] |
| Chemicals | Oxidizing agents, aldehydes, and acids and alkali disinfectants | It depends on the chemical nature of the agent but may include: DNA, inner membrane, germination enzymes, and core proteins | SASPs, coat, low permeability | Hospital and industry facilities, and surfaces | Toxic and corrosive effects; residue generation | [57,74,75,76,77] |
| Plasma | DNA damage | Further studies are needed | Packing material and food | Food oxidation; plasma source is relatively small and must be in proximity | [58,78,79,80] | |
| Supercritical fluids | Supercritical CO2 | Spore cell wall, coat, cortex, and membrane | Further studies are needed | Packing material and food | Small scale | [81,82,83] |
| Germinants | Induction of germination with enzymes, nutrients, or heat and pressure | Expensive; heterogeneous germination; superdormancy | [67,84,85,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Rodríguez, A.; Ruiz-Villafán, B.; Martínez-de la Peña, C.F.; Sánchez, S. Targeting the Impossible: A Review of New Strategies against Endospores. Antibiotics 2023, 12, 248. https://doi.org/10.3390/antibiotics12020248
Romero-Rodríguez A, Ruiz-Villafán B, Martínez-de la Peña CF, Sánchez S. Targeting the Impossible: A Review of New Strategies against Endospores. Antibiotics. 2023; 12(2):248. https://doi.org/10.3390/antibiotics12020248
Chicago/Turabian StyleRomero-Rodríguez, Alba, Beatriz Ruiz-Villafán, Claudia Fabiola Martínez-de la Peña, and Sergio Sánchez. 2023. "Targeting the Impossible: A Review of New Strategies against Endospores" Antibiotics 12, no. 2: 248. https://doi.org/10.3390/antibiotics12020248
APA StyleRomero-Rodríguez, A., Ruiz-Villafán, B., Martínez-de la Peña, C. F., & Sánchez, S. (2023). Targeting the Impossible: A Review of New Strategies against Endospores. Antibiotics, 12(2), 248. https://doi.org/10.3390/antibiotics12020248






