Implementation of MRSA Nasal Swabs as an Antimicrobial Stewardship Intervention to Decrease Anti-MRSA Therapy in COVID-19 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
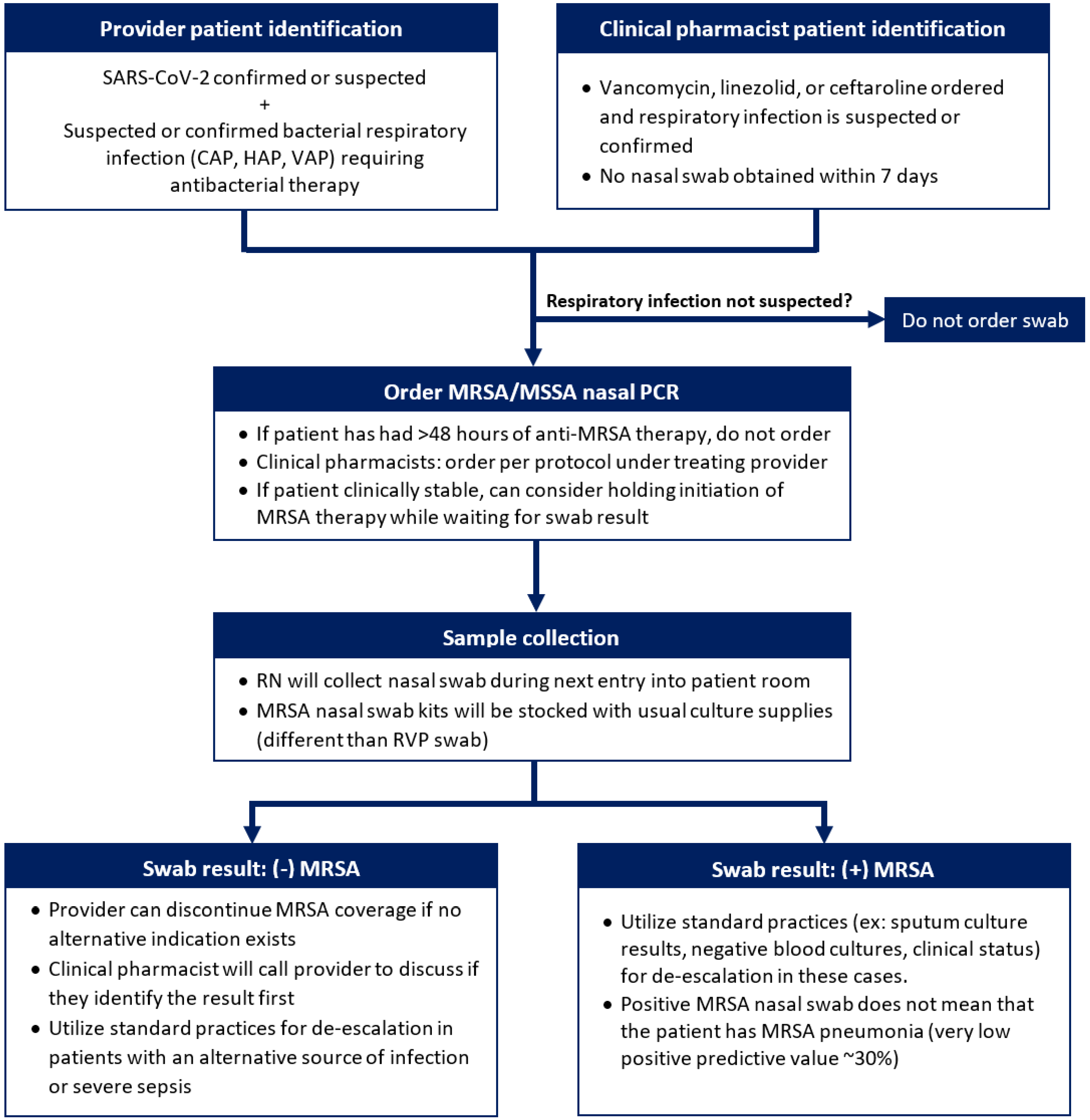
2.2. Intervention
2.3. Data Collection and Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111, Correction in Clin. Infect. Dis. 2017, 64, 1298; Correction in Clin. Infect. Dis. 2017, 65, 1435; Correction in Clin. Infect. Dis. 2017, 65, 2161. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.-C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with (COVID-19). Clin. Infect. Dis. 2020, v1.0.4. ciaa478. [Google Scholar] [CrossRef]
- Johnson, J.A.; E Wright, M.; A Sheperd, L.; Musher, D.M.; Dang, B.N. Nasal Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction: A Potential Use in Guiding Antibiotic Therapy for Pneumonia. Perm. J. 2015, 19, 34–36. [Google Scholar] [CrossRef]
- Giancola, S.E.; Nguyen, A.T.; Le, B.; Ahmed, O.; Higgins, C.; Sizemore, J.A.; Orwig, K.W. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn. Microbiol. Infect. Dis. 2016, 86, 307–310. [Google Scholar] [CrossRef]
- Smith, M.N.; Erdman, M.J.; Ferreira, J.A.; Aldridge, P.; Jankowski, C.A. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J. Crit. Care 2017, 38, 168–171. [Google Scholar] [CrossRef]
- Parente, D.M.; Cunha, C.; Mylonakis, E.; Timbrook, T.T. The Clinical Utility of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia: A Diagnostic Meta-analysis With Antimicrobial Stewardship Implications. Clin. Infect. Dis. 2018, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.; Daley, M.J.; Merkel, K.G.; Rose, D.T. Clinical Utility of Methicillin-Resistant Staphylococcus aureus Nasal Screening for Antimicrobial Stewardship: A Review of Current Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, K.A.; Starr, K.E.; Wattengel, B.A.; Lesse, A.J.; Sumon, Z.; Sellick, J.A. Determining the Utility of Methicillin-Resistant Staphylococcus aureus Nares Screening in Antimicrobial Stewardship. Clin. Infect. Dis. 2020, 71, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Baden, L.; Cheng, V.C.C.; Edwards, K.M.; Gallagher, J.C.; Gandhi, R.T.; Muller, W.J.; Nakamura, M.M.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2022, v10.0.0. ciac724. [Google Scholar] [CrossRef]
- COVID-19: U.S. Impact on Antimicrobial Resistance; Special Report 2022; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022. [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Staub, M.B.; Beaulieu, R.M.; Graves, J.; Nelson, G.E. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect. Control Hosp. Epidemiol. 2021, 42, 1–7. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical Characteristics of 3,062 COVID-19 Patients: A Meta-Analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef]
- Auld, S.C.; Caridi-Scheible, M.; Blum, J.M.; Robichaux, C.; Kraft, C.; Jacob, J.T.; Jabaley, C.S.; Carpenter, D.; Kaplow, R.; Hernandez-Romieu, A.C.; et al. ICU and ventilator mortality among critically ill adults with COVID-19. Crit. Care Med. 2020, 48, e799–e804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Batlle, D.; Soler, M.J.; Sparks, M.A.; Hiremath, S.; South, A.M.; Welling, P.A.; Swaminathan, S.; COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group. Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J. Am. Soc. Nephrol. 2020, 31, 1380–1383. [Google Scholar] [CrossRef]
- Fabrizi, F.; Alfieri, C.M.; Cerutti, R.; Lunghi, G.; Messa, P. COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Pathogens 2020, 9, 1052. [Google Scholar] [CrossRef]
- van Hal, S.J.; Paterson, D.L.; Lodise, T.P. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 2013, 57, 734–744. [Google Scholar] [CrossRef]
- Sinha Ray, A.; Haikal, A.; Hammoud, K.A.; Yu, A.S. Vancomycin and the risk of AKI: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2132–2140. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, E.; Cho, Y.-J.; Lee, Y.J.; Rhie, S.J. Linezolid-induced thrombocytopenia increases mortality risk in intensive care unit patients, a 10 year retrospective study. J. Clin. Pharm. Ther. 2019, 44, 84–90. [Google Scholar] [CrossRef]
- Campioli, C.C.; Barth, D.; Garrigos, Z.E.; Abu Saleh, O.; Sohail, R.M.; Sia, I.G. Linezolid and fentanyl: An underrecognized drug-to-drug interaction. J. Clin. Pharm. Ther. 2020, 45, 825–827. [Google Scholar] [CrossRef]

| All | Discontinued | Continued | ||||
|---|---|---|---|---|---|---|
| N = 122 | %, IQR | N = 99 | %, IQR | N = 23 | %, IQR | |
| Male | 76 | (62.3) | 63 | (63.6) | 13 | (56.5) |
| Age * | 65 | (56, 72) | 65 | (55, 72) | 65 | (57, 72) |
| COVID-19 diagnosis | 103 | (84.4) | 81 | (81.8) | 22 | (95.7) |
| Length of stay * (days) | 12 | (6, 19) | 11 | (5, 20) | 11 | (6, 19) |
| ICU admission | 92 | (75.4) | 73 | (73.7) | 19 | (82.6) |
| ICU LOS * (days) | 12 | (7, 17) | 12 | (6, 18) | 9 | (6, 13) |
| Admit to ICU * (days) | 1 | (0, 3) | 1 | (0, 3) | 1 | (0, 3) |
| Ventilated | 61 | (50.0) | 46 | (46.5) | 15 | (68.2) |
| Mortality | 53 | (43.4) | 42 | (42.4) | 11 | (47.8) |
| Time from admit to swab * (days) | 2 | (1, 7) | 2 | (1, 6) | 2 | (1, 9) |
| Swab ordering user | ||||||
| Physician | 70 | (57.3) | 61 | (61.6) | 9 | (39.1) |
| Pharmacist | 45 | (36.9) | 32 | (32.3) | 13 | (56.5) |
| Nurse | 7 | (5.7) | 6 | (6.1) | 1 | (4.3) |
| MRSA agent | ||||||
| Vancomycin | 50 | (41.0) | 34 | (34.3) | 16 | (70.0) |
| Linezolid | 33 | (27.0) | 27 | (27.3) | 6 | (26.1) |
| Ceftaroline | 4 | (3.3) | 3 | (3.0) | 1 | (4.3) |
| None | 35 | (28.7) | 35 | (35.4) | 0 | (0.0) |
| Positive blood cultures | 13 | (10.7) | 11 | (11.1) | 2 | (8.7) |
| Time to positive * (days) | 3 | (0, 12) | 8 | (0, 12) | 0 | (0, 0) |
| Sputum culture collected | 35 | (28.7) | 26 | (26.2) | 9 | (39.1) |
| Sputum pathogen # | 16 | (13.1) | 10 | (10.1) | 6 | (26.1) |
| Time to positive * (days) | 9 | (6, 13) | 10 | (6, 17) | 6 | (2, 10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeKerlegand, A.; Johnston, E.; Mellor, B.; Schrack, M.R.; O’Neal, C. Implementation of MRSA Nasal Swabs as an Antimicrobial Stewardship Intervention to Decrease Anti-MRSA Therapy in COVID-19 Infection. Antibiotics 2023, 12, 253. https://doi.org/10.3390/antibiotics12020253
DeKerlegand A, Johnston E, Mellor B, Schrack MR, O’Neal C. Implementation of MRSA Nasal Swabs as an Antimicrobial Stewardship Intervention to Decrease Anti-MRSA Therapy in COVID-19 Infection. Antibiotics. 2023; 12(2):253. https://doi.org/10.3390/antibiotics12020253
Chicago/Turabian StyleDeKerlegand, Alaina, Emily Johnston, Britney Mellor, Melanie Rae Schrack, and Catherine O’Neal. 2023. "Implementation of MRSA Nasal Swabs as an Antimicrobial Stewardship Intervention to Decrease Anti-MRSA Therapy in COVID-19 Infection" Antibiotics 12, no. 2: 253. https://doi.org/10.3390/antibiotics12020253
APA StyleDeKerlegand, A., Johnston, E., Mellor, B., Schrack, M. R., & O’Neal, C. (2023). Implementation of MRSA Nasal Swabs as an Antimicrobial Stewardship Intervention to Decrease Anti-MRSA Therapy in COVID-19 Infection. Antibiotics, 12(2), 253. https://doi.org/10.3390/antibiotics12020253





