Therapeutic Options and Outcomes for the Treatment of Children with Gram-Positive Bacteria with Resistances of Concern: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction and Assessment of Study Quality
- Study characteristics (authors, year of publication, study design, study location, and country);
- Patient characteristics (age, care setting, and inclusion and exclusion criteria);
- Type of MDR;
- Setting;
- Main results with accuracy measures;
- Health outcomes (e.g., mortality, clinical response, and microbiological eradication);
- Main results.
2.5. Summary Measures
3. Results
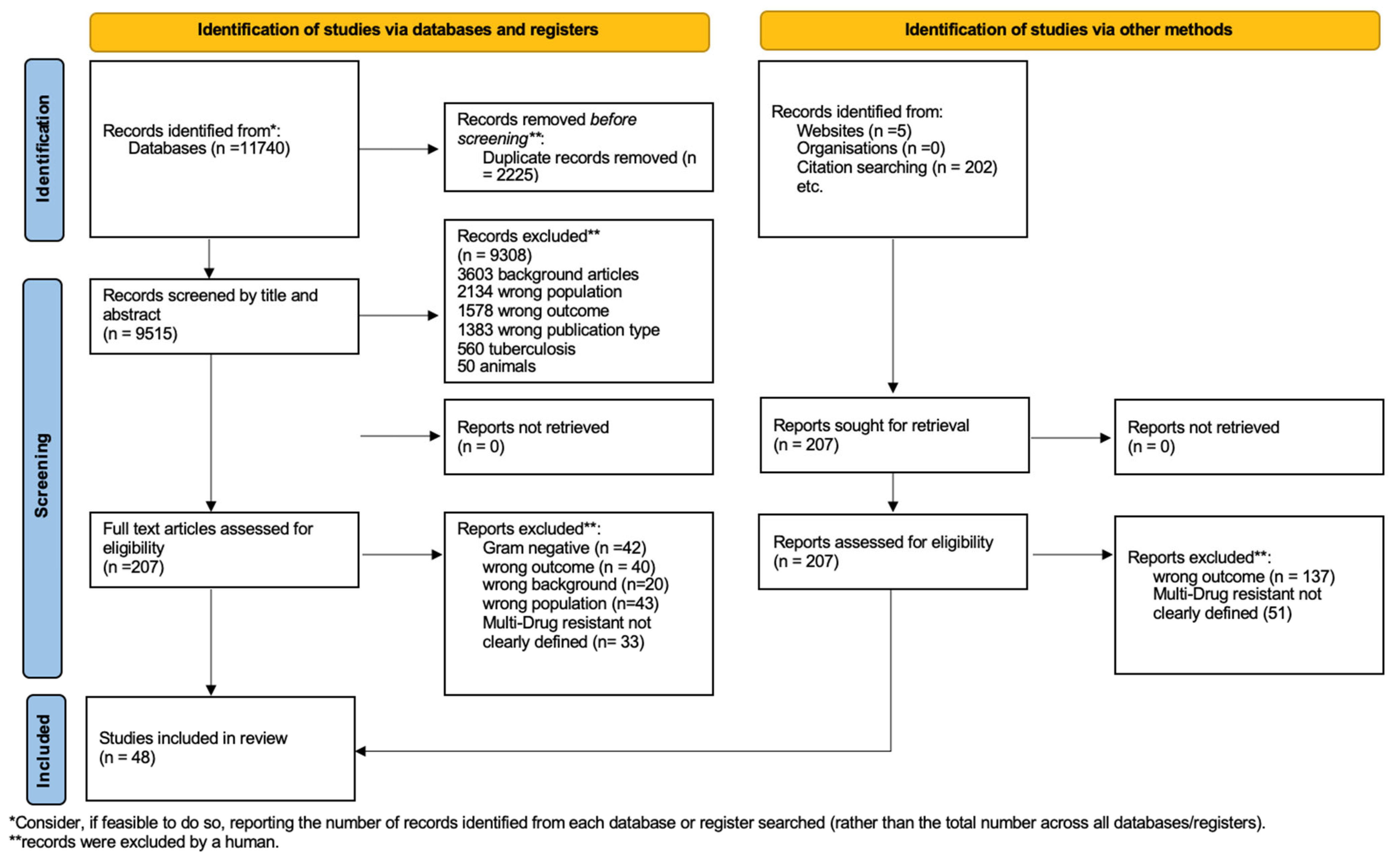
3.1. Study Selection
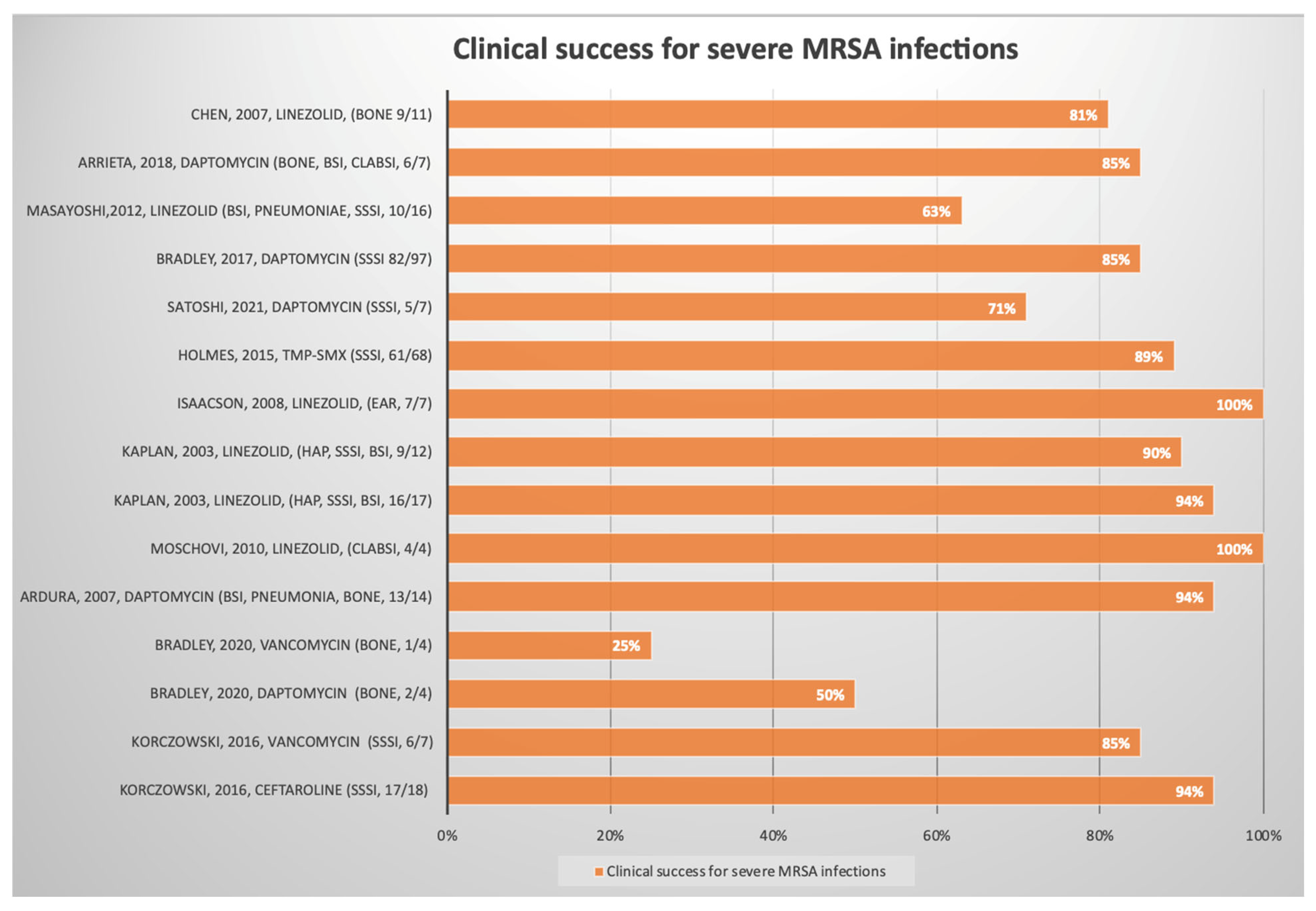
3.2. Methicillin-Resistant Staphylococcus aureus (MRSA)
3.3. Vancomycin-Resistant Enterococcus (VRE)
3.4. Methicillin-Resistant Coagulase-Negative Staphylococci (MR-CoNS)
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Site of Infection | Antimicrobial Treatment | Route | Outcomes Measures | Outcomes Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Korczowski, Bartosz [20] | Randomized, observer-blinded, active-controlled | 2016 | Worldwide | Multicenter | Inpatient | 25 | Not indicated # | MRSA | SSSIs | Ceftaroline | iv | Clinical success, microbiological eradication | Absolute value | Clinical success: ceftaroline 17/18 (94%) vs. vancomycin 6/7 (86%) Microbiological response: 16/18 (89%) ceftaroline vs. 4/7 (57%) vancomycin | Good |
| John S. Bradley [21] | Randomized, controlled, double-blind | 2020 | Worldwide | Multicenter | Inpatient, outpatient | 8 | 9 | MRSA | Bone | Daptomycin | iv | Clinical success, microbiological eradication | Absolute value | Clinical success: daptomycin 2/4 (50.0%) vs. vancomycin1/4 (25.0%) Microbiological eradication: daptomycin 2/4 (50.0%) vs. vancomycin 3/4 (75.05) | Good |
| Monica I. Ardura [22] | Retrospective | 2007 | USA | Monocenter | Inpatient, outpatient | 14 | 6 | MRSA | BSIs, endocarditis, pneumonia, pyomyositis, and osteoarthritis | Daptomycin | iv | Clinical success | Absolute value | Clinical success: daptomycin 13/14 (92%) | Fair |
| Maria Moschovi [23] | Prospective | 2010 | Greece | Monocenter | Oncoematological | 4 | 4 | MRSA | CLABSIs | Linezolid | iv | Clinical success | Absolute value | Clinical success: 4/4 (100%) | Fair |
| Sheldon L. Kaplan [25] | Randomized trial, comparator-controlled | 2003 | USA e Mexico | Multicenter | Inpatient, outpatient | 29 | 6 | MRSA | HAP, SSSIs, BSIs | Linezolid vs. vancomycin | iv | Clinical success | Absolute value | Clinical success Linezolid 16/17 (94%) vs. vancomycin 9/12 (90%) | Good |
| Glenn Isaacson [26] | Retrospective | 2008 | USA | Monocenter | Outpatient | 7 | 2 | MRSA | Ear | Linezolid | Oral | Clinical success | Absolute value | Clinical success: 100% (7/7) | Poor |
| Adem YılmAz [27] | Retrospective | 2010 | Turkey | Monocenter | Inpatient | 1 | 11 | MRSA | CNS | Linezolid | iv | Clinical success | Absolute value | Clinical success: 1/1 | Fair |
| Tae-Jung Sung [28] | Case report | 2008 | Korea | Monocenter | Inpatient | 1 | Premature | MRSA | Endocarditis | Linezolid | iv | Clinical success | Absolute value | Clinical response: 0/1 | Poor |
| Joshua I. Chan [29] | Case report | 2020 | USA | Monocenter | Inpatient | 1 | Premature | MRSA | BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Zakaria Jalal [30] | Case report | 2013 | Turkey | Monocenter | Inpatient | 1 | 12 | MRSA | Endocarditis, BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Aaron e. Chen [31] | Randomized trial | 2010 | USA | Monocenter | Outpatient | 133 | Not indicated # | MRSA | SSSIs | Cephalexin Vs Clindamycin | Oral | Clinical success | Absolute value | Clinical success: cephalexin 63/63 (100%) vs. clindamycin 66/70 (94%) | Good |
| Stefan Borgmann [32] | Case report | 2016 | Germany | Monocenter | Inpatient | 1 | 9 | MRSA | BSIs | Ceftaroline | iv | Clinical success | Absolute value | Clinical success: BSIs resolved | Poor |
| Al Zabem [33] | Case series | 2016 | Jordan | Monocenter | Inpatient, outpatient | 5 | 5.8 | MRSA | Bone | Vancomycin + rifampicin, then Tmp/Smx + rifampicin | iv/oral | Clinical success | Absolute value | Clinical success: 4/5 (80%) | Poor |
| Lucy Holmes [34] | Randomized trial | 2015 | USA | Monocenter | Inpatient | 137 | Not indicated # | MRSA | SSSIs | TMP-SMX | Oral | Clinical success | Absolute value | Clinical success: 61/68 (89%) 3 days of therapy vs. 68/69 (98%) 10 days of therapy | Good |
| Satoshi Iwata [39] | Open-label, single-arm phase 2 study | 2021 | Japan | Multicenter | Inpatient, outpatient | 8 | 7 | MRSA | cSSIs, BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: cSSIs 5/7 (71%), BSI 1/1 (100%) | Good |
| Nicholas M. Fusco [37] | Retrospective | 2019 | USA | Monocenter | Cystic fibrosis | 122 * | 18 | MRSA | Pneumonia | Linezolid vs. vancomycin | iv | Clinical success, adverse effects | Absolute value | Clinical success: vancomycin 53/66 (80.3%) vs. linezolid 50/66 (76%) Adverse effect: vancomycin 10/66 (15.2%) vs. linezolid 2/66 (3%) | Good |
| John Bradley [38] | Randomized trial | 2017 | Worldwide | Multicenter | Inpatient, outpatient | 97 | Not indicated # | MRSA | SSSIs | Daptomycin vs. standard of care | iv | Clinical success, adverse effects | Absolute value | Clinical success: daptomycin 82/97 (85%) and SOC 41/46 (89%) Adverse effect: 14% of daptomycin vs. 17% of SOC | Good |
| Aaron Cook [40] | Case report | 2005 | USA | Monocenter | Inpatient | 1 | 4 | MRSA | CNS | Linezolid + rifampicin | iv | Clinical and microbiological success | Absolute value | Clinical success and microbiological success: 1/1 | Poor |
| masayoshi shinjoh [35] | Retrospective | 2012 | Japan | Monocenter | Inpatient | 16 | 4 | MRSA | BSIs, skin, lung, CNS | Linezolid | iv/oral | Clinical success | Absolute value | Clinical success: 10/16 (63%) | Fair |
| Loeffler A. [36] | Prospective | 2002 | USA | Multicenter | Not specified | 8 | 7 | MRSA | BSIs, skin, pneumonia, joints, bone, CLABSI | Quinupristin/dalfopristin | iv | Clinical success | Absolute value | Clinical success: 5/8 (62%) | Fair |
| Antonio c. Arrieta [48] | Randomized multicenter | 2018 | Worldwide | Multicenter | Not specified | 10 | 8 | MRSA | Bone, joints, BSIs, CLABSIs, intrabdominal | Daptomycin vs. SOC | iv/oral | Clinical success | Absolute value | Clinical success: daptomycin 6/7 (85%) vs. 2/3 (67%) SOC | Good |
| Kenneth Wible [24] | Randomized controlled trial | 2003 | USA | Multicenter | Not specified | 20 | 10 | MRSA | SSSIs | Linezolid | oral | Clinical success | Absolute value | Clinical success: linezolid 12/13 (92%) vs. cefadroxil 6/7 (85%) | Good |
| Ram Yogev [41] | Open label, randomized | 2003 | USA | Multicenter | Inpatient | 18 | 3 | MRSA | SSSIs | Linezolid vs. vancomycin | iv/oral | Clinical success | Absolute value | Clinical success: linezolid 9/10 (90%) vs. vancomycin 6/8 (75%) | Good |
| Gallagher [42] | Case report | 2008 | UK | Monocenter | Inpatient | 1 | 4 | MRSA-VISA | CNS | Rifampicin, linezolid | iv/oral | Clinical and microbiological success | Absolute value | Clinical success: 1/1 | Poor |
| Chih-Jung Chen [43] | Retrospective | 2007 | Taiwan | Monocenter | Not specified | 11 | 6 | MRSA | Bone | Linezolid iv/os | iv/oral | Clinical and microbiological success | Absolute value | Clinical success: 9/11 (81%) | Fair |
| Lara Jacobson [44] | Case report | 2009 | USA | Monocenter | Inpatient | 1 | 15 | MRSA | BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 0/1 | Poor |
| Salerno [45] | Case report | 2017 | USA | Monocenter | Inpatient | 1 | Premature | MRSA | Pneumonia | Ceftaroline + rifampicin | iv | Clinical and microbiological success | Absolute value | Clinical and microbiological success: 1/1 | Poor |
| Hussain [47] | Case report | 2011 | United Kingdom | Monocenter | NICU | 1 | Premature | MRSA | BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Palma [46] | Case series | 2013 | Italy | Monocenter | PICU | 3 | Not indicated | MRSA | BSIs, SSSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 3/3 | Poor |
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Bacteria | Site of Infection | Antimicrobial Treatment | Route | Outcomes Measures | OutcoMes Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monica I. Ardura [22] | Retrospective | 2007 | USA | Monocenter | Inpatient | 1 | 10 | VRE | E. faecium | UTIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: daptomycin 0/1 | Fair |
| Maria Moschovi [23] | Prospective | 2010 | Greece | Monocenter | Oncoematological | 10 | 2.8 | VRE | Enterococcus spp. | BSIs, stool | Linezolid | iv | Clinical success | Absolute value | Clinical success: 10/10 (100%) | Fair |
| Ayse Şahin [49] | Case report | 2019 | Turkey | Monocenter | Inpatient | 1 | 2 months | VRE | E. faecium | CNS | Linezolid iv + daptomycin iv | iv + ivt | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Ayse Sahina [50] | Case report | 2019 | Turkey | Monocenter | Inpatient | 1 | 5 months | VRE | E. faecium | CNS | Tigecycline | iv + ivt | Microbiological eradication | Absolute value | Microbiological eradication: 1/1 | Poor |
| Heather B. Jaspan [51] | Case report | 2010 | USA | Monocenter | Inpatient | 1 | 21 months | VRE | Enterococcus faecium | CNS | Linezolid + daptomycin + tigecycline + daptomycin IVT | iv + ivt | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Rene Hoehn [52] | Case report | 2006 | Germany | Monocenter | NICU | 2 | Preterm | VRE | Enterococcus spp. | BSIs | Linezolid | iv | Clinical success | Absolute value | Clinical and success 2/2 | Poor |
| Adem YılmAz [27] | Retrospective | 2010 | Turkey | Monocenter | Inpatient | 1 | 11 | VRE | E. faecium | CNS | Linezolid | iv | Clinical success | Absolute value | Clinical success: 1/1 | Fair |
| Sheldon L. Kaplan [25] | Trial randomized | 2003 | USA and central America | Multicenter | Inpatient | 3 | 3 | VRE | E. faecium | BSIs | Linezolid | iv | Clinical success | Absolute value | Clinical success: 2/3 (66%) | Good |
| Marco Fossati [53] | Case report | 2010 | Italy | Inpatient | Oncoematological | 1 | 11 | VRE | E. faecium | BSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success 0/1 | Poor |
| Loeffler A. [36] | Prospective | 2002 | USA | Multicenter | Not specified | 101 | 7 | VRE | Enterococcus spp. | BSIs, skin, pneumonia, joint, bone, CLABSI | Quinupristin/dalfopristin | iv | Clinical success | Absolute value | Clinical success: 71/101 (70%) | Fair |
| Kevin Paul [54] | Case report | 2021 | Germany | Monocenter | Inpatient | 1 | 10 months | VRE | Abdominal | Bacteriophage therapy | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor | |
| James W. Gray [55] | Case Series | 2000 | UK | Monocenter | Inpatient and outpatient | 8 | 7 | VRE | Enterococcus spp. | BSIs, abdominal | Quinupristin/dalfopristin | iv | Clinical success | Absolute value | Clinical success: 7/8 (87%) | Fair |
| Jocelyn Ang [56] | Case report | 2003 | USA | Monocenter | NICU | 1 | Premature | VRE | E. faecium | Endocarditis | Linezolid | iv | Clinical and microbiological success | Absolute value | Clinical and microbiological success: 1/1 | Poor |
| Mehmet Baysallar [57] | Case report | 2006 | Turkey | Monocenter | Inpatient | 1 | 7 months | VRE | E. faecium | CNS | Chloramphenicol, rifampin, and meropenem | iv | Clinical and microbiological success | Absolute value | Clinical and microbiological success: 1/1 | Poor |
| M. Travaglianti [58] | Retrospective | 2007 | Argentina | Monocenter | Inpatient | 15 | 7 years | VRE | Enterococcus spp. | BSIs, UTIs, abdominal, endocarditis | Linezolid | iv/oral | Clinical and microbiological success | Absolute value | Clinical and microbiological success: 13/15 (87%) | Poor |
| Graham [59] | Case report | 2002 | USA | Monocenter | NICU | 1 | Preterm | VRE | E. faecium | CNS | Linezolid | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Beneri [60] | Case report | 2008 | USA | Monocenter | Inpatient | 1 | Neonate | VRE | E. faecium | BSIs | Daptomycin + doxyxyxline | Iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Maranich [61] | Case report | 2008 | USA | Monocenter | Inpatient | 1 | 17 months | VRE | E. faecium | CNS | Linezolid | Iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Palma [46] | Case series | 2013 | Italy | Monocenter | PICU | 1 | Not indicated | VRE | E. faecium | BSIs, SSSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
| Ergaz [62] | Case report | 2009 | Israel | Monocenter | NICU | 3 | Preterm | VRE | E. faecium | BSIs, CNS | Linezolid | Iv | Clinical success | Absolute value | Clinical success: 3/3 | Poor |
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Bacteria | Site of Infection | Antimicrobial Treatment | Route | Outcomes Measures | Outcomes Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shanti [63] | Case report | 2009 | Malesia | Monocenter | Inpatient | 1 | 1 | MR | S. epidermis | CNS | iv teicoplanin + IVT teicoplanin 10 mg daily | iv+ivt | Microbiological eradication | Absolute value | Microbiological eradication: 1/1 | Poor |
| Sheldon L. Kaplan [25] | Trial randomized | 2003 | USA e central America | Multicenter | Inpatient | 46 | 2 | MR | S. epidermis | BSI | Linezolid vs. vancomycin | iv | Clinical success | Absolute value | Clinical success: linezolid 29/34 (85.3) vs. vancomycin 10/12 (83.3) | Good |
| Adem YılmAz [27] | Retrospective | 2010 | Turkey | Monocenter | Inpatient | 4 | 11 | MR | S. epidermidis | CNS | Linezolid | iv | Clinical success | Absolute value | Clinical success: 4/4 | Fair |
| C. Minotti [64] | Case report | 2022 | Italy | Monocenter | NICU | 1 | Preterm | MR | S. epidermidis | CLABSI | Daptomycin | iv | Clinical success | Clinical success | Clinical success: 1/1 | Poor |
| Fumihiro ochi [65] | Case series | 2018 | Japan | Monocenter | Inpatient | 2 | 2 | MR | S. epidermidis | CNS | Linezolid | iv | Clinical success | Absolute value | Clinical success: 2/2 | Fair |
| Palma [46] | Case series | 2013 | Italy | Monocenter | PICU | 3 | Not indicated | MR | S. epidermidis | BSI, SSSIs | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 3/3 | Poor |
| Gawronski [66] | Case report | 2015 | Ohio | Monocenter | NICU | 1 | Preterm | MR | S. epidermidis | BSI | Daptomycin | iv | Clinical success | Absolute value | Clinical success: 1/1 | Poor |
4. Discussion
4.1. MRSA, VRE
4.2. MR-CoNS
4.3. Neonatal Safety
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; Department of Health and Human Services: Washington, DC, USA, 2019; pp. 1–140. [CrossRef] [Green Version]
- ECDC. Antimicrobial resistance in the EU/EEA (EARS-Net) Annual Epidemiological Report for 2019. Epidemiolog. Antimicrob. Resist EU/EEA 2020, 174, 341. [Google Scholar]
- McMullan, B.J.; Bowen, A.; Blyth, C.C.; Van Hal, S.; Korman, T.M.; Buttery, J.; Voss, L.; Roberts, S.; Cooper, C.; Tong, S.Y.C.; et al. Epidemiology and mortality of Staphylococcus aureus Bacteremia in Australian and New Zealand children. JAMA Pediatr. 2016, 170, 979–986. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; Word Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Dantes, R.; Mu, Y.; Belflower, R.; Aragon, D.; Dumyati, G.; Harrison, L.H.; Lessa, F.C.; Lynfield, R.; Nadle, J.; Petit, S.; et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern. Med. 2013, 173, 1970–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortuin-de Smidt, M.C.; Singh-Moodley, A.; Badat, R.; Quan, V.; Kularatne, R.; Nana, T.; Lekalakala, R.; Govender, N.P.; Perovic, O. Staphylococcus aureus bacteraemia in Gauteng academic hospitals, South Africa. Int. J. Infect. Dis. 2015, 30, e41–e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Karageorgopoulos, D.E.; Leptidis, J.; Korbila, I.P. MRSA in Africa: Filling the Global Map of Antimicrobial Resistance. PLoS ONE 2013, 8, e68024. [Google Scholar] [CrossRef] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2018, 19, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Gladstone, B.P.; Azzini, A.M.; Göpel, S.; Tacconelli, E. Gross national income and antibiotic resistance in invasive isolates: Analysis of the top-ranked antibiotic-resistant bacteria on the 2017 WHO priority list. J. Antimicrob. Chemother. 2019, 74, 3619–3625. [Google Scholar] [CrossRef]
- Panesso, D.; Reyes, J.; Rincón, S.; Díaz, L.; Galloway-Peña, J.; Zurita, J.; Carrillo, C.; Merentes, A.; Guzmán, M.; Adachi, J.A.; et al. Molecular epidemiology of vancomycin-resistant Enterococcus faecium: A prospective, multicenter study in South American hospitals. J. Clin. Microbiol. 2010, 48, 1562–1569. [Google Scholar] [CrossRef]
- Alemayehu, T.; Hailemariam, M. Prevalence of vancomycin-resistant enterococcus in Africa in one health approach: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 20542. [Google Scholar] [CrossRef] [PubMed]
- Marincola, G.; Liong, O.; Schoen, C.; Abouelfetouh, A.; Hamdy, A.; Wencker, F.D.R.; Marciniak, T.; Becker, K.; Köck, R.; Ziebuhr, W. Antimicrobial Resistance Profiles of Coagulase-Negative Staphylococci in Community-Based Healthy Individuals in Germany. Front. Public Health 2021, 9, 684456. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Klein, E.Y.; Rothman, R.E.; Laxminarayan, R. Trends in antibiotic resistance in coagulase-negative staphylococci in the United States, 1999 to 2012. Antimicrob. Agents Chemother. 2014, 58, 1404–1409. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Black, C.G.; Tavares, L.; Stachel, A.; Ratner, A.J.; Randis, T.M. Distribution of Late-Onset Neonatal Sepsis Pathogens Differs in Inpatient and Outpatient Settings. Am. J Perinatol. 2019, 36, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 November 2022).
- Korczowski, B.; Antadze, T.; Giorgobiani, M.; Stryjewski, M.; Jandourek, A.; Smith, A.; O’Neal, T.; Bradley, J.S. A Multicenter, Randomized, Observer-Blinded, Active-Controlled Study to Evaluate the Safety and Efficacy of Ceftaroline versus Comparator in Pediatric Patients with Acute Bacterial Skin and Skin Structure Infection. Pediatr. Infect. Dis. J. 2016, 35, e239–e247. [Google Scholar] [CrossRef]
- Bradley, J.S.; Arrieta, A.C.; Digtyar, V.A.; Popejoy, M.W.; Grandhi, A.; Bokesch, P.; Hershberger, E.; Dorr, M.B.; Tan, C.M.; Murata, Y.; et al. Daptomycin for Pediatric Gram-Positive Acute Hematogenous Osteomyelitis. Pediatr. Infect. Dis. J. 2020, 39, 814–823. [Google Scholar] [CrossRef]
- Ardura, M.I.; Mejías, A.; Katz, K.S.; Revell, P.; McCracken, G.H.; Sánchez, P.J. Daptomycin Therapy for Invasive Gram-Positive Bacterial Infections in Children. Pediatr. Infect. Dis. J. 2007, 26, 1128–1132. [Google Scholar] [CrossRef]
- Moschovi, M.; Trimis, G.; Tsotra, M.; Chatzi, F.; Karamolegou, K.; Santou, A.; Tourkantoni, N.; Chrousos, G. Efficacy and safety of linezolid in immunocompromised children with cancer. Pediatr. Int. 2010, 52, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Wible, K.; Tregnaghi, M.; Bruss, J.; Fleishaker, D.; Naberhuis-Stehouwer, S.; Hilty, M. Linezolid versus cefadroxil in the treatment of skin and skin structure infections in children. Pediatr. Infect. Dis J. 2003, 22, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.L.; Deville, J.G.; Yogev, R.; Morfin, M.R.; Wu, E.; Adler, S.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr. Infect. Dis. J. 2003, 22, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, G.; Aronoff, S.C. Linezolid for tympanostomy tube otorrhea caused by methicillin-resistant Staphylococcus aureus and multiple drug-resistant Streptococcus pneumoniae. Int. J. Pediatr Otorhinolaryngol. 2008, 72, 647–651. [Google Scholar] [CrossRef]
- Yılmaz, A.; Dalgic, N.; Müslüman, M.; Sancar, M.; Çolak, I.; Aydın, Y. Linezolid treatment of shunt-related cerebrospinal fluid infections in children. J. Neurosurg. Pediatr. 2010, 5, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.-J.; Kim, H.-M.; Kim, M.-J. Methicillin-Resistant Staphylococcus aureus Endocarditis in an Extremely Low-Birth-Weight Infant Treated With Linezolid. Clin. Pediatr. 2008, 47, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.I.; Noor, A.; Clauss, C.; Aggarwal, R.; Nayak, A. Methicillin-Resistant Staphylococcus aureus Endovascular Infection in a Neonate: Prolonged, Safe, and Effective Use of Daptomycin and Enoxaparin. J. Pediatr. Pharmacol. Ther. 2020, 25, 68–74. [Google Scholar] [CrossRef]
- Jalal, Z.; Camou, F.; Roubertie, F.; Iriart, X.; Thambo, J.-B. Pediatric case of MRSA right-sided infective endocarditis with septic pulmonary embolism successfully treated by daptomycin in combination with gentamicin and rifampin. J. Pediatr. Infect. Dis. 2013, 8, 139–142. [Google Scholar] [CrossRef]
- Chen, A.E.; Carroll, K.C.; Diener-West, M.; Ross, T.; Ordun, J.; Goldstein, M.A.; Kulkarni, G.; Cantey, J.B.; Siberry, G.K. Randomized Controlled Trial of Cephalexin Versus Clindamycin for Uncomplicated Pediatric Skin Infections. Pediatrics 2011, 127, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Borgmann, S.; Rieß, B.; Von Wernitz-Keibel, T.; Bühler, M.; Layer, F.; Strommenger, B. Recovery of a 10-year-old girl from methicillin- resistant Staphylococcus aureus sepsis in response to low-dose ceftaroline treatment. Ther. Clin. Risk Manag. 2016, 12, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Al-Zaben, R.M.; Al Majali, A.; Rahayma, J.; Al-Zaben, A. The treatment of inappropriately treated Methicillin Resistant Staph Aureus osteomyelitis in pediatric age group. Rawal Med. J. 2016, 41, 212–215. [Google Scholar]
- Holmes, L.; Ma, C.; Qiao, H.; Drabik, C.; Hurley, C.; Jones, D.; Judkiewicz, S.; Faden, H. Trimethoprim-Sulfamethoxazole Therapy Reduces Failure and Recurrence in Methicillin-Resistant Staphylococcus aureus Skin Abscesses after Surgical Drainage. J. Pediatr. 2015, 169, 128–134.e1. [Google Scholar] [CrossRef] [PubMed]
- Shinjoh, M.; Iketani, O.; Watanabe, K.; Kudo, M.; Sugita, K.; Mori, T.; Hasegawa, N.; Iwata, S.; Yamagishi, H.; Shimada, H.; et al. Safety and efficacy of linezolid in 16 infants and children in Japan. J. Infect. Chemother. 2012, 18, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, A.M.; Drew, R.H.; Perfect, J.R.; Grethe, N.I.; Stephens, J.W.; Gray, S.L.; Talbot, G.H. Safety and efficacy of quinupristin/dalfopristin for treatment of invasive Gram-positive infections in pediatric patients. Pediatr. Infect. Dis. J. 2002, 21, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.M.; Meaney, C.J.; Frederick, C.A.; Prescott, W.A. Comparative Effectiveness of Vancomycin Versus Linezolid for the Treatment of Acute Pulmonary Exacerbations of Cystic Fibrosis. Ann. Pharmacother. 2020, 54, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Glasser, C.; Patino, H.; Arnold, S.R.; Arrieta, A.; Congeni, B.; Daum, R.S.; Kojaoghlanian, T.; Yoon, M.; Anastasiou, D.; et al. Daptomycin for Complicated Skin Infections: A Randomized Trial. Pediatrics 2017, 139, e20162477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, S.; Koyama, H.; Murata, Y. Efficacy and safety of daptomycin in Japanese pediatric participants with complicated skin and soft tissue infections or bacteremia caused by gram-positive cocci. J. Infect. Chemother. 2022, 28, 406–412. [Google Scholar] [CrossRef]
- Cook, A.M.; Ramsey, C.N.; Martin, C.A.; Pittman, T. Linezolid for the Treatment of a Heteroresistant Staphylococcus aureus Shunt Infection. Pediatr. Neurosurg. 2005, 41, 102–104. [Google Scholar] [CrossRef]
- Yogev, R.; Patterson, L.E.; Kaplan, S.L.; Adler, S.; Morfin, M.R.; Martin, A.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B. Linezolid for the treatment of complicated skin and skin structure infections in children. Pediatr. Infect. Dis J. 2003, 22, 172–177. [Google Scholar] [CrossRef]
- Gallagher, R.; Pizer, B.; Ellison, J.; Riordan, F. Glycopeptide insensitive Staphylococcus aureus subdural empyema treated with linezolid and rifampicin. J. Infect. 2008, 57, 410–413. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chiu, C.-H.; Lin, T.-Y.; Lee, Z.-L.; Yang, W.-E.; Huang, Y.-C. Experience With Linezolid Therapy in Children With Osteoarticular Infections. Pediatr. Infect. Dis. J. 2007, 26, 985–988. [Google Scholar] [CrossRef]
- Jacobson, L.M.; Milstone, A.; Zenilman, J.; Carroll, K.C.; Arav-Boger, R. Daptomycin therapy failure in an adolescent with methicillin-resistant Staphylococcus aureus bacteremia. Pediatr. Infect. Dis. J. 2009, 28, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Salerno, S.N.; Bernhardt, J.; Laughon, M.; Jhaveri, R.; Massaro, M.; Gonzalez, D. Pharmacokinetics of Ceftaroline in a Preterm Infant with Methicillin-Resistant Staphylococcus aureus Pneumonia. J. Pediatr. Infect. Dis. Soc. 2018, 7, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.M.; Giordano, S.; Cracchiolo, A.N.; Zangara, V.; Coffaro, G.; Tetamo, R. Daptomycin in the treatment of invasive Gram-positive bacterial infections in children: Personal experience. Minerva Pediatr. 2013, 65, 173–178. [Google Scholar]
- Hussain, A.; Kairamkonda, V.; Jenkins, D.R. Successful treatment of meticillin-resistant Staphylococcus aureus bacteraemia in a neonate using daptomycin. J. Med. Microbiol. 2011, 60, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, A.C.; Bradley, J.S.; Popejoy, M.W.; Bensaci, M.; Grandhi, A.; Bokesch, P.; Glasser, C.; Du, L.; Patino, H.; Kartsonis, N.A. Randomized Multicenter Study Comparing Safety and Efficacy of Daptomycin Versus Standard-of-care in Pediatric Patients With Staphylococcal Bacteremia. Pediatr. Infect. Dis. J. 2018, 37, 893–900. [Google Scholar] [CrossRef]
- Şahin, A.; Dalgic, N. Intravenous and intraventricular daptomycin plus intravenous linezolid treatment of an infant with Vancomycin-Resistant Enterococci induced ventriculoperitoneal shunt infection. World Neurosurg. 2019, 124, 328–330. [Google Scholar] [CrossRef]
- Şahin, A.; Dalgic, N. Intraventricular plus Intravenous Tigecycline for the Treatment of Daptomycin Nonsusceptible Vancomycin-Resistant Enterococci in an Infant with Ventriculoperitoneal Shunt Infection. World Neurosurg. 2019, 130, 470–473. [Google Scholar] [CrossRef]
- Jaspan, H.B.; Brothers, A.W.; Campbell, A.J.P.; McGuire, J.K.; Browd, S.R.; Manley, T.J.; Pak, D.; Weissman, S.J. Multidrug-Resistant Enterococcus faecium Meningitis in a Toddler: Characterization of the Organism and Successful Treatment with Intraventricular Daptomycin and Intravenous Tigecycline Heather. Pediatr. Infect. Dis. J. 2016, 29, 379–381. [Google Scholar] [CrossRef] [Green Version]
- Hoehn, R.; Groll, A.; Schaefer, V.; Bauer, K.; Schloesser, R. Linezolid treatment of glycopeptide-resistant Enterococcus faecium in very low birth weight premature neonates. Int. J. Antimicrob. Agents 2006, 27, 256–258. [Google Scholar] [CrossRef]
- Fossati, M.; Cappelli, B.; Biral, E.; Chiesa, R.; Biffi, A.; Ossi, C.; Moro, M.; Cirillo, D.M.; Clementi, M.; Soliman, C.; et al. Case Report Fatal vancomycin- and linezolid-resistant Enterococcus faecium sepsis in a child undergoing allogeneic haematopoietic stem cell transplantation for beta-thalassaemia major. J. Med. Microbiol. 2010, 59, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Paul, K.; Merabishvili, M.; Hazan, R.; Christner, M.; Herden, U.; Gelman, D.; Khalifa, L.; Yerushalmy, O.; Coppenhagen-Glazer, S.; Harbauer, T.; et al. Bacteriophage Rescue Therapy of a Vancomycin-Resistant Enterococcus faecium Infection in a One-Year-Old Child following a Third Liver Transplantation. Viruses 2021, 13, 1785. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.W.; Darbyshire, P.J.; Beath, S.V.; Kelly, D. Experience with quinupristin/dalfopristin in treating infections with vancomycin-resistant Enterococcus faecium in children. Pediatr. Infect. Dis. J. 2000, 19, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.Y.; Lua, J.L.; Turner, D.R.; Asmar, B.I. Vancomycin-resistant Enterococcus faecium endocarditis in a premature infant successfully treated with linezolid. Pediatr. Infect. Dis. J. 2003, 22, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Baysallar, M.; Izci, Y.; Kilic, A.; Avci, I.Y.; Senses, Z.; Doganci, L.; Tamma, P.D.; Hsu, A.J.; Wang, J.S.; Muzevich, K.; et al. A Case of Ventricular Drainage Infection with a Rare Pathogen in Cerebrospinal Fluid: Vancomycin-Resistant Enterococcus faecium. Microbiol. Drug Resist. 2006, 12, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Travaglianti, M.; Pérez, M.; Sberna, N.; Rousseau, M.; Calle, G.; Gómez, S. Tratamiento de infecciones por Enterococcus resistente a vancomicina con linezolid en un hospital pediátrico. Farm. Hosp. 2007, 31, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.L., III; Ampofo, K.; Saiman, L. Linezolid treatment of vancomycin-resistant Enterococcus faecium ventriculitis. Pediatr. Infect. Dis. J. 2002, 21, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Beneri, C. Successful treatment of a neonate with persistent vancomycin-resistant enterococcal bacteremia with a daptomycin-containing regimen. Infect. Drug Resist. 2008, 1, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Maranich, A.M.; Rajnik, M. Successful treatment of vancomycin-resistant enterococcal ventriculitis in a pediatric patient with linezolid. Mil. Med. 2008, 173, 927–929. [Google Scholar] [CrossRef] [Green Version]
- Ergaz, Z.; Arad, I.; Bar-Oz, B.; Peleg, O.; Benenson, S.; Minster, N.; Moses, A.; Block, C. Elimination of vancomycin-resistant enterococci from a neonatal intensive care unit following an outbreak. J. Hosp. Infect. 2010, 74, 370–376. [Google Scholar] [CrossRef]
- Shanti, R.; Ic, S.; Ariffin, H. Intraventricular teicoplanin. J. Health Transl. Med. 2009, 12, 35–38. [Google Scholar]
- Minotti, C.; Zuccon, I.; Priante, E.; Bonadies, L.; Di Chiara, C.; Donà, D.; Baraldi, E.; Costenaro, P. Daptomycin for Treatment of S. epidermidis Endocarditis in an Extremely Preterm Neonate—Outcome and Perspectives. Children 2022, 9, 457. [Google Scholar] [CrossRef] [PubMed]
- Ochi, F.; Tauchi, H.; Nagai, K.; Moritani, K.; Tezuka, M.; Jogamoto, T.; Aibara, K.; Motoki, T.; Ishii, E. Therapeutic Effect of Linezolid in Children With Health Care—Associated Meningitis or Ventriculitis. Clin. Pediatr. 2018, 57, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, K.M. Successful Use of Daptomycin in a Preterm Neonate With Persistent Methicillin-Resistant Staphylococcus epidermidis Bacteremia. J. Pediatr. Pharmacol. Ther. 2015, 20, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [Green Version]
- McMullan, B.J.; Campbell, A.J.; Blyth, C.C.; McNeil, J.C.; Montgomery, C.P.; Tong, S.Y.; Bowen, A.C. Clinical Management of Staphylococcus aureus Bacteremia in Neonates, Children, and Adolescents. Pediatrics 2020, 146, e20200134. [Google Scholar] [CrossRef]
- FDA Rationale for Recognition Decision: Ceftaroline Fosamil. Published 17 April 2020. Available online: https://www.fda.gov/drugs/development-resources/fda-rationale-recognition-decision-ceftaroline-fosamil (accessed on 25 November 2022).
- European Medicines Agency (EMA). Zinforo (Ceftaroline Fosamil). 2022. Last update 27 July 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zinforo (accessed on 25 November 2022).
- Bradley, J.S.; Stone, G.G.; Chan, P.L.S.; Raber, S.R.; Riccobene, T.; Casullo, V.M.; Yan, J.L.; Hendrick, V.M.; Hammond, J.; Leister-Tebbe, H.K. Phase 2 study of the safety, pharmacokinetics and efficacy of ceftaroline fosamil in neonates and very young infants with late-onset sepsis. Pediatr. Infect. Dis. J. 2020, 39, 411–418. [Google Scholar] [CrossRef]
- Branstetter, J.; Searcy, H.; Benner, K.; Yarbrough, A.; Crowder, C.; Troxler, B. Ceftaroline vs vancomycin for the treatment of acute pulmonary exacerbations in pediatric patients with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 3337–3342. [Google Scholar] [CrossRef]
- Yim, J.; Molloy, L.M.; Newland, J.G. Use of Ceftaroline Fosamil in Children: Review of Current Knowledge and its Application. Infect. Dis. Ther. 2017, 6, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus Standard Therapy for Bacteremia and Endocarditis Caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Arbeit, R.D.; Maki, D.; Tally, F.P.; Campanaro, E.; Eisenstein, B.I.; Daptomycin 98-01 and 99-01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Infect. Dis. Clin. Pract. 2004, 38, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Drug Approval Package. Zyvox (Linezolid). 2002. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-130s003_21131s003_21132s003_ZyvoxTOC.cfm (accessed on 23 November 2022).
- Kaplan, S.L.; Afghani, B.; Lopez, P.; Wu, E.; Fleishaker, D.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B. Linezolid for the treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr. Infect. Dis. J. 2003, 22 (Suppl. 9), 178–185. [Google Scholar] [CrossRef] [PubMed]
- Deville, J.G.; Adler, S.; Azimi, P.H.; Jantausch, B.A.; Morfin, M.R.; Beltran, S.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B. Linezolid versus vancomycin in the treatment of known or suspected resistant Gram-positive infections in neonates. Pediatr. Infect. Dis. J. 2003, 22 (Suppl. 9), 158–163. [Google Scholar] [CrossRef] [PubMed]
- Minotti, C.; Bonadies, L.; Liberati, C.; De Pieri, M.; Giaquinto, C.; Baraldi, E.; Donà, D. Enteral Linezolid as an Effective Option to Treat an Extremely Preterm Infant with Bacillus cereus Sepsis. Children 2022, 9, 415. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef]
- Wu, Q.; Sabokroo, N.; Wang, Y.; Hashemian, M.; Karamollahi, S.; Kouhsari, E. Systematic review and meta-analysis of the epidemiology of vancomycin-resistance Staphylococcus aureus isolates. Antimicrob. Resist. Infect. Control. 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Wiesch, P.A.Z.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Sivextro, Tedizolid Phosphate. 2015. Last update 16 December 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/sivextro (accessed on 9 January 2023).
- Bradley, J.S.; Antadze, T.; Ninov, B.; Tayob, M.S.; Broyde, N.; Butterton, J.R.; Chou, M.Z.; De Anda, C.S.; Kim, J.Y.; Sears, P.S. Safety and Efficacy of Oral and/or Intravenous Tedizolid Phosphate from a Randomized Phase 3 Trial in Adolescents with Acute Bacterial Skin and Skin Structure Infections. Pediatr. Infect. Dis. J. 2021, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.; Hachem, R.; Hanna, H.; Afif, C.; Escalante, C.; Kantarjian, H.; Rolston, K. Prospective, randomized study comparing quinupristin-dalfopristin with linezolid in the treatment of vancomycin-resistant Enterococcus faecium infections. J. Antimicrob. Chemother. 2004, 53, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.P.; Lee, S.-O.; Song, E.H.; Lee, E.J.; Jang, E.-Y.; Kim, S.-H.; Choi, S.-H.; Kim, M.-N.; Jeong, J.-Y.; Woo, J.H.; et al. Quinupristin-dalfopristin versus linezolid for the treatment of vancomycin-resistant Enterococcus faecium bacteraemia: Efficacy and development of resistance. Scand. J. Infect. Dis. 2010, 42, 491–499. [Google Scholar] [CrossRef]
- Koulenti, D.; Xu, E.; Yin Sum Mok, I.; Song, A.; Karageorgopoulos, D.E.; Armaganidis, A.; Lipman, J.; Tsiodras, S. Novel Antibiotics for Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms 2019, 7, 270. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, S.; Decano, A.G.; Bandali, A.; Lai, D.; E Malat, G.; E Bias, T. Oritavancin (Orbactiv) a new-generation lipoglycopeptide for the treatment of acute bacterial skin and skin structure infections. Pharm. Ther. 2018, 43, 143–147. [Google Scholar]
- Open-Label, Dose-Finding, Pharmacokinetics, Safety and Tolerability Study of Oritavancin in Pediatric Patients with Suspected or Confirmed Bacterial Infections (NCT02134301). ClinicalTrials.gov. Last update 17 January 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT02134301 (accessed on 21 January 2023).
- European Medicines Agency (EMA). Xydalba, Dalbavancin. 2015. Last update 14 December 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xydalba (accessed on 9 January 2023).
- Dalbavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections in Children, Known or Suspected to Be Caused by Susceptible Gram-Positive Organisms, Including MRSA-Full Text View-ClinicalTrials.gov. Last update 17 October 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02814916 (accessed on 25 November 2022).
- Bradley, J.S.; Benziger, D.; Bokesch, P.; Jacobs, R. Single-dose pharmacokinetics of daptomycin in pediatric patients 3-24 months of age. Pediatr. Infect. Dis. J. 2014, 33, 936–939. [Google Scholar] [CrossRef]
- Mohzari, Y.; Aljobair, F.; Alrashed, A.; Asdaq, S.; Alshuraim, R.; Asfour, S.; Al-Mouqdad, M.; Bamogaddam, R.; Al-Anazi, D.; Zeilinger, C.; et al. Safety and efficacy of daptomycin in neonates with coagulase-negative staphylococci: Case series analysis. Antibiotics 2021, 10, 168. [Google Scholar] [CrossRef]
- Garazzino, S.; Tovo, P.-A. Clinical experience with linezolid in infants and children. J. Antimicrob. Chemother. 2011, 66 (Suppl. 4), iv23–iv41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiusaroli, L.; Liberati, C.; Rulli, L.; Barbieri, E.; De Pieri, M.; Di Chiara, C.; Mengato, D.; Giaquinto, C.; Donà, D. Therapeutic Options and Outcomes for the Treatment of Children with Gram-Positive Bacteria with Resistances of Concern: A Systematic Review. Antibiotics 2023, 12, 261. https://doi.org/10.3390/antibiotics12020261
Chiusaroli L, Liberati C, Rulli L, Barbieri E, De Pieri M, Di Chiara C, Mengato D, Giaquinto C, Donà D. Therapeutic Options and Outcomes for the Treatment of Children with Gram-Positive Bacteria with Resistances of Concern: A Systematic Review. Antibiotics. 2023; 12(2):261. https://doi.org/10.3390/antibiotics12020261
Chicago/Turabian StyleChiusaroli, Lorenzo, Cecilia Liberati, Luigi Rulli, Elisa Barbieri, Marica De Pieri, Costanza Di Chiara, Daniele Mengato, Carlo Giaquinto, and Daniele Donà. 2023. "Therapeutic Options and Outcomes for the Treatment of Children with Gram-Positive Bacteria with Resistances of Concern: A Systematic Review" Antibiotics 12, no. 2: 261. https://doi.org/10.3390/antibiotics12020261
APA StyleChiusaroli, L., Liberati, C., Rulli, L., Barbieri, E., De Pieri, M., Di Chiara, C., Mengato, D., Giaquinto, C., & Donà, D. (2023). Therapeutic Options and Outcomes for the Treatment of Children with Gram-Positive Bacteria with Resistances of Concern: A Systematic Review. Antibiotics, 12(2), 261. https://doi.org/10.3390/antibiotics12020261






