Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality
Abstract
1. Introduction
2. Results
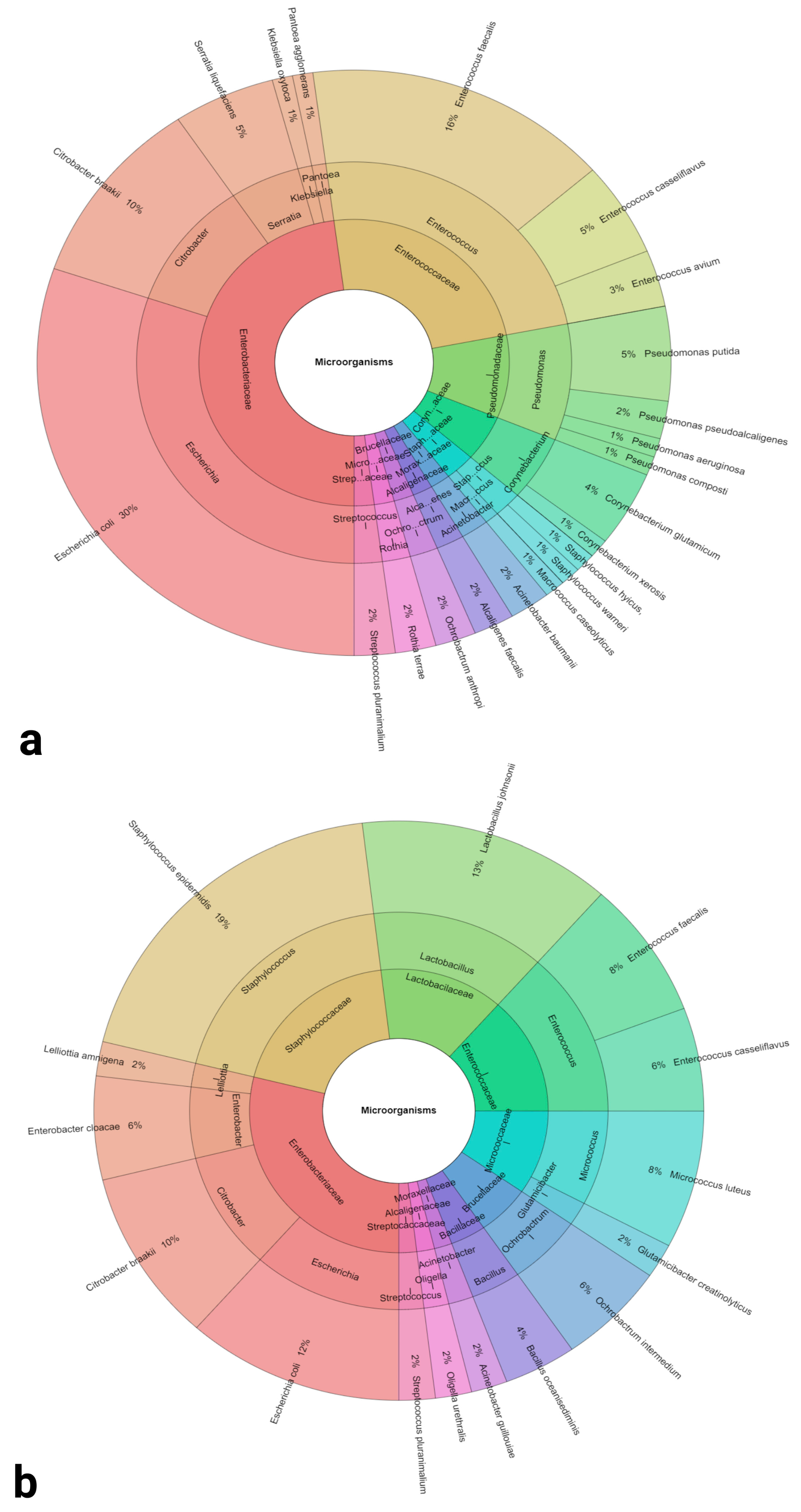
2.1. Identification of Bacteria
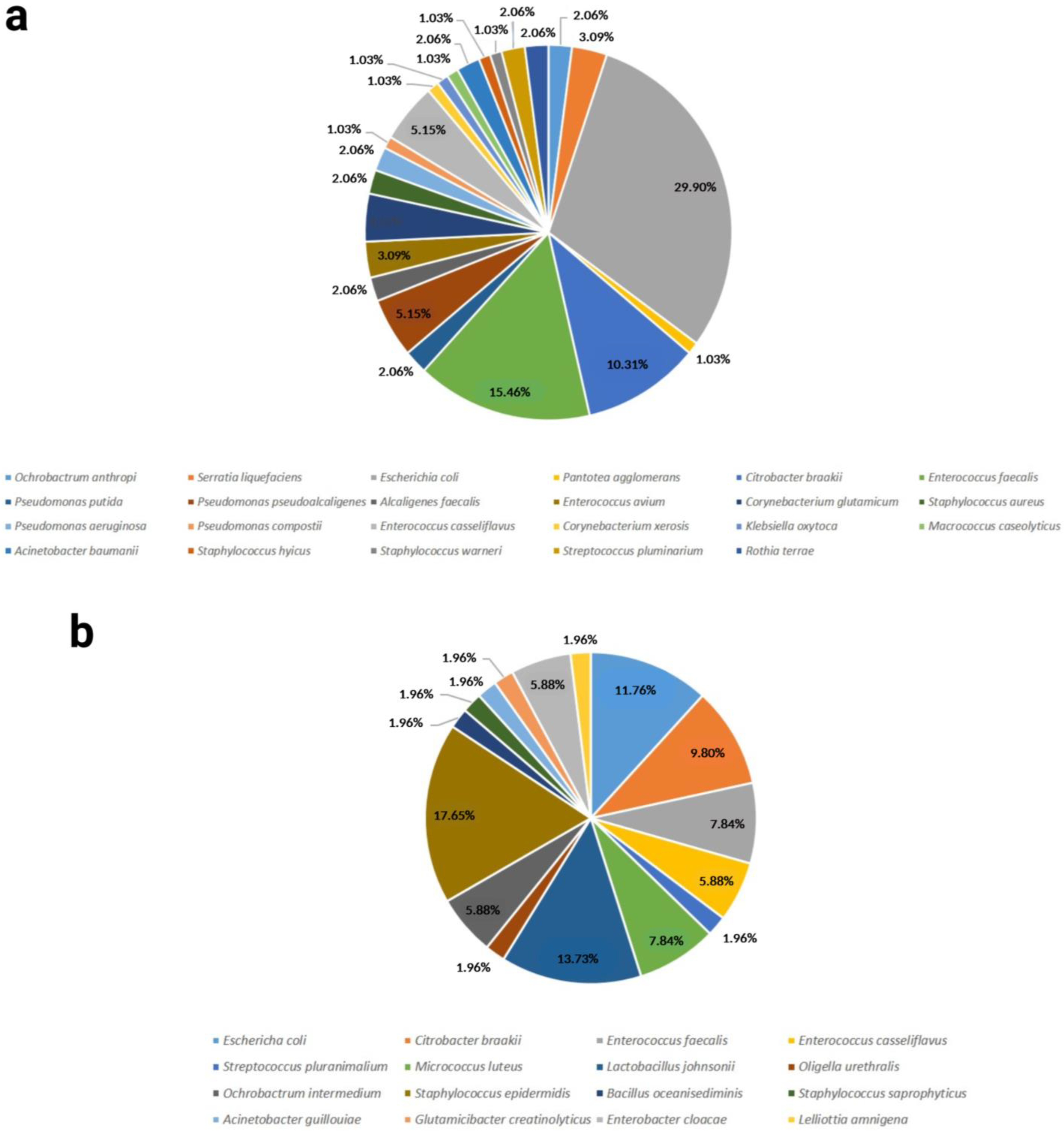
2.2. Biodiversity Assessment
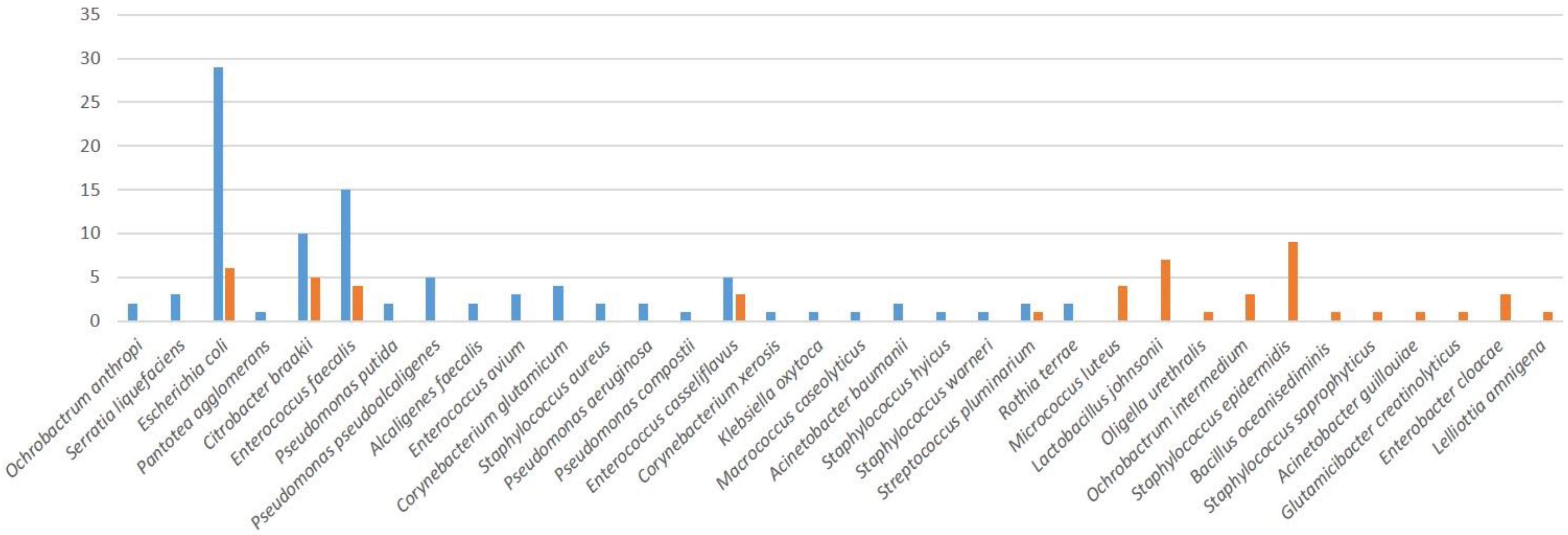
2.3. Bacterial Resistence
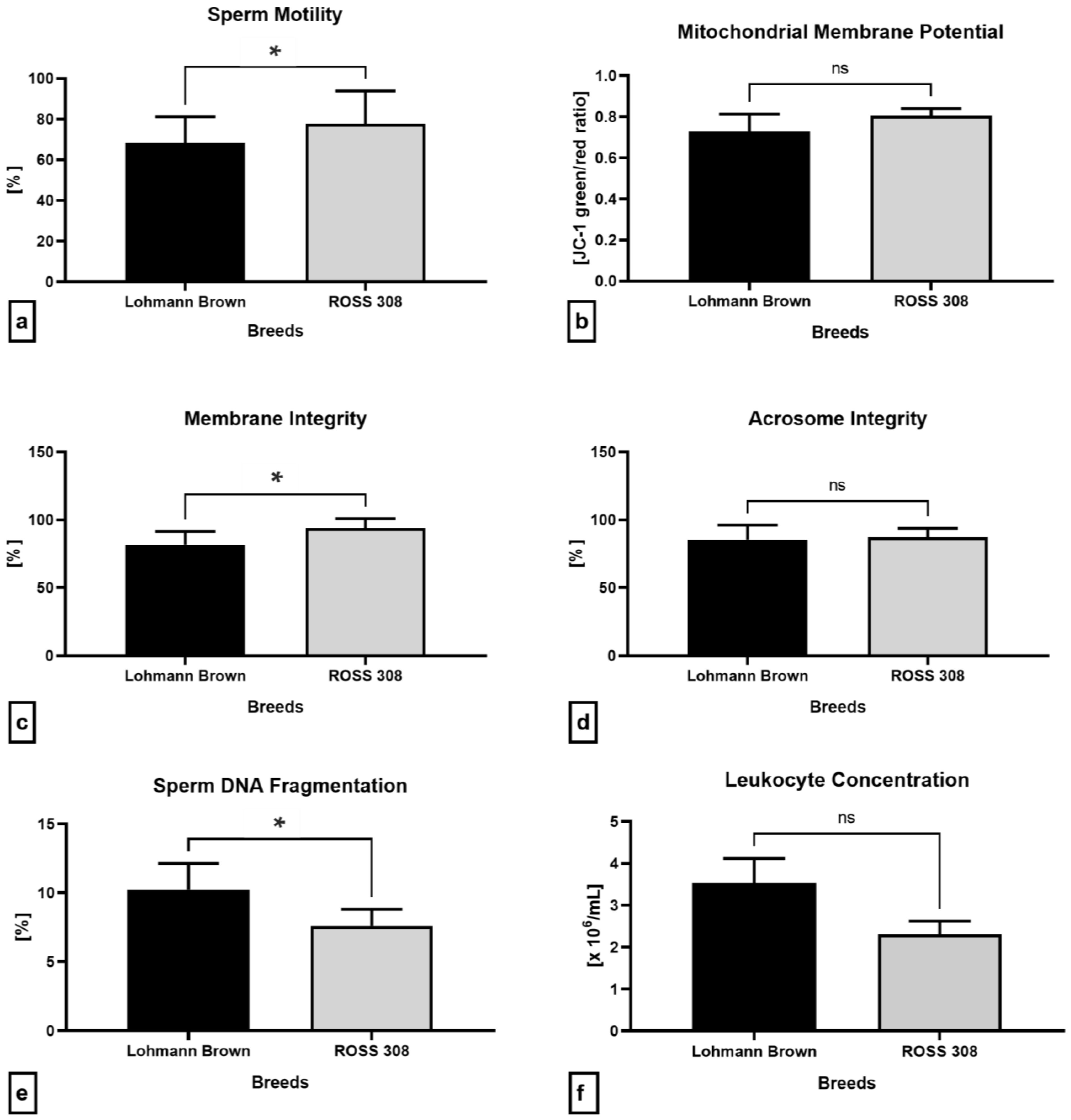
2.4. Semen Quality Parameters
2.5. Oxidative Profile
2.6. Immunological Profile of Semen
2.7. Antibacterial Proteins
3. Discussion
4. Materials and Methods
4.1. Semen Samples
4.2. Bacteriological Analysis
4.3. Biodiversity Analysis
4.4. Antibiotic Resistance Analysis
4.5. Conventional Semen Quality Parameters
4.6. Oxidative Profile
4.7. ELISA Assays
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebsa, Y.A.; Harpal, S.; Negia, G.G. Challenges and chicken production status of poultry producers in Bishoftu, Ethiopia. Poult. Sci. 2019, 98, 5452–5455. [Google Scholar] [CrossRef]
- Attia, Y.A.; Rahman, M.T.; Hossain, M.J.; Basiouni, S.; Khafaga, A.F.; Shehata, A.A.; Hafez, H.M. Poultry Production and Sustainability in Developing Countries under the COVID-19 Crisis: Lessons Learned. Animals 2022, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Roiter, L.M.; Vedenkina, I.V.A.; Eremeeva, N. Analysis of the market potential of poultry meat and its forecast. IOP Conf. Series Earth Environ. Sci. 2021, 937, 022104. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Oliveira, C.A. Epididymal lithiasis in roosters: In the middle of the way there was a stone. Life Sci. 2011, 89, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, A.; Gong, L.; Chen, Z.; Zhang, B.; Li, X. The Microbiome, an Important Factor That Is Easily Overlooked in Male Infertility. Front. Microbiol. 2022, 13, 831272. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kačániová, M.; Baláži, A.; Vašíček, J.; Vozaf, J.; Jurčík, R.; Ďuračka, M.; Žiarovská, J.; Kováč, J.; Chrenek, P. The Impact of Bacteriocenoses on Sperm Vitality, Immunological and Oxidative Characteristics of Ram Ejaculates: Does the Breed Play a Role? Animals 2022, 12, 54. [Google Scholar] [CrossRef]
- Lenický, M.; Slanina, T.; Kačániová, M.; Galovičová, L.; Petrovičová, M.; Ďuračka, M.; Benko, F.; Kováč, J.; Tvrdá, E. Identification of Bacterial Profiles and Their Interactions with Selected Quality, Oxidative, and Immunological Parameters of Turkey Semen. Animals 2021, 11, 1771. [Google Scholar] [CrossRef]
- Medo, J.; Žiarovská, J.; Ďuračka, M.; Tvrdá, E.; Baňas, Š.; Gábor, M.; Kyseľ, M.; Kačániová, M. Core Microbiome of Slovak Holstein Friesian Breeding Bulls’ Semen. Animals 2021, 11, 3331. [Google Scholar] [CrossRef]
- Masarikova, M.; Mrackova, M.; Sedlinska, M. Application of Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry in Identification of Stallion Semen Bacterial Contamination. J. Equine Vet. Sci. 2014, 34, 833–836. [Google Scholar] [CrossRef]
- Omprakash, A.; Venkatesh, G. Effect of vaginal douching and different semen extenders on bacterial load and fertility in turkeys. Br. Poult. Sci. 2006, 47, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus Faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef]
- Tvrdá, E.; Bučko, O.; Rojková, K.; Ďuračka, M.; Kunová, S.; Kováč, J.; Benko, F.; Kačániová, M. The Efficiency of Selected Extenders against Bacterial Contamination of Boar Semen in a Swine Breeding Facility in Western Slovakia. Animals 2021, 11, 3320. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.F.; Warner, L.; King, C.C.; Sobel, J.D.; Klein, R.S.; Cu-Uvin, S.; Rompalo, A.M.; Jamieson, D.J. Association between Semen Exposure and Incident Bacterial Vaginosis. Infect. Dis. Obstet. Gynecol. 2011, 2011, 842652. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.E.; Karcher, D.M. Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 5023–5027. [Google Scholar] [CrossRef] [PubMed]
- Moyle, T.; Drake, K.; Gole, V.; Chousalkar, K.; Hazel, S. Bacterial contamination of eggs and behaviour of poultry flocks in the free range environment. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al Musharafi, S.K.; Paulson, J.R. Consumption of Contaminated Eggs: A Public Health Con-cern. Med. Res. Arch. 2015, 2, 22–28. [Google Scholar] [CrossRef]
- Berkhoff, J.; Alvarado-Gilis, C.; Keim, J.P.; Alcalde, J.A.; Vargas-Bello-Pérez, E.; Gandarillas, M. Consumer preferences and sensory characteristics of eggs from family farms. Poult. Sci. 2020, 99, 6239–6246. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nat. Commun. 2021, 12, 5109. [Google Scholar] [CrossRef]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- Akpan, U.E.; Ofongo-Abule, R.T.S. Preliminary results on sources of bacteria of economic importance from three broiler chicken farms in Uyo metropolis of Akwa Ibom State. Niger. J. Anim. Sci. 2019, 21, 99–105. [Google Scholar]
- Christensen, H.; Bachmeier, J.; Bisgaard, M. New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathol. 2021, 50, 370–381. [Google Scholar] [CrossRef]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Maasjost, J.; Mühldorfer, K.; de Jäckel, S.C.; Hafez, H.M. Antimicrobial Susceptibility Patterns of Enterococcus faecalis and Enterococcus faecium Isolated from Poultry Flocks in Germany. Avian Dis. 2015, 59, 143–148. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Roesler, U.; Hafez, H.M.; Tomaso, H.; Neubauer, H.; El-Adawy, H. Evolution of Antibiotic Resistance of Coagulase-Negative Staphylococci Isolated from Healthy Turkeys in Egypt: First Report of Linezolid Resistance. Microorganisms 2019, 7, 476. [Google Scholar] [CrossRef]
- El-Adawy, H.; Ahmed, M.F.E.; Hotzel, H.; Tomaso, H.; Tenhagen, B.-A.; Hartung, J.; Neubauer, H.; Hafez, H.M. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli recovered from organic turkey farms in Germany. Poult. Sci. 2015, 94, 2831–2837. [Google Scholar] [CrossRef]
- Morrell, J.M.; Wallgren, M. Alternatives to Antibiotics in Semen Extenders: A Review. Pathogens 2014, 3, 934–946. [Google Scholar] [CrossRef]
- Shanmugam, M.; Vinoth, A.; Rajaravindra, K.S.; Rajkumar, U. Evaluation of semen quality in roosters of different age during hot climatic condition. Anim. Reprod. Sci. 2014, 145, 81–85. [Google Scholar] [CrossRef]
- Fouad, A.M.; El-Senousey, H.K.; Ruan, D.; Xia, W.; Chen, W.; Wang, S.; Zheng, C. Nutritional modulation of fertility in male poultry. Poult. Sci. 2020, 99, 5637–5646. [Google Scholar] [CrossRef]
- Prabakar, G.; Gopi, M.; Kolluri, G.; Rokade, J.J.; Pavulraj, S.; Pearlin, B.V.; Sudamrao Khillare, G.; Madhupriya, V.; Tyagi, J.S.; Mohan, J. Seasonal variations on semen quality attributes in turkey and egg type chicken male breeders. Int. J. Biometeorol. 2022, 66, 1547–1560. [Google Scholar] [CrossRef]
- Froman, D.P.; Rhoads, D.D. Breeding and Genetics Symposium: A systems biology definition for chicken semen quality. J. Anim. Sci. 2013, 91, 523–529. [Google Scholar] [CrossRef]
- Cox, N.A.; Stern, N.J.; Wilson, J.L.; Musgrove, M.T.; Buhr, R.J.; Hiett, K.L. Isolation of Campylobacter spp. from Semen Samples of Commercial Broiler Breeder Roosters. Avian Dis. 2002, 46, 717–720. [Google Scholar] [CrossRef]
- Haines, M.D.; Parker, H.M.; McDaniel, C.D.; Kiess, A.S. Impact of 6 different intestinal bacteria on broiler breeder sperm motility in vitro. Poult. Sci. 2013, 92, 2174–2181. [Google Scholar] [CrossRef]
- Vizzier-Thaxton, Y.; Cox, N.A.; Richardson, L.J.; Buhr, R.J.; McDaniel, C.D.; Cosby, D.E.; Wilson, J.L.; Bourassa, D.V.; Ard, M.B. Apparent Attachment of Campylobacter and Salmonella to Broiler Breeder Rooster Spermatozoa. Poult. Sci. 2006, 85, 619–624. [Google Scholar] [CrossRef]
- Hutchings, L.M.; Andrews, F.N. Isolation of Brucella suis from boar’s semen. J. Bacteriol. 1945, 50, 715–718. [Google Scholar] [CrossRef]
- Rosenthal, L. Agglutinating Properties of Escherichia coli: Agglutination of erythroces, leucocytes, thrombocytes, spermatozoa, spores of molds, and pollen by strains of E. coli. J. Bacteriol. 1943, 45, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Reiber, M.A.; McInroy, J.A.; Conner, D.E. Enumeration and Identification of Bacteria in Chicken Semen. Poult. Sci. 1995, 74, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K. Bacterial Flora of Poultry Semen and Their Antibiotic Sensitivity Pattern. Int. J. Appl. Pure Sci. Agric. 2015, 1, 39–41. [Google Scholar]
- Tesfay, H.H.; Sun, Y.; Li, Y.; Shi, L.; Fan, J.; Wang, P.; Zong, Y.; Ni, A.; Ma, H.; Mani, A.I.; et al. Comparative studies of semen quality traits and sperm kinematic parameters in relation to fertility rate between 2 genetic groups of breed lines. Poult. Sci. 2020, 99, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Buzala, M.; Janicki, B. Review: Effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult. Sci. 2016, 95, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.-M.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Lenický, M.; Kačániová, M.; Ďuračka, M.; Zajacová, Ž.; Muráňová, K.; Tvrdá, E. Bovine spermatozoa and Lactobacilli interactions: Deterioration or improvement of the sperm vitality? Anim. Reprod. Sci. 2022, 247, 107131. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in Female Infertility Via Modulating Sperm Agglutination and Immobilization. Front. Cell. Infect. Microbiol. 2021, 10, 620529. [Google Scholar] [CrossRef]
- Benoff, S.; Cooper, G.W.; Centola, G.M.; Jacob, A.; Hershlag, A.; Hurley, I.R. Metal ions and human sperm mannose receptors. Andrologia 2000, 32, 317–329. [Google Scholar] [CrossRef]
- Agarwal, J.; Srivastava, S.; Singh, M. Pathogenomics of uropathogenic Escherichia coli. Indian, J. Med. Microbiol. 2012, 30, 141–149. [Google Scholar] [CrossRef]
- Wolff, H.; Panhans, A.; Stolz, W.; Meurer, M. Adherence of Escherichia coli to sperm: A mannose mediated phenomenon leading to agglutination of sperm and E. coli. Fertil. Steril. 1993, 60, 154–158. [Google Scholar] [CrossRef]
- Fraczek, M.; Piasecka, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kazienko, A.; Lenart, S.; Laszczynska, M.; Kurpisz, M. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia 2012, 44, 315–329. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Sanocka, D.; Frączek, M.; Jędrzejczak, P.; Szumała-Kąkol, A.; Kurpisz, M. Male genital tract infection: An influence of leukocytes and bacteria on semen. J. Reprod. Immunol. 2004, 62, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Kurpisz, M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: Potential inflammatory markers in semen. Folia Histochem. Cytobiol. 2015, 53, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian, J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef]
- Payan-Carreira, R.; Santana, I.; Pires, M.A.; Holst, B.S.; Rodriguez-Martinez, H. Localization of tumor necrosis factor in the canine testis, epididymis and spermatozoa. Theriogenology 2012, 77, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Vera, O.; Vásqucz, L.A.; Muñoz, M.G. Semen quality and presence of cytokines in seminal fluid of bull ejaculates. Theriogenology 2003, 60, 553–558. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Pérez-Patiño, C.; Vazquez, J.M.; Martínez, E.A.; Rodríguez-Martínez, H.; Roca, J.; Parrilla, I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Dworacki, G.; Sanocka, D.; Kurpisz, M. In vitro reconstruction of inflammatory reaction in human semen: Effect on sperm DNA fragmentation. J. Reprod. Immunol. 2013, 100, 76–85. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Ďuračka, M.; Albertová, M.; Lukáč, N. Associations between inflammatory factors, lipid peroxidation and antioxidant capacity in bovine seminal plasma. Sci. Papers Anim. Sci. Biotechnol. 2016, 49, 38–44. [Google Scholar]
- Tvrdá, E.; Benko, F.; Ďuračka, M. Oxidative Stress as an Underlying Mechanism of Bacteria-Inflicted Damage to Male Gametes. Oxygen 2022, 2, 547–569. [Google Scholar] [CrossRef]
- Bording-Jorgensen, M.; Alipour, M.; Danesh, G.; Wine, E. Inflammasome Activation by ATP Enhances Citrobacter rodentium Clearance through ROS Generation. Cell. Physiol. Biochem. 2017, 41, 193–204. [Google Scholar] [CrossRef]
- Schulte, M.; Frick, K.; Gnandt, E.; Jurkovic, S.; Burschel, S.; Labatzke, R.; Aierstock, K.; Fiegen, D.; Wohlwend, D.; Gerhardt, S.; et al. A mechanism to prevent production of reactive oxygen species by Escherichia coli respiratory complex I. Nat. Commun. 2019, 10, 2551. [Google Scholar] [CrossRef] [PubMed]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell. Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Uchiyama, K.; Kinukawa, M.; Tagami, T.; Kaneda, M.; Watanabe, S. Evaluation of sperm DNA damage in bulls by TUNEL assay as a parameter of semen quality. J. Reprod. Dev. 2015, 61, 185–190. [Google Scholar] [CrossRef]
- Collodel, G.; Baccetti, B.; Capitani, S.; Moretti, E. Necrosis in human spermatozoa. I. Ultrastructural features and FISH study in semen from patients with urogenital infections. J. Submicrosc. Cytol. Pathol. 2007, 37, 67–73. [Google Scholar]
- Al Azad, M.A.R.; Rahman, M.M.; Amin, R.; Begum, M.I.A.; Fries, R.; Husna, A.; Khairalla, A.S.; Badruzzaman, A.T.M.; El Zowalaty, M.E.; Na Lampang, K.; et al. Susceptibility and Multidrug Resistance Patterns of Escherichia coli Isolated from Cloacal Swabs of Live Broiler Chickens in Bangladesh. Pathogens 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Sting, R.; Popp, C.; Rau, J.; Tenhagen, B.-A.; Guerra, B.; Hafez, H.M.; Fetsch, A. Prevalence of types of methicillin-resistant Staphylococcus aureus in turkey flocks and personnel attending the animals. Epidemiol. Infect. 2012, 140, 2223–2232. [Google Scholar] [CrossRef]
- Słowińska, M.; Nynca, J.; Arnold, G.J.; Fröhlich, T.; Jankowski, J.; Kozłowski, K.; Mostek, A.; Ciereszko, A. Proteomic identification of turkey (Meleagris gallopavo) seminal plasma proteins. Poult. Sci. 2017, 96, 3422–3435. [Google Scholar] [CrossRef]
- Zhang, G.; Sunkara, L.T. Avian Antimicrobial Host Defense Peptides: From Biology to Therapeutic Applications. Pharmaceuticals 2014, 7, 220–247. [Google Scholar] [CrossRef]
- Das, S.C.; Isobe, N.; Yoshimura, Y. Expression of Toll-like receptors and avian β-defensins and their changes in response to bacterial components in chicken sperm. Poult. Sci. 2011, 90, 417–425. [Google Scholar] [CrossRef]
- Duracka, M.; Galovičová, L.; Kunová, S.; Kačániová, M.; Lukáč, N.; Tvrdá, E. The bacterial presence in bovine semen and expression of seminal plasma proteins. Reprod. Domest. Anim. 2022, 57, 65–106. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed. Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Brown, K.L.; Poon, G.F.T.; Birkenhead, D.; Pena, O.N.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.E.W.; Johnson, P. Host Defense Peptide LL-37 Selectively Reduces Proinflammatory Macrophage Responses. J. Immunol. 2011, 186, 5497–5505. [Google Scholar] [CrossRef]
- Choi, K.-Y.G.; Mookherjee, N. Multiple Immune-Modulatory Functions of Cathelicidin Host Defense Peptides. Front. Immunol. 2012, 3, 149. [Google Scholar] [CrossRef] [PubMed]
- Horvatić, A.; Guillemin, N.; Kaab, H.; McKeegan, D.; O’Reilly, E.; Bain, M.; Kuleš, J.; Eckersall, P.D. Quantitative proteomics using tandem mass tags in relation to the acute phase protein response in chicken challenged with Escherichia coli lipopolysaccharide endotoxin. J. Proteom. 2019, 192, 64–77. [Google Scholar] [CrossRef]
- Kuz’min, M.D.; Ivanov, I.uB.; Bukharin, O.V. Use of lysozyme in the treatment of male infertility. Urol. Nefrol. 1998, 3, 46–48. [Google Scholar]
- Sotirov, L.; Dimitrov, S.; Jeliazkov, E. Semen lysozyme levels and semen quality in Turkeys (Meleagris gallopavo) fed with variousdietary protein levels. Revue Méd. Vét. 2002, 153, 815–818. [Google Scholar]
- Rowe, M.; Czirják, G.Á.; Ligjeld, J.T.; Giraudeau, M. Lysozyme-associated bactericidal activity in the ejaculate of a wild pas-serine. Biol. J. Linn. Soc. 2013, 109, 92–100. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Štefániková, J.; Žiarovská, J.; Savitskaya, T.; Grinshpan, D.; Kowalczewski, P.Ł.; Vukovic, N.; Tvrdá, E. Chemical Composition and Antimicrobial Activity of Selected Essential Oils against Staphylococcus spp. Isolated from Human Semen. Antibiotics 2020, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Petrovičová, M.; Benko, F.; Ďuračka, M.; Galovičová, L.; Slanina, T.; Kačániová, M. Curcumin Attenuates Damage to Rooster Spermatozoa Exposed to Selected Uropathogens. Pharmaceutics 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.H.; Lee, T.K.Y.; Montaño, M.A. Improved Chemiluminescence Assay for Measuring Antioxidant Capacity of Seminal Plasma. Methods Mol. Biol. 2013, 927, 363–376. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef] [PubMed]



| Groups | Lohmann Brown (n = 30) | ROSS 308 (n = 30) |
|---|---|---|
| Bacterial Load (log10 CFU/mL) | 7.23 ± 0.64 | 13.44 ± 1.97 * |
| Identified Bacterial Species and Sample Positivity | Escherichia coli (93.00%) | Staphylococcus epidermidis (30.00%) |
| Enterococcus faecalis (50.00%) | Lactobacillus johnsonii (23.30%) | |
| Citrobacter braakii (33.30%) | Escherichia coli (20.00%) | |
| Enterococcus casseliflavus (16.70%) | Citrobacter braakii (16.70%) | |
| Pseudomonas putida (17.70%) | Enterococcus faecalis (13.30%) | |
| Corynebacterium glutamicum (13.30%) | Micrococcus luteus (13.30%) | |
| Enterococcus avium (10.00%) | Enterobacter cloacae (10.00%) | |
| Serratia liquefaciens (10.00%) | Enterococcus casseliflavus (10.00%) | |
| Acinetobacter baumannii (6.70%) | Ochrobactrum intermedium (10.00%) | |
| Alcaligenes faecalis (6.70%) | Oligella urethralis (3.33%) | |
| Ochrobactrum anthropi (6.70%) | Acinetobacter guillouiae (3.33%) | |
| Pseudomonas aeruginosa (6.70%) | Bacillus oceanisediminis (3.33%) | |
| Pseudomonas pseudoalcaligenes (6.70%) | Glutamicibacter creatinolyticus (3.33%) | |
| Rothia terrae (6.70%) | Lelliottia amnigena (3.33%) | |
| Staphylococcus aureus (6.70%) | Staphylococcus saprophyticus (3.33%) | |
| Streptococcus pluranimalium (6.70%) | Streptococcus pluranimalium (3.33%) | |
| Corynebacterium xerosis (3.33%) | ||
| Klebsiella oxytoca (3.33%) | ||
| Macrococcus caseolyticus (3.33%) | ||
| Pantoea agglomerans (3.33%) | ||
| Pseudomonas composti (3.33%) | ||
| Staphylococcus hyicus (3.33%) | ||
| Staphylococcus warneri (3.33%) |
| Groups | Lohman Brown | ROSS 308 |
|---|---|---|
| Richness (R) | 23 | 16 |
| Berger Parker Dominance Index | 0.29 | 0.18 |
| Shannon α-diversity | 0.03 | 0.02 |
| Simpson dominance | 0.14 | 0.1 |
| Bacterium | Sensitivity | AMP | GEN | C | TET | IMP | TOB | TGC | LEV |
|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | S | 100% | 50% | 100% | 100% | 100% | 50% | 100% | 100% |
| I | 0% | 50% | 0% | 0% | 0% | 50% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Acinetobacter guillouiae | S | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Alcaligenes faecalis | S | 100% | 0% | 100% | 0% | 100% | 100% | 100% | 100% |
| I | 0% | 100% | 0% | 100% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Bacillus oceanisediminis | S | 0% | 100% | ND | 0% | 100% | ND | 100% | 100% |
| I | 100% | 0% | 100% | 0% | 0% | 0% | |||
| R | 0% | 0% | 0% | 0% | 0% | 0% | |||
| Citrobacter braakii | S | 0% | 100% | 66% | 100% | 100% | 33% | 100% | 83% |
| I | 25% | 0% | 17% | 0% | 0% | 50% | 0% | 17% | |
| R | 75% | 0% | 17% | 0% | 0% | 17% | 0% | 0% | |
| Corynebacterium glutamicum | S | 100% | 100% | ND | 100% | 100% | ND | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | |||
| R | 0% | 0% | 0% | 0% | 0% | 0% | |||
| Corynebacterium xerosis | S | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Enterobacter cloacae | S | 0% | 100% | 100% | 100% | 100% | 50% | 100% | 100% |
| I | 50% | 0% | 0% | 0% | 0% | 50% | 0% | 0% | |
| R | 50% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Enterococcus casseliflavus | S | 40% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 60% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Enterococcus avium | S | 50% | 50% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 50% | 50% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Enterococcus faecalis | S | 50% | 100% | 100% | 100% | 75% | 100% | 75% | 100% |
| I | 25% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 25% | 0% | 0% | 0% | 25% | 0% | 25% | 0% | |
| Escherichia coli | S | 18% | 100% | 64% | 64% | 100% | 82% | 100% | 100% |
| I | 36% | 0% | 0% | 36% | 0% | 0% | 0% | 0% | |
| R | 46% | 0% | 36% | 0% | 0% | 18% | 0% | 0% | |
| Glutamicibacter creatinolyticus | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Klebsiella oxytoca | S | 100% | 0% | 100% | 100% | 100% | 0% | 100% | 100% |
| I | 0% | 100% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 100% | 0% | 0% | |
| Lactobacillus johnsonii | S | 50% | 100% | ND | 33.3% | 100% | 100% | 100% | 100% |
| I | 50% | 0% | 33.3% | 0% | 0% | 0% | 0% | ||
| R | 0% | 0% | 33.3% | 0% | 0% | 0% | 0% | ||
| Lelliottia amnigena | S | 67% | 67% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 33% | 33% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Macrococcus caseolyticus | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Micrococcus luteus | S | 100% | 100% | ND | 100% | 100% | ND | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | |||
| R | 0% | 0% | 0% | 0% | 0% | 0% | |||
| Ochrobactrum anthropi | S | 0% | 100% | ND | 100% | 100% | ND | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | |||
| R | 100% | 0% | 0% | 0% | 0% | 0% | |||
| Ochrobactrum intermedium | S | 0% | 100% | ND | 100% | 100% | ND | 100% | 100% |
| I | 50% | 0% | 0% | 0% | 0% | 0% | |||
| R | 50% | 0% | 0% | 0% | 0% | 0% | |||
| Oligella urethralis | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 0% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 100% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Pantoea agglomerans | S | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Pseudomonas aeruginosa | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Pseudomonas composti | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Pseudomonas pseudoalcaligenes | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Pseudomonas putida | S | 33% | 67% | 100% | 100% | 33% | 100% | 100% | 100% |
| I | 67% | 33% | 0% | 0% | 67% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Rothia terrae | S | 100% | 0% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 100% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Serratia liquefaciens | S | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Staphylococcus aureus | S | 100% | 100% | 100% | 0% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 100% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Staphylococcus epidermidis | S | 40% | 100% | 100% | 20% | 100% | 20% | 100% | 100% |
| I | 40% | 0% | 0% | 20% | 0% | 40% | 0% | 0% | |
| R | 20% | 0% | 0% | 60% | 0% | 40% | 0% | 0% | |
| Staphylococcus hyicus | S | 0% | 100% | 100% | 0% | 100% | 100% | 100% | 0% |
| I | 100% | 0% | 0% | 100% | 0% | 0% | 0% | 100% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Staphylococcus saprophyticus | S | 0% | 100% | 100% | 100% | 100% | 100% | 100% | 0% |
| I | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 100% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| Staphylococcus warneri | S | 0% | 100% | 100% | 100% | 100% | 0% | 100% | 100% |
| I | 100% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 100% | 0% | 0% | |
| Streptococcus pluranimalium | S | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| I | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | |
| R | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvrdá, E.; Petrovičová, M.; Benko, F.; Ďuračka, M.; Kováč, J.; Slanina, T.; Galovičová, L.; Žiarovská, J.; Kačániová, M. Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality. Antibiotics 2023, 12, 336. https://doi.org/10.3390/antibiotics12020336
Tvrdá E, Petrovičová M, Benko F, Ďuračka M, Kováč J, Slanina T, Galovičová L, Žiarovská J, Kačániová M. Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality. Antibiotics. 2023; 12(2):336. https://doi.org/10.3390/antibiotics12020336
Chicago/Turabian StyleTvrdá, Eva, Michaela Petrovičová, Filip Benko, Michal Ďuračka, Ján Kováč, Tomáš Slanina, Lucia Galovičová, Jana Žiarovská, and Miroslava Kačániová. 2023. "Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality" Antibiotics 12, no. 2: 336. https://doi.org/10.3390/antibiotics12020336
APA StyleTvrdá, E., Petrovičová, M., Benko, F., Ďuračka, M., Kováč, J., Slanina, T., Galovičová, L., Žiarovská, J., & Kačániová, M. (2023). Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality. Antibiotics, 12(2), 336. https://doi.org/10.3390/antibiotics12020336









