ESBL-Positive Enterobacteriaceae from Dogs of Santiago and Boa Vista Islands, Cape Verde: A Public Health Concern
Abstract
1. Introduction
2. Results
2.1. Characterization of the Isolates’ Antimicrobial-Resistance Profile
2.2. Characterization of the Isolates’ Virulence Profile
3. Discussion
4. Materials and Methods
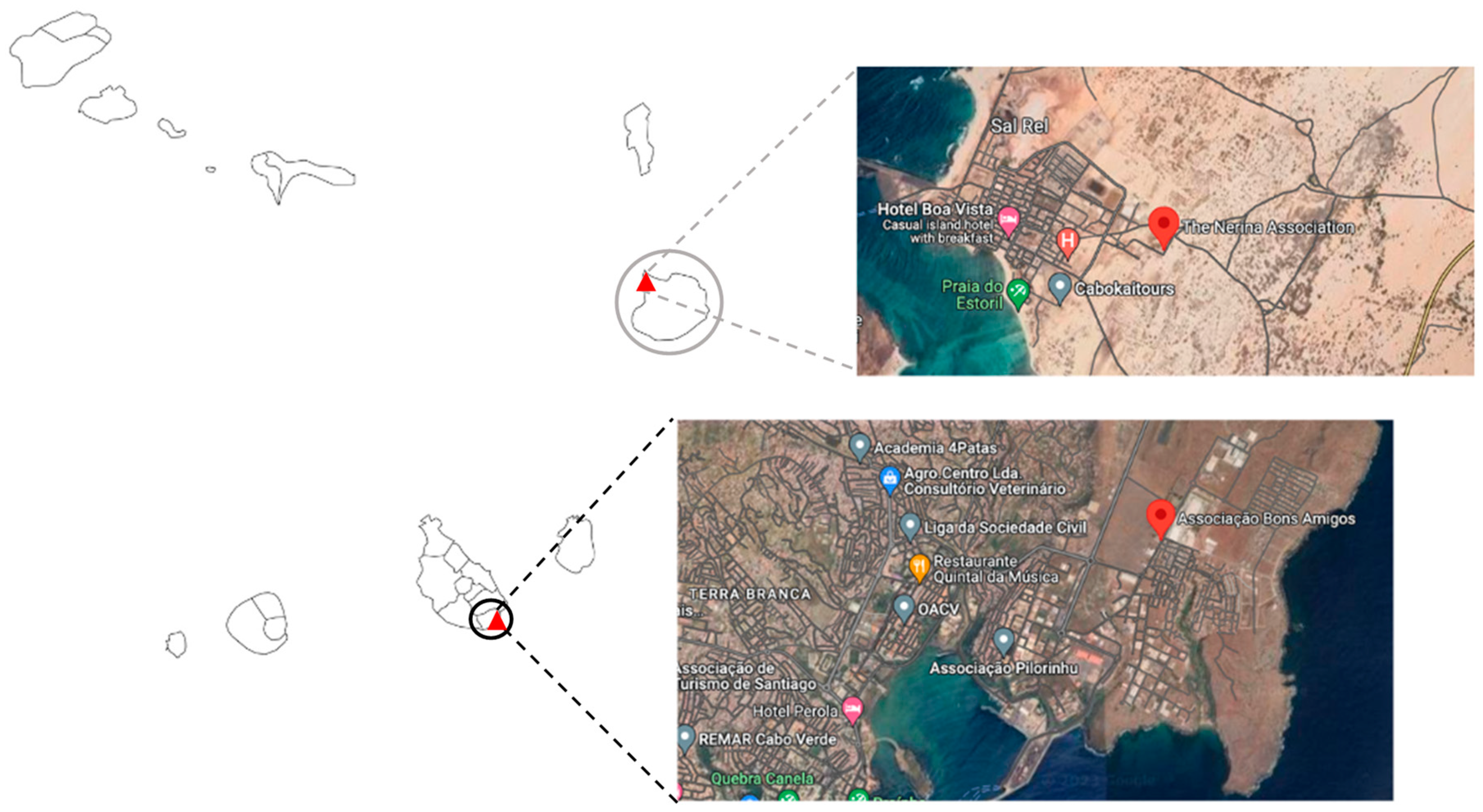
4.1. Sampling Area
4.2. Sample Collection
4.3. Isolation and Identification of ESBL-Positive Bacteria
4.4. Evaluation of Isolates’ Antibiotic-Resistance Profile
4.5. Phenotypic Evaluation of Isolates’ Virulence Profile
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Antimicrobial Resistance: Fact Sheet on Sustainable Development Goals (SDGs): Health Targets; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- WHO. Pubishes List of Bacteria for which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 20 September 2022).
- CDC. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Presention: Atlanta, GA, USA, 2019. [Google Scholar]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Rawat, D.; Nair, D. Extended-Spectrum β-Lactamases in Gram Negative Bacteria. J. Glob. Infect. Dis. 2010, 2, 263. [Google Scholar] [CrossRef]
- Monteiro, T.; Wysocka, M.; Tellez, E.; Monteiro, O.; Spencer, L.; Veiga, E.; Monteiro, S.; de Pina, C.; Gonçalves, D.; de Pina, S.; et al. A Five-Year Retrospective Study Shows Increasing Rates of Antimicrobial Drug Resistance in Cabo Verde for Both Staphylococcus aureus and Escherichia coli. J. Glob. Antimicrob. Resist. 2020, 22, 483–487. [Google Scholar] [CrossRef]
- Sanchez, S.; Stevenson, M.A.M.; Hudson, C.R.; Maier, M.; Buffington, T.; Dam, Q.; Maurer, J.J. Characterization of Multidrug-Resistant Escherichia coli Isolates Associated with Nosocomial Infections in Dogs. J. Clin. Microbiol. 2002, 40, 10. [Google Scholar] [CrossRef]
- Lanza, V.F.; Tedim, A.P.; Martínez, J.L.; Baquero, F.; Coque, T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015, 3, PLAS-0039-2014. [Google Scholar] [CrossRef]
- Furtado da Luz, E.; Braga, J.U. Under-Reporting of Tuberculosis in Praia, Cape Verde, from 2006 to 2012. Int. J. Tuberc. Lung Dis. 2018, 22, 258–263. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- The World Bank in Cape Verde. Available online: https://www.worldbank.org/pt/country/caboverde/overview#1 (accessed on 20 September 2022).
- Fan, N.-C.; Chen, H.-H.; Chen, C.-L.; Ou, L.-S.; Lin, T.-Y.; Tsai, M.-H.; Chiu, C.-H. Rise of Community-Onset Urinary Tract Infection Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli in Children. J. Microbiol. Immunol. Infect. 2014, 47, 399–405. [Google Scholar] [CrossRef]
- Kassakian, S.Z.; Mermel, L.A. Changing Epidemiology of Infections Due to Extended Spectrum Beta-Lactamase Producing Bacteria. Antimicrob. Resist. Infect. Control 2014, 3, 9. [Google Scholar] [CrossRef]
- Goyal, D.; Dean, N.; Neill, S.; Jones, P.; Dascomb, K. Risk Factors for Community-Acquired Extended-Spectrum Beta-Lactamase–Producing Enterobacteriaceae Infections—A Retrospective Study of Symptomatic Urinary Tract Infections. Open Forum Infect. Dis. 2019, 6, ofy357. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. IDR 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- Data for Lower Middle Income, Cabo Verde. Available online: https://data.worldbank.org/?locations=XN-CV (accessed on 20 September 2022).
- Marques, C.; Belas, A.; Aboim, C.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.T.; Pomba, C. Evidence of Sharing of Klebsiella pneumoniae Strains between Healthy Companion Animals and Cohabiting Humans. J. Clin. Microbiol. 2019, 57, e01537-18. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable Sources of Community-Acquired Carriage of Escherichia coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef]
- Toombs-Ruane, L.J.; Benschop, J.; French, N.P.; Biggs, P.J.; Midwinter, A.C.; Marshall, J.C.; Chan, M.; Drinković, D.; Fayaz, A.; Baker, M.G.; et al. Carriage of Extended-Spectrum-Beta-Lactamase- and AmpC Beta-Lactamase-Producing Escherichia coli Strains from Humans and Pets in the Same Households. Appl. Environ. Microbiol. 2020, 86, e01613-20. [Google Scholar] [CrossRef]
- Schmitt, K.; Kuster, S.P.; Zurfluh, K.; Jud, R.S.; Sykes, J.E.; Stephan, R.; Willi, B. Transmission Chains of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae at the Companion Animal Veterinary Clinic–Household Interface. Antibiotics 2021, 10, 171. [Google Scholar] [CrossRef]
- Platell, J.L.; Trott, D.J.; Johnson, J.R.; Heisig, P.; Heisig, A.; Clabots, C.R.; Johnston, B.; Cobbold, R.N. Prominence of an O75 Clonal Group (Clonal Complex 14) among Non-ST131 Fluoroquinolone-Resistant Escherichia coli Causing Extraintestinal Infections in Humans and Dogs in Australia. Antimicrob. Agents Chemother. 2012, 56, 3898–3904. [Google Scholar] [CrossRef]
- Marques, C.; Belas, A.; Aboim, C.; Trigueiro, G.; Cavaco-Silva, P.; Gama, L.T.; Pomba, C. Clonal Relatedness of Proteus Mirabilis Strains Causing Urinary Tract Infections in Companion Animals and Humans. Vet. Microbiol. 2019, 228, 77–82. [Google Scholar] [CrossRef]
- Brilhante, M.; Menezes, J.; Belas, A.; Feudi, C.; Schwarz, S.; Pomba, C.; Perreten, V. OXA-181-Producing Extraintestinal Pathogenic Escherichia coli Sequence Type 410 Isolated from a Dog in Portugal. Antimicrob. Agents Chemother. 2020, 64, e02298-19. [Google Scholar] [CrossRef]
- Perestrelo, S.; Correia Carreira, G.; Valentin, L.; Fischer, J.; Pfeifer, Y.; Werner, G.; Schmiedel, J.; Falgenhauer, L.; Imirzalioglu, C.; Chakraborty, T.; et al. Comparison of Approaches for Source Attribution of ESBL-Producing Escherichia coli in Germany. PLoS ONE 2022, 17, e0271317. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A.L.; Delavari, P. Canine Feces as a Reservoir of Extraintestinal Pathogenic Escherichia Coli. Infect. Immun. 2001, 69, 1306–1314. [Google Scholar] [CrossRef]
- Damborg, P.; Morsing, M.K.; Petersen, T.; Bortolaia, V.; Guardabassi, L. CTX-M-1 and CTX-M-15-Producing Escherichia coli in Dog Faeces from Public Gardens. Acta Vet. Scand. 2015, 57, 83. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-Resistant Escherichia coli Isolated from Canine Faeces in a Public Park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef]
- Salinas, L.; Loayza, F.; Cárdenas, P.; Saraiva, C.; Johnson, T.J.; Amato, H.; Graham, J.P.; Trueba, G. Environmental Spread of Extended Spectrum Beta-Lactamase (ESBL) Producing Escherichia Coli and ESBL Genes among Children and Domestic Animals in Ecuador. Environ. Health Perspect. 2021, 129, 027007. [Google Scholar] [CrossRef]
- Omulo, S.; Lofgren, E.T.; Lockwood, S.; Thumbi, S.M.; Bigogo, G.; Ouma, A.; Verani, J.R.; Juma, B.; Njenga, M.K.; Kariuki, S.; et al. Carriage of Antimicrobial-Resistant Bacteria in a High-Density Informal Settlement in Kenya Is Associated with Environmental Risk-Factors. Antimicrob. Resist. Infect. Control 2021, 10, 18. [Google Scholar] [CrossRef]
- INE. Estatísticas das Famílias e Condições de Vida: Inquérito Multi-Objetivo Contínuo 2019; Instituto Nacional de Estatística: Praia, Cabo Verde, 2020. [Google Scholar]
- Lopes Antunes, A.C.; Ducheyne, E.; Bryssinckx, W.; Vieira, S.; Malta, M.; Vaz, Y.; Nunes, T.; Mintiens, K. The Dog and Cat Population on Maio Island, Cape Verde: Characterisation and Prediction Based on Household Survey and Remotely Sensed Imagery. Geospat Health 2015, 10, 386. [Google Scholar] [CrossRef]
- Tiwari, H.K.; Robertson, I.D.; O’Dea, M.; Vanak, A.T. Demographic Characteristics of Free-Roaming Dogs (FRD) in Rural and Urban India Following a Photographic Sight-Resight Survey. Sci. Rep. 2019, 9, 16562. [Google Scholar] [CrossRef]
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Ogeer-Gyles, J.; Mathews, K.A.; Sears, W.; Prescott, J.F.; Weese, J.S.; Boerlin, P. Development of Antimicrobial Drug Resistance in Rectal Escherichia coli Isolates from Dogs Hospitalized in an Intensive Care Unit. J. Am. Vet. Med. Assoc. 2006, 229, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.L.; Rankin, S.C.; Byrne, B.A.; Weese, J.S. Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control. J. Vet. Intern. Med. 2011, 25, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Monteiro, J.L.; Rana, S.; Vilela, C.L. Antimicrobial Resistance in Gram-Positive Bacteria from Timorese River Buffalo (Bubalus bubalis) Skin Microbiota. Trop. Anim. Health Prod. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.P.; Crichton, M. Cefpodoxime Screening of Escherichia coli and Klebsiella spp. by Vitek for Detection of Organisms Producing Extended-Spectrum -Lactamases. Diagn. Microbiol. Infect. Dis. 2000, 3, 255–257. [Google Scholar] [CrossRef]
- Färber, J.; Moder, K.-A.; Layer, F.; Tammer, I.; König, W.; König, B. Extended-Spectrum Beta-Lactamase Detection with Different Panels for Automated Susceptibility Testing and with a Chromogenic Medium. J. Clin. Microbiol. 2008, 46, 3721–3727. [Google Scholar] [CrossRef]
- Girlich, D.; Bouihat, N.; Poirel, L.; Benouda, A.; Nordmann, P. High Rate of Faecal Carriage of Extended-Spectrum β-Lactamase and OXA-48 Carbapenemase-Producing Enterobacteriaceae at a University Hospital in Morocco. Clin. Microbiol. Infect. 2014, 20, 350–354. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Third Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Jarlier, V.; Nicolas, M.-H.; Fournier, G.; Philippon, A. Extended Broad-Spectrum -Lactamases Conferring Transferable Resistance to Newer -Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns. Clin. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Drieux, L.; Brossier, F.; Sougakoff, W.; Jarlier, V. Phenotypic Detection of Extended-Spectrum β-Lactamase Production in Enterobacteriaceae: Review and Bench Guide. Clin. Microbiol. Infect. 2008, 14, 90–103. [Google Scholar] [CrossRef]
- Kaur, J.; Chopra, S.; Sheevani; Mahajan, G. Modified Double Disc Synergy Test to Detect ESBL Production in Urinary Isolates of Escherichia coli and Klebsiella pneumoniae. J. Clin. Diagn. Res. 2013, 7, 229–233. [Google Scholar] [CrossRef]
- EUCAST. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance Version 2.0; European Committee on Antimicrobial Susceptibility Testing: Stockholm, Sweden, 2017. [Google Scholar]
- Hordijk, J.; Schoormans, A.; Kwakernaak, M.; Duim, B.; Broens, E.; Dierikx, C.; Mevius, D.; Wagenaar, J.A. High Prevalence of Fecal Carriage of Extended Spectrum β-Lactamase/AmpC-Producing Enterobacteriaceae in Cats and Dogs. Front. Microbiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; Samir, A. Occurrence of Extended Spectrum β–Lactamase-Producing Enterobacteriaceae among Pet Dogs and Cats: An Emerging Public Health Threat Outside Health Care Facilities. Am. J. Infect. Control 2014, 42, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing Prevalence of ESBL-Producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 19044. [Google Scholar] [CrossRef] [PubMed]
- Punia, M.; Kumar, A.; Charaya, G.; Kumar, T. Pathogens Isolated from Clinical Cases of Urinary Tract Infection in Dogs and Their Antibiogram. Vet. World 2018, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Otto, C.M. Systemic Inflammatory Response Syndrome, Sepsis, and Multiple Organ Dysfunction. Vet. Clin. North Am. Small Anim. Pract. 2001, 31, 1147–1162. [Google Scholar] [CrossRef]
- Keynan, Y.; Rubinstein, E. The Changing Face of Klebsiella pneumoniae Infections in the Community. Int. J. Antimicrob. Agents 2007, 30, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Milo, S.; Heylen, R.A.; Glancy, J.; Williams, G.T.; Patenall, B.L.; Hathaway, H.J.; Thet, N.T.; Allinson, S.L.; Laabei, M.; Jenkins, A.T.A. A Small-Molecular Inhibitor against Proteus mirabilis Urease to Treat Catheter-Associated Urinary Tract Infections. Sci. Rep. 2021, 11, 3726. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharm. 2017, 33, 300. [Google Scholar] [CrossRef]
- Romo-Ibáñez, Á.; Calatrava-Hernández, E.; Gutiérrez-Soto, B.; Pérez-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. High Clinical Impact of Rapid Susceptibility Testing on CHROMID ESBL® Medium Directly from Swabs. Ann. Transl. Med. 2020, 8, 604. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Parajuli, N.P.; Maharjan, P.; Parajuli, H.; Joshi, G.; Paudel, D.; Sayami, S.; Khanal, P.R. High Rates of Multidrug Resistance among Uropathogenic Escherichia coli in Children and Analyses of ESBL Producers from Nepal. Antimicrob. Resist. Infect. Control 2017, 6, 9. [Google Scholar] [CrossRef]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in Antimicrobial Resistance and Emergence of Major International High-Risk Clonal Lineages in Dogs and Cats with Urinary Tract Infection: 16 Year Retrospective Study. J. Antimicrob. Chemother. 2018, 73, 377–384. [Google Scholar] [CrossRef]
- Kantele, A.; Mero, S.; Kirveskari, J.; Lääveri, T. Fluoroquinolone Antibiotic Users Select Fluoroquinolone-Resistant ESBL-Producing Enterobacteriaceae (ESBL-PE)—Data of a Prospective Traveller Study. Travel Med. Infect. Dis. 2017, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Johansson, V.; Nykäsenoja, S.; Myllyniemi, A.-L.; Rossow, H.; Heikinheimo, A. Genomic Characterization of ESBL/AmpC-Producing and High-Risk Clonal Lineages of Escherichia coli and Klebsiella pneumoniae in Imported Dogs with Shelter and Stray Background. J. Glob. Antimicrob. Resist. 2022, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Götsch, S.; Leschnik, M.; Duscher, G.; Burgstaller, J.P.; Wille-Piazzai, W.; Joachim, A. Ticks and Haemoparasites of Dogs from Praia, Cape Verde. Vet. Parasitol. 2009, 166, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Leshem, E.; Meltzer, E.; Schwartz, E. Travel-Associated Zoonotic Bacterial Diseases. Curr. Opin. Infect. Dis. 2011, 24, 457–463. [Google Scholar] [CrossRef]
- Taheri, A.R.; Rad, S.S.; Molkara, S. Systemic Treatments of Leishmaniasis: A Narrative Review. Rev. Clin. Med. 2019, 6, 91–97. [Google Scholar]
- Onduru, O.G.; Aboud, S.; Nyirenda, T.S.; Rumisha, S.F.; Mkakosya, R.S. Antimicrobial Susceptibility Testing Profiles of ESBL-Producing Enterobacterales Isolated from Hospital and Community Adult Patients in Blantyre, Malawi. IJID Reg. 2021, 1, 47–52. [Google Scholar] [CrossRef]
- Ortiz-Díez, G.; Mengíbar, R.L.; Turrientes, M.-C.; Artigao, M.-R.B.; Gallifa, R.L.; Tello, A.M.; Pérez, C.F.; Santiago, T.A. Prevalence, Incidence and Risk Factors for Acquisition and Colonization of Extended-Spectrum Beta-Lactamase- and Carbapenemase-Producing Enterobacteriaceae from Dogs Attended at a Veterinary Hospital in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101922. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Thornsberry, C.; Jones, M.E.; Sahm, D.F. Susceptibility of Antimicrobial-Resistant Urinary Escherichia coli Isolates to Fluoroquinolones and Nitrofurantoin. Clin. Infect. Dis. 2003, 36, 183–187. [Google Scholar] [CrossRef]
- Raja, N.S. Oral Treatment Options for Patients with Urinary Tract Infections Caused by Extended Spectrum Βeta-Lactamase (ESBL) Producing Enterobacteriaceae. J. Infect. Public Health 2019, 12, 843–846. [Google Scholar] [CrossRef]
- McKinnell, J.A.; Stollenwerk, N.S.; Jung, C.W.; Miller, L.G. Nitrofurantoin Compares Favorably to Recommended Agents as Empirical Treatment of Uncomplicated Urinary Tract Infections in a Decision and Cost Analysis. Mayo Clin. Proc. 2011, 86, 480–488. [Google Scholar] [CrossRef]
- Leuin, A.S.; Hartmann, F.; Viviano, K. Administration of Nitrofurantoin in Dogs with Lower Urinary Tract Infections: 14 Cases (2013–2019). J. Small Anim. Pr. 2021, 62, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Spiegel, C.A.; Gilmore, M.S. Bacteremia Caused by Hemolytic, High-Level Gentamicin-Resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 1991, 35, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Leener, E.D.; Decostere, A.; De Graef, E.M.; Moyaert, H.; Haesebrouck, F. Presence and Mechanism of Antimicrobial Resistance among Enterococci from Cats and Dogs. Microb. Drug Resist. 2005, 11, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Dadfarma, N.; Imani Fooladi, A.A.; Oskoui, M.; Mahmoodzadeh Hosseini, H. High Level of Gentamicin Resistance (HLGR) among Enterococcus Strains Isolated from Clinical Specimens. J. Infect. Public Health 2013, 6, 202–208. [Google Scholar] [CrossRef]
- Aslanta, Ö. Investigation of Faecal Carriage of High-Level Gentamicin Resistant Enterococci in Dogs and Cats. Isr. J. Vet. Med. 2022, 77, 27–37. [Google Scholar]
- Magwenzi, M.T.; Gudza-Mugabe, M.; Mujuru, H.A.; Dangarembizi-Bwakura, M.; Robertson, V.; Aiken, A.M. Carriage of Antibiotic-Resistant Enterobacteriaceae in Hospitalised Children in Tertiary Hospitals in Harare, Zimbabwe. Antimicrob. Resist. Infect. Control 2017, 6, 10. [Google Scholar] [CrossRef]
- Maeyama, Y.; Taniguchi, Y.; Hayashi, W.; Ohsaki, Y.; Osaka, S.; Koide, S.; Tamai, K.; Nagano, Y.; Arakawa, Y.; Nagano, N. Prevalence of ESBL/AmpC Genes and Specific Clones among the Third-Generation Cephalosporin-Resistant Enterobacteriaceae from Canine and Feline Clinical Specimens in Japan. Vet. Microbiol. 2018, 216, 183–189. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem Resistance in Enterobacteriaceae: Here Is the Storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- van Duin, D.; Kaye, K.S.; Neuner, E.A.; Bonomo, R.A. Carbapenem-Resistant Enterobacteriaceae: A Review of Treatment and Outcomes. Diagn. Microbiol. Infect. Dis. 2013, 75, 115–120. [Google Scholar] [CrossRef]
- Kelly, A.M.; Mathema, B.; Larson, E.L. Carbapenem-Resistant Enterobacteriaceae in the Community: A Scoping Review. Int. J. Antimicrob. Agents 2017, 50, 127–134. [Google Scholar] [CrossRef]
- Cole, S.D.; Peak, L.; Tyson, G.H.; Reimschuessel, R.; Ceric, O.; Rankin, S.C. New Delhi Metallo-β-Lactamase-5–Producing Escherichia coli in Companion Animals, United States. Emerg. Infect. Dis. 2020, 26, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S. Companion Animals Emerged as an Important Reservoir of Carbapenem-Resistant Enterobacteriaceae: A Report from India. Curr. Microbiol. 2021, 78, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W. Mechanisms of Bacterial Pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, B.D.; Costerton, J.W. Bacterial Resistance to Antibiotics: The Role of Biofilms. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès des Recherches Pharmaceutiques; Jucker, E., Ed.; Birkhäuser: Basel, Switzerland, 1991; pp. 91–105. ISBN 978-3-7643-2626-5. [Google Scholar]
- Johnson, D.I. Bacterial Pathogens and Their Virulence Factors; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-67650-0. [Google Scholar]
- Mah, T.-F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- De Jesus, M.B.; Ehlers, M.M.; Dos Santos, R.F.; Kock, M.M. Review—Understanding β-Lactamase Producing Klebsiella Pneumoniae. In Antimicrobial Resistance—An Open Challenge; Ossiprandi, M.C., Ed.; InTech: Nappanee, Indiana, 2015; ISBN 978-953-51-2222-7. [Google Scholar]
- Cinquepalmi, V.; Monno, R.; Fumarola, L.; Ventrella, G.; Calia, C.; Greco, M.; de Vito, D.; Soleo, L. Environmental Contamination by Dog’s Faeces: A Public Health Problem? Int. J. Environ. Res. Public Health 2012, 10, 72–84. [Google Scholar] [CrossRef]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D.; et al. Bacterial Zoonoses Transmitted by Household Pets: State-of-the-Art and Future Perspectives for Targeted Research and Policy Actions. J. Comp. Pathol. 2016, 155, S27–S40. [Google Scholar] [CrossRef]
- Low, D.A.; Braaten, B.A.; Ling, G.V.; Johnson, D.L.; Ruby, A.L. Isolation and Comparison of Escherichia Coli Strains from Canine and Human Patients with Urinary Tract Infections. Infect. Immun. 1988, 56, 2601–2609. [Google Scholar] [CrossRef]
- Wedley, A.L.; Dawson, S.; Maddox, T.W.; Coyne, K.P.; Pinchbeck, G.L.; Clegg, P.; Nuttall, T.; Kirchner, M.; Williams, N.J. Carriage of Antimicrobial Resistant Escherichia coli in Dogs: Prevalence, Associated Risk Factors and Molecular Characteristics. Vet. Microbiol. 2017, 199, 23–30. [Google Scholar] [CrossRef]
- van den Bunt, G.; Fluit, A.C.; Spaninks, M.P.; Timmerman, A.J.; Geurts, Y.; Kant, A.; Scharringa, J.; Mevius, D.; Wagenaar, J.A.; Bonten, M.J.M.; et al. Faecal Carriage, Risk Factors, Acquisition and Persistence of ESBL-Producing Enterobacteriaceae in Dogs and Cats and Co-Carriage with Humans Belonging to the Same Household. J. Antimicrob. Chemother. 2020, 75, 342–350. [Google Scholar] [CrossRef]
- INE. V Recenseamento Geral da População e Habitação (RGPH 2021): Resultados Preliminares; Instituto Nacional de Estatística: Praia, Cape Verde, 2021. [Google Scholar]
- ONU-Habitat. Perfil Urbano da Cidade da Praia Ilha de Santiago República de Cabo Verde; Programa das Nações Unidas para os Assentamentos Humanos: Nairobi, Kenya, 2013. [Google Scholar]
- Eisenstein, M. The Growth of Slums in the Developing World’s Rapidly Expanding Cities Is Creating New Opportunities for Infectious Disease to Flourish and Spread; Nature Publishing Group: Berlin, Germany, 2016. [Google Scholar]
- Tal, S.; Tikhonov, E.; Aroch, I.; Hefetz, L.; Turjeman, S.; Koren, O.; Kuzi, S. Developmental Intestinal Microbiome Alterations in Canine Fading Puppy Syndrome: A Prospective Observational Study. NPJ Biofilms Microbiomes 2021, 7, 52. [Google Scholar] [CrossRef]
- Fernandes, M.; Nóbrega Carneiro, C.; Villada Rosales, A.M.; Grilo, M.; Ramiro, Y.; Cunha, E.; Nunes, T.; Tavares, L.; Sandi, J.; Oliveira, M. Antimicrobial Resistance and Virulence Profiles of Enterobacterales Isolated from Two-Finger and Three-Finger Sloths (Choloepus hoffmanni and Bradypus variegatus) of Costa Rica. PeerJ 2022, 10, e12911. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; Prata, I.; Rebelo, I.; Nunes, T.; Pires, A.; Carneiro, C.; Bexiga, R. Antimicrobial (ESBL) Resistance Genes in Faecal E. Coli of Calves Fed Waste Milk with Antimicrobial Residues. J. Dairy Res. 2022, 89, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Grilo, M.L.; Carneiro, C.; Cunha, E.; Tavares, L.; Patino-Martinez, J.; Oliveira, M. Antibiotic Resistance and Virulence Profiles of Gram-Negative Bacteria Isolated from Loggerhead Sea Turtles (Caretta caretta) of the Island of Maio, Cape Verde. Antibiotics 2021, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Grilo, M.L.; Pereira, A.; Sousa-Santos, C.; Robalo, J.I.; Oliveira, M. Climatic Alterations Influence Bacterial Growth, Biofilm Production and Antimicrobial Resistance Profiles in Aeromonas spp. Antibiotics 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
| Spay/Neuter Status | Presence of External Parasites | Body Condition Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| YES | NO | YES | NO | 1 | 2 | 3 | 4 | 5 | |
| BA (n = 50) | n = 0 | n = 50 (100%) | n = 46 (92%) | n = 4 (8%) | n = 6 (12%) | n = 22 (44%) | n = 20 (40%) | n = 2 (4%) | n = 0 |
| Ne (n = 50) | n = 35 (70%) | n = 15 (30%) | n = 12 (24%) | n = 38 (76%) | n = 1 (2%) | n = 12 (24%) | n = 34 (68%) | n = 3 (6%) | n = 0 |
| Antimicrobial Class | Antimicrobial Compound | Concentration (µg) | Bacterial Isolates (n = x (%)) | ||
|---|---|---|---|---|---|
| S | I | R | |||
| β-lactams | AMP | 10 | 0 | 0 | 48 (100) |
| CTX | 30 | 0 | 1 (2) | 47 (98) | |
| CPT | 30 | 0 | 3 (6) | 45 (94) | |
| AMC | 30 | 24 (50) | 9 (19) | 15 (31) | |
| MEM | 10 | 48 (100) | 0 | 0 | |
| Fluroquinolones | CIP | 5 | 26 (54) | 9 (19) | 13 (27) |
| ENR | 5 | 10 (21) | 13 (27) | 25 (52) | |
| Tetracyclines | DO | 30 | 18 (38) | 4 (8) | 26 (54) |
| Aminoglycosides | CN | 10 | 29 (60) | 1 (2) | 18 (38) |
| CN | 120 | 48 (100) | 0 | 0 | |
| Nitrofurans | F | 100 | 41 (85) | 0 | 7 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.; Cunha, E.; Baptista, L.; Tavares, L.; Oliveira, M. ESBL-Positive Enterobacteriaceae from Dogs of Santiago and Boa Vista Islands, Cape Verde: A Public Health Concern. Antibiotics 2023, 12, 447. https://doi.org/10.3390/antibiotics12030447
Matos A, Cunha E, Baptista L, Tavares L, Oliveira M. ESBL-Positive Enterobacteriaceae from Dogs of Santiago and Boa Vista Islands, Cape Verde: A Public Health Concern. Antibiotics. 2023; 12(3):447. https://doi.org/10.3390/antibiotics12030447
Chicago/Turabian StyleMatos, Alice, Eva Cunha, Lara Baptista, Luís Tavares, and Manuela Oliveira. 2023. "ESBL-Positive Enterobacteriaceae from Dogs of Santiago and Boa Vista Islands, Cape Verde: A Public Health Concern" Antibiotics 12, no. 3: 447. https://doi.org/10.3390/antibiotics12030447
APA StyleMatos, A., Cunha, E., Baptista, L., Tavares, L., & Oliveira, M. (2023). ESBL-Positive Enterobacteriaceae from Dogs of Santiago and Boa Vista Islands, Cape Verde: A Public Health Concern. Antibiotics, 12(3), 447. https://doi.org/10.3390/antibiotics12030447






