Abstract
Klebsiella pneumoniae (K. pneumoniae) is an opportunistic bacterium that has drawn attention due to its resistance to carbapenem antibiotics. The treatment of patients with severe infections has been challenging. Thus, silver nanoparticles (AgNPs) have been applied for their antimicrobial effects. This work aims to analyze the synergistic effect of the carbapenem antibiotic Imipenem with AgNPs against different susceptibility clinical profiles of K. pneumoniae. The silver nanoparticles were synthesized by bottom-up methodology and capped with alpha-lipoic acid. Susceptibility tests were performed using four K. pneumoniae strains with different susceptibility profiles to Imipenem. The strains were induced to form a biofilm for 48 h. Crystal violet and Resazurin assays were performed to determine biofilm formation and minimal inhibitory concentration, respectively. The reduction in Imipenem concentration with the association of nanoparticles was found in all strains studied in planktonic form, and the synergism between silver nanoparticles and Imipenem was demonstrated through the analysis of the fractional inhibitory concentration index. The viability percentage was reduced at rates ≥80% in the biofilm analysis, characterized by the minimal biofilm inhibitory concentration. The study’s proposed association resulted in inhibitory effects on different K. pneumoniae profiles, both in planktonic forms and biofilm, with peculiar behavior in the Imipenem-resistant profile.
1. Introduction
K. pneumoniae is an opportunistic Gram-negative bacterium that colonizes the gastrointestinal tract, the nasopharynx of humans, the mucosa of animals, and environments such as water and soil. The species has strains with diverse antimicrobial susceptibility profiles, that are naturally resistant to penicillins and often exhibit acquired resistance. Currently, attention has been drawn to the emergence and dissemination of hypervirulent strains and those resistant to carbapenem antibiotics, with reports of severe infections. Intensivists have been constantly challenged when treating such strains with current antibiotics, increasing the risk of patient complications, length of hospital stay, morbidity and mortality rates, and associated costs [1,2,3,4,5,6].
The World Health Organization (WHO) has classified carbapenem-resistant K. pneumoniae as a high-priority pathogen for the research, discovery, and development of new antibiotics [7]. The Food and Drug Administration (FDA) also raised the need for new drugs for the treatment of severe bacterial infections and recently approved Recarbrio®, the brand name for the combination of the active principles Imipenem, Cilastatin, and Relebactam [8]. Imipenem is a carbapenem antibiotic that inhibits bacterial cell wall synthesis, whereas Cilastatin and Relebactam work to keep Imipenem from being inactivated by renal enzymes and beta-lactamase degradation enzymes, respectively [9]. Despite the efforts, antimicrobial resistance has been considered one of the greatest threats to public health and a subject of constant discussion among the scientific community. The inappropriate use of antibiotics in human medicine and food production has put the population at risk [10,11].
The global pandemic caused by SARS-CoV-2 also had a major impact on antimicrobial resistance due to the excessive administration of antibiotics for the treatment [12]. The consequences are difficult to predict and may vary in the short, medium, and long term with persistent challenges in various spheres of society [13].
Nanotechnology applied to the study of antimicrobials has been relevant due to the use of nanoscale materials, which can result in greater contact and absorption with the bacteria. Among the nanomaterials, silver nanoparticles (AgNPs) have been extensively studied as antimicrobial agents [14,15], which led to their use in medical devices, such as contraceptives, dressings, surgical equipment, and catheters [16]. The combination of AgNPs with other antimicrobial agents has also been reported as enhancing the microbicidal effect [17]. In this context, the present study aims to analyze the synergistic effect of the carbapenem antibiotic Imipenem with AgNPs against different susceptibility profiles of K. pneumoniae.
2. Results and Discussion
2.1. Synthesis and Characterization of AgNPs
The synthesis of AgNPs coated with α-Lipoic Acid (α-LA) used the bottom-up chemical method, in which NaBH4 reduced AgNO3 to form the colloidal silver solution with yellow color. The exchange of the stabilizing agent occurs by incubating the nanoparticles with the α-LA solution for 24 h, where the bond with the disulfide group present in this molecule suggests a potential capped interaction in the particle (Figure 1a). The α-LA molecule is synthesized by mitochondria [18], leading to reduced cytotoxicity in mammalian cells [19], contributing to the intravenous applicability of the nanopharmaceuticals, as α-LA demonstrates biocompatibility involving endothelium, platelets, and red blood cells [20]. Furthermore, the coating with α-LA not only provides stability to the biological system, in which there is a series of biomacromolecules that bind to the surface of AgNPs, inducing agglomeration and preventing their interaction [21,22], but also promotes an antimicrobial activity, leading to the inhibitory effect that occurs through inducing excessive permeability of the cell membrane with changes in intracellular adenosine triphosphate (ATP) concentration and a decrease in cytoplasmic pH [23]. AgNPs coated with biocompatible materials have recently been studied in vitro and in vivo [24,25]. In vitro, they did not show cytotoxicity, with cell viability values greater than 90% in the cell line L929 [24]. In vivo, the results proved to be an excellent bionanomaterial; histological analyses with sections of various organs of the mice showed no changes in the treated groups compared to the control group, and in the hemocompatibility assay, a low rate of hemolysis was presented [25].
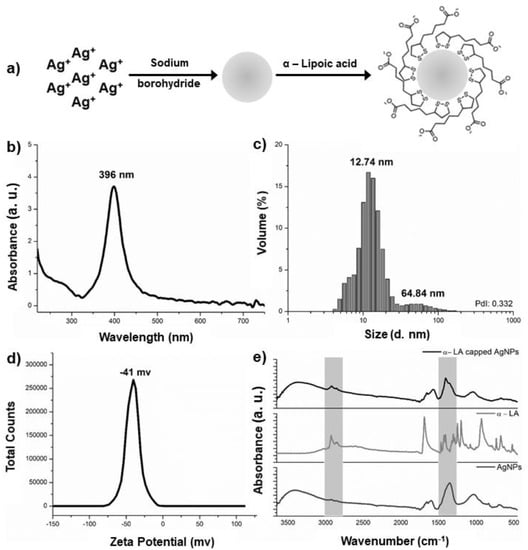
Figure 1.
Synthesis and characterization of α-LA capped AgNPs. (a) Scheme of synthesis and functionalization of AgNPs; (b) UV-Vis absorption spectra of α-LA capped AgNPs; (c) Hydrodynamic size measured by DLS of α-LA capped AgNPs; (d) Zeta potential of α-LA capped AgNps; (e) FT-IR spectra of bare nanoparticles, α-LA, and α-LA capped AgNPs.
Figure 1b shows the UV–visible (UV-Vis) spectrum of colloids solution capped α-LA, in which a single band centered at 396 nm with a maximum intensity of 3.706 a.u. is observed. The morphology of nanoparticles has a direct correlation with the band absorbance maxima (λMax) in the UV-Vis spectrum and the color of the colloidal solution. Spherical nanoparticles with diameters of up to 20 nm have the λMax between 350 and 400 nm and exhibit yellow shades [26]. Therefore, from the observed results, it is possible to affirm that the morphologies of the AgNPs synthetized are spherical.
Factors such as the difference in intensity, bandwidth, and shift of the maximum absorption wavelength are instrumental in emphasizing the presence of the coating [21,27]. Furthermore, bare nanoparticles with approximately 10 nm hydrodynamic size exhibit an original surface plasmon band centered around 392 nm [20]. This fact corroborates with results obtained in dynamic light scattering (DLS), where the capped nanoparticles showed a hydrodynamic diameter of 12.74 nm with 93.3% volume distribution and a polydispersity index (PdI) of 0.332 and indicated high antimicrobial efficiency (Figure 1c) [28]. The capped AgNPs showed a slight hydrodynamic increase in the particles, which aids in reducing polydispersion due to the increased stability generated by the coating, given that the zeta potential analysis is −41 mV (Figure 1d), and a value greater than +30 mv or less than −30 mv indicates chemical stability against aggregation of α-LA capped AgNPs [29].
The Fourier-Transform Infrared (FT-IR) spectra highlight the biochemical differences between the bare nanoparticle and capped AgNPs (Figure 1e). The spectra of the capped nanoparticles showed identical frequencies to the synthesized nanoparticles with slight shifts, conformational changes, and band intensities. The bands are found between 3000 and 2800 cm−1. The spectrum of the α-LA capped AgNPs nanoparticles show an increase in intensity compared to the same region of the uncapped AgNPs where a discrete shoulder is found. This increase in intensity can be attributed to the contributions of the asymmetric and symmetric stretching (ν) of (C-H) of α-LA at 2926 cm−1, 2864 cm−1, and 2843 cm−1. Contributions from the post-synthesis adsorbed borohydride, such as the H–B–H bending vibrational mode, are observed at 1500–1200 cm−1, in addition to the α-LA vibrational modes, such as deformation (δ) (CH2) scissoring at 1465 cm−1, C–O–H in-plane bending at 1425 cm−1, ν(C–H) at 1304 cm−1, and ν(C–O)/δ(O-H) out-of-plane at 1248 cm−1. The peaks in the hatched region are at 1404 cm−1 assigned to ν(C–C), associated with the changes in the 3000–2800 cm−1 regions, evidenced by the capping of AgNPs with α-LA [30,31,32,33], corroborating the α-LA molecule spectrum and also the UV-Vis and DLS analyses.
2.2. Efficiency of Imipenem Combined with Capped Silver Nanoparticles on K. pneumoniae
The efficacy of an antimicrobial agent depends on the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) assay results. Table 1 presents the results of the four strains studied with the susceptibility profiles susceptible (Kp-S), intermediate (Kp-I), and resistant (Kp-R) to Imipenem. The MIC of AgNPs in the different profiles of K. pneumoniae was 4 × 1011 particles/mL, and that of Imipenem performed under the same experimental conditions confirmed the automation profiles (Table 2) according to the breakpoints in the guidelines BrCAST/EUCAST [34]. The combination of AgNPs with Imipenem and the search for a possible synergistic effect led to reduced doses for the treated wells. The inhibition for the ATCC 700603, Kp-S and Kp-I strains was determined by the minimal concentration tested of 0.015 µg/mL, while for the resistant strain (Kp-R), inhibition was achieved with only 0.5 µg/mL combined with 2 × 1011 particles/mL of AgNPs. A reduction in Imipenem concentration was observed associating the nanoparticles with all the strains studied, in which the MBC values are very close to the values determined in the MIC; this result was also found by Parvekar and collaborators [35] when using silver nanoparticles against Staphylococcus aureus.

Table 1.
Minimal inhibitory concentration and minimal bactericidal concentration assay data of silver nanoparticles, Imipenem, and their association against different profiles of K. pneumoniae.

Table 2.
Antibiotic susceptibility profiles of K. pneumoniae clinical isolate strains isolated from catheter tip (Kp-S and Kp-I) and urine sample (Kp-R).
The association of AgNPs and Imipenem in the MIC assay reduced antibiotic concentrations by an approximate fold change from 16- to 256-fold (Table 1). The susceptible strains showed a 32-fold reduction in Imipenem concentration, whereas the intermediate strain showed a 256-fold reduction. This fact may relate to the intermediary category that includes isolates with antimicrobial agent MICs that usually approach achievable blood and tissue levels and for which response rates may be lower than for susceptible isolates [36]. Moreover, intermediate strains also include a buffer zone, which should prevent small, uncontrolled, technical factors from causing major discrepancies in interpretations, especially for drugs with narrow pharmacotoxicity margins [36]. The strain with a resistant profile presented a 16-fold reduction when the association between AgNPs and Imipenem was used. Considering that the susceptibility profile of this strain does not favor inhibition with therapeutic doses, this reduction is significant. Several studies propose the combination of antibiotics with AgNPs, including the work of Li et al. [37], who used AgNPs combined with Amoxicillin against Escherichia coli. Deng and colleagues [38] also used several classes of antibiotics associated with AgNPs against multidrug-resistant (MDR) Salmonella typhimurium, and Wan et al. [39] combined AgNPs with Polymyxin B, Rifampicin, and Tigecycline against MDR Acinetobacter baumannii.
Synergistic interactions between AgNPs associated with Imipenem were found in all studied profiles with values of the Fractional Inhibitory Concentration Index (FICI) and the Fractional Bactericidal Concentration Index (FBCI) equal to 0.5 (Table 1), which are commonly used to define the interaction of antimicrobials. Synergy was defined as an FICI or FBCI ≤ 0.5; partial synergy = 0.5 ≤ FICI or FBCI < 1; additive = 1; indifferent = 2 ≤ FICI or FBCI < 4; and antagonism was defined as an index > 4 [40,41]. This result suggests a possible reduction in the viability of bacterial strains at lower antibiotic concentrations. Lopes-Carrizales and co-authors [40] studied the effects of AgNPs combined with Amikacin and Ampicillin on resistant uropathogens in Gram-positive and Gram-negative bacteria. In Gram-negative bacteria, the study showed 2- to 32-fold reductions in Amikacin concentration and 4- to 32-fold reductions in Ampicillin. Regarding the interaction of AgNPs with antimicrobials in different bacteria, synergistic, partially synergistic, and additive combination results have been found [40]. Malawong et al. [41] studied three clinical isolates of Burkholderia pseudomallei, a Gram-negative bacteria that was submitted with a combination of silver nanoparticles and different antibiotics, including Ceftazidime, Imipenem, Meropenem, and Gentamicin. The associations between the drugs showed fold changes of 2 to 16 times and synergistic combinations [41].
An MIC is usually associated with laboratory investigations of antibiotic susceptibility profiles against microorganisms. However, nanoparticle characteristics, particularly opacity and insolubility in culture medium, can hinder MIC assay and application in clinical laboratories that rely on the visual observation of turbidity [42]. In this context, because it is a simple and high-throughput method, the Resazurin assay was used to quantify the performance of the nanomaterial associated with the antibiotic, allowing the determination of the MIC to be confirmed. Figure 2 shows the viability of planktonic cells subjected to action with AgNPs combined with Imipenem. The metabolically active bacteria convert the weakly fluorescent redox dye Resazurin into the fluorescent product Resorufin [43]. Therefore, the relative fluorescence units (RFU) were used to calculate the viability percentage. The strains with susceptible and intermediate profiles do not show growth in any concentration of the association tested. In contrast, the strain with a resistant profile showed growth inhibition only at a concentration of 0.5 µg/mL combined with nanoparticles, proving the reduction in bacterial viability and correlating with the MIC results.

Figure 2.
Metabolic activity of the Resazurin assay in planktonic cells of K. pneumoniae. (a) ATCC; (b) Kp-S; (c) Kp-I; and (d) Kp-R. Data are expressed as the mean ± Standard Deviation (SD) of triplicate determinations.
AgNPs and Imipenem are bactericidal agents and present different mechanisms of action. The results found in the MIC, MBC, and Resazurin assays demonstrate the synergistic effect between the nanoparticles and the antibiotic proven by FICI and FBCI, considering that each agent’s isolated concentration was not enough to conduct the bacteriostatic or bactericidal effect. Thus, the action mechanism and the synergistic effect of AgNPs and Imipenem on bacteria are presented in Figure 3.
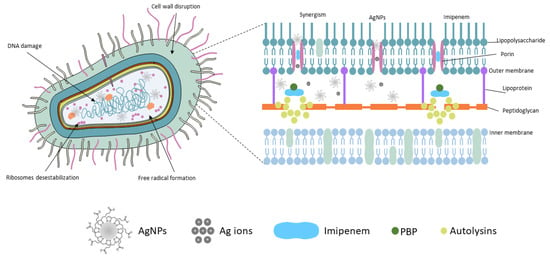
Figure 3.
Schematic representation describing the interaction and antimicrobial action of silver nanoparticles, Imipenem, and the agents’ association on the Gram-negative bacterium.
The effect of AgNPs on bacteria can be explained initially by adhesion to the bacterial cell wall surface, penetration into the cell, and disruption of organelles and intracellular biomolecules. Protein synthesis is altered, destabilizing the cellular outer membrane composition with the induction of reactive oxygen species’ (ROS) release, causing cellular toxicity, oxidative stress, free radical formation, and damage to mitochondria and DNA. This antimicrobial effect is also potentiated with Ag+ ions. Ions and nanoparticles smaller than 2 nm penetrate cells through porin channels in the outer membrane of Gram-negative bacteria by binding to proteins and nucleic acids, causing a confluence of bacterial structural changes [17,44,45]. In contrast, Imipenem has a β-lactam ring in its structure that gives them the ability to bind to and inactivate relevant transpeptidases, known as penicillin-binding proteins (PBPs), which are responsible for the elongation and cross-linking of the bacterial cell walls’ peptidoglycan. In response to the inactivation of the PBPs, the autolysins are activated, interfering with cell wall formation. Carbapenems, such as Imipenem, can bind to a specific PBP (PBP-1), resulting in faster lysis than other beta-lactams and higher bactericidal activity [46,47,48]. The association of these events leads to cell death regardless of the susceptibility profile of the microorganism.
2.3. Antimicrobial Effects of Imipenem Combined with AgNPs on Biofilm K. pneumoniae
The crystal violet assay was carried out to determine the biomass during the biofilm production by K. pneumoniae (Figure 4). The biofilm formation ability of the four strains, in which the ATCC strain formed a biofilm with moderate adhesion and optical density at 570 nm (OD570) values of 1.07 ± 0.02 after 48 h of incubation, was analyzed (Figure 4b). When analyzing the clinical strains with a different susceptibility profile, it was observed that the biofilm formation showed moderate adhesion regardless of the profile, with OD570 values for Kp-S of 1.08 ± 0.01, Kp-I of 1.11 ± 0.01, and for Kp-R of 1.06 ± 0.01 (Figure 4b). Micrographs provided evidence that corroborated biofilm formation activity (Figure 4a).

Figure 4.
K. pneumoniae biofilm formation: (a) The crystal violet assay was used to visualize biofilm formation on polystyrene plates. The scale bars represent 50 μm, and the magnification is ×200; (b) graph of the optical density reading (OD570) expressed as mean ± SD of the biofilms with the association of the photograph of the respective wells before the OD measurement.
The optical density of the biofilm can reflect its ability to adhere compared to the optical density of the control group [49]. Moderate adhesion can be interpreted as positive biofilm production according to the analysis proposed by Stepanovic et al. [50]. The quantification of biofilm biomass is presented in the study of Oleksy-Wawrzyniak and co-authors [51], where they analyzed the adherence of 118 isolates of K. pneumoniae on polystyrene plates from the crystal violet assay, and 51% of isolates showed biofilms with moderate adherence. This study also demonstrated K. pneumoniae ATCC 700603 adherence with the obtained value of OD 1.00 unit ± 0.35, corroborating the results shown in Figure 4b.
The results of biofilm formation by the Resazurin assay met the criteria established by the Z-factor, which is an indicator of assay quality (Figure 5) [52,53,54]. The K. pneumoniae biofilms indicate a high-quality assay exhibiting a wide separation between positive and negative control and acceptable data variability (Figure 5a). The values obtained demonstrate data suitable for bioassay (Figure 5b), where the Z-factor is greater than 0.5, the signal window is greater than 2, and the coefficient of variation is less than 20%. Thus, a high degree of precision and sensitivity in the assay is essential, considering the variability of measurements [54].
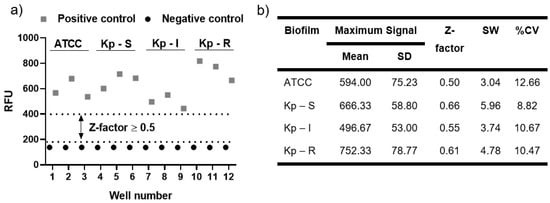
Figure 5.
Indicator of assay quality of biofilm formation of K. pneumoniae. (a) Distribution chart of the positive and negative controls of the studied biofilms and the Z-factor presentation; (b) Mean and SD in RFU of the positive control biofilms, with the determination of the Z-factor, signal window (SW), and percent coefficient of variation (%CV). The negative control had a mean of 137.67 RFU with an SD of 0.58 RFU.
The impacts on the inhibition of biofilms formed with moderate adhesion were demonstrated by combining AgNPs (2 × 1011 particles/mL) and Imipenem (16 µg/mL to 0.25 µg/mL) followed by the Resazurin assay. The percentage of biofilm viability was determined, followed by normality and lognormality tests for the appropriate statistical test analysis. A regular distribution in the quantile–quantile (QQ) plot graphs was observed, and the Shapiro–Wilk test determined approval of the normality test (alpha = 0.05). The results of the one-way ANOVA are presented in Figure 6, in which significant differences are found between the tested concentrations in the different susceptibility profiles. At the concentration of 0.25 µg/mL of the antibiotic-associated AgNPs, there was an increase in the viability of bacteria present in the biofilm compared to the control group, especially in the susceptible (Kp-S) and intermediate (Kp-I) profiles. Sub-inhibitory concentrations of antibiotics have been reported as increasing their metabolic activity. There is growing evidence that bacteria respond specifically and defensively to these concentrations. In particular, Gram-negative bacteria can respond with numerous strategies to combat antibiotics and, more recently, AgNPs [22,55,56,57].
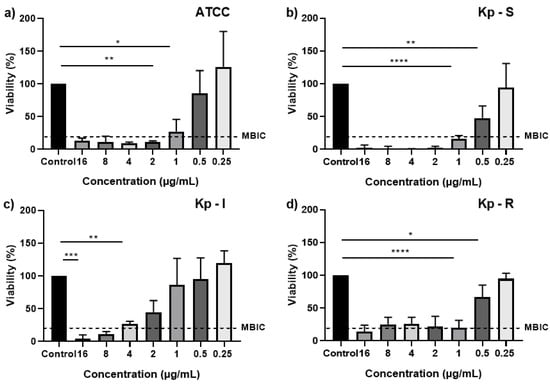
Figure 6.
Viable cells in biofilms determined via the Resazurin assay after administration with AgNPs combined with Imipenem. (a) ATCC; (b) Kp-S; (c) Kp-I; and (d) Kp-R. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
The viability reduction was found with an increased Imipenem concentration associated with AgNPs in all biofilms. The clinical strain with a susceptible susceptibility profile (Kp-S) showed a viability < 2% for 2 µg/mL concentrations up to 16 µg/mL. The strain with a resistant profile (Kp-R) showed viability of 25.4% when the MIC alone of the antibiotic was used at 8 µg/mL.
The minimal biofilm inhibitory concentration (MBIC) is defined as the concentration that inhibits ≥80% of the metabolic activity of each treated biofilm compared to the positive control [58]. Thus, it can be defined that the MBIC for the ATCC strain was 2 µg/mL, and Kp-S was set at 1 µg/mL for the clinical strain. However, for clinical strains Kp-I, increasing concentrations of Imipenem associated with AgNPs showed a dose-dependent aspect of growth inhibition, with the MBIC defined as 8 µg/mL to contain at least 80% of viability. However, when analyzing the biofilm with a resistant profile (Kp-R) regarding the inhibitory effect generated by the association of AgNPs and Imipenem, a behavior similar to that of the susceptible biofilm with an MBIC equal to 1 µg/mL was observed presenting an eight-fold reduction compared to the MIC value of the antibiotic alone, whereas for the susceptible profile, there was an increase in concentration for biofilm inhibition compared to planktonic strains. This find could be attributed to a possible synergistic effect generated by both antibacterial agents, implying that the action mechanism causes the resistant biofilm to lose its inhibition effectiveness to Imipenem. Moreover, AgNPs can easily penetrate the biofilm, and their association with the antibiotic increases the anti-biofilm activity [17,59].
Overall, this study demonstrated that Imipenem has a synergistic effect with AgNPs in planktonic cells. The association reduced the concentration of Imipenem in MIC tests, which is an assay that guides the appropriate antibiotic choice by the intensivist and infectious disease specialist for administration to patients. A lower dose of Imipenem associated with AgNPs may reduce the drug’s neurotoxic and nephrotoxic side effects. Furthermore, it was observed that the association of the antibiotic with AgNPs exhibited an inhibitory effect on biofilms with similar concentrations and even reduced the MIC.
3. Material and Methods
3.1. Synthesis of Silver Nanoparticles
AgNPs were synthesized using the bottom-up chemical method by reducing silver nitrate (AgNO3; Sigma-Aldrich (St. Louis, MO, USA), 209139) by sodium borohydride (NaBH4; Sigma-Aldrich, 452882) to obtain the colloidal solution. Thus, all glassware and magnetic stirring bars used were cleaned with aqua regia with a 1:3 ratio of nitric acid (HNO3, ≥ 65%; Sigma-Aldrich, 84378) and hydrochloric acid (HCl, 37%; Neon (São Paulo, Brazil), 02618) to remove contaminating metals and then rinsed in ultra-pure water prior to use. In an ultra-thermostatic bath (Marconi, MA-184, Piracicaba, Brazil), a 30 mL aliquot of the 1 mM NaBH4 solution was cooled and stirred for 1 h at 0 °C. Subsequently, 10 mL of 2 mM AgNO3 was added to the solution, and after seven minutes in the dark, colloidal silver with a yellow coloration was observed [60]. The solution was left in the light for 24 h. Subsequently, α-LA (Sigma-Aldrich, T5625) at a concentration of 7.75 mM was introduced into the colloidal solution under constant stirring (IKA-MS3, Staufen, Germany) for 24 h to stabilize the AgNPs in a biological medium. The chemical by-products of the synthesis were removed by centrifugation at 15,000 rpm for ten minutes (Multifuge X1R; Thermo Scientific, Langenselbold, Hessen, Germany), the supernatant was discarded, and the pellet was resuspended in ultrapure water. The sample was stored in the dark and characterized by UV-Vis, DLS, and FT-IR spectroscopy.
3.1.1. UV-Visible Spectroscopy
The UV-Vis absorption spectra were acquired on a DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA) with a resolution of 1.5 nm. The microvolume mode’s spectral region of 220–750 nm was used with 2 µL of each sample in the pedestal. The data were plotted using OriginPro, version 8.5.1 (Origin Lab., Northampton, MA, USA).
3.1.2. Dynamic Light Scattering (DLS)
The hydrodynamic diameter of the nanoparticles and the Zeta potential were determined by the Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK). The average diameter and PdI analyses were performed in the size mode, using a 400 µL aliquot of AgNPs deposited in a disposable polystyrene cuvette (ZEN0118). The three measurements of each sample at 90° were taken at 25 °C with an equilibration time of 120 s. Zeta potential was measured in zeta mode using the same parameters cited above with 1 mL of AgNPs deposited in disposable, folded capillary cells (DTS 1070). The values correspond to the average of three independent measurements.
3.1.3. Fourier Transform Infrared (FTIR) Spectroscopy
The mid-infrared spectra were collected on the Spectrum 400 FT-IR/FIR equipment (Perkin Elmer, Waltham, MA, USA) using a GladiATR (PIKE Technologies, Fitchburg, WI, USA) accessory. The average spectrum was obtained in the range of 4000–450 cm−1 by collecting three spectra with 16 scans in absorbance mode at a resolution of 4 cm−1. Spectral normalization was performed in OPUS version 4.2 (Bruker, Ettlingen, Germany) and plotted in OriginPro version 8.5.1 (Origin Lab., USA).
3.2. Dilution of the Antibiotic Imipenem
The antibiotic Imipenem (C12H17N3O4S-H2O, Sigma-Aldrich, PHR-1796) was previously diluted in phosphate buffer.
3.3. Bacterial Strain and Culture Conditions
In this study, the strain standard (ATCC 700603) and clinical strains (Kp-S, Kp-I, and Kp-R) of K. pneumoniae with different susceptibility profiles were evaluated (Table 2). Clinical isolates were obtained from Laboratório Oswaldo Cruz (Kp-S and Kp-I) and the Hospital de Clínicas Sul (Kp-R), located in São José dos Campos, SP, Brazil. The identification and susceptibility profiles of the strains were performed using the BD Phoenix equipment (Becton Dickinson, Franklin Lakes, NJ, USA). Bacteria on the storage surface used brain heart infusion (BHI) broth (Oxoid, Basingstoke, Hampshire, England, CM 1135) with 20% glycerol (LGC, 13-1325-10) in the deep ultra-freezer (−80 °C). Strains were reactivated in BHI broth, being incubated in a bacteriological oven (Heratherm; Thermo Scientific, Dreieich, Hesse, Germany) for 24 h at 35 °C (±2 °C) and seeded onto BHI agar (Oxoid, CM 1136) for 24 h at 35°C (±2 °C). The colony morphology and Gram staining were analyzed.
3.4. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration Assay
The broth microdilution method was used to determine the MIC according to International Organization for Standardization (ISO) standard 20776-1 [61]. The Mueller-Hinton broth (MHB—Himedia, Dindhori, India, M391) was dispensed in a 96-well plate, and different concentrations of the antibiotic Imipenem were tested, following a serial dilution starting from a concentration of 16 µg/mL to 0.007 µg/mL. The concentration of AgNPs in the association was set at 2 × 1011 particles/mL, with a concentration below the MIC of AgNPs alone. The inoculum was prepared in saline solution (0.9%) initially on the McFarland 0.5 scale (1 × 108 CFU/mL) (Densichek plus; BioMerieux, Craponne, France), followed by a 1:20 dilution (5 × 106 CFU/mL) with a concentration of 5 × 105 CFU/mL at the end of the test. Sterility control (Imipenem, AgNPs, and MHB) and growth control were also included in the microplate. The plates were incubated at 35 °C (± 2 °C) for 18 h. After incubation, there was a visual investigation of the MIC, which was confirmed by reading the optical density by absorbance at a wavelength of 600 nm (OD600) in the Synergy HTX spectrophotometer (BioTek Instruments, Winooski, VT, USA). The MIC was defined as the minimal concentration capable of inhibiting bacterial growth. The experiment was carried out in triplicate.
The MBC was determined by the microdrop method, in which a 10 μL volume of the MIC concentrations assay was deposited on the surface of BHI agar. After absorbing the microdrop, the plates were inverted and incubated at 35 °C (±2 °C) for 24 h. The absence of bacterial growth on the agar indicated the bactericidal effect.
3.5. Quantification of Planktonic Cell Viability
In the Resazurin assay (Resazurin sodium salt—Sigma-Aldrich, R7017), 2 µL of the reagent at 6.75 mg/mL was added to each well; then, tested in the MIC and incubated for two hours in a bacteriological oven. The whole procedure was carried out in the dark. The relative fluorescence units (RFU) reading was performed with a Synergy HTX spectrophotometer (BioTek Instruments, USA), with excitation at 528 nm and emission at 645 nm. At least three independent replications were performed for each sample. The percentage of the viability of grown planktonic cells relative to the positive control (PC) was determined according to Equation (1), in which the absorption value of the material control (MC) group was subtracted:
3.6. Biofilm Formation
Biofilms were formed using 100 µL of BHI broth and 100 µL of the standardized inoculum in saline (0.9%) at 107 CFU/mL in polystyrene 96-well microplates. These microplates were incubated at 35 °C (±2 °C) in a bacteriological oven. The medium was renewed every 24 h during incubation. The biofilm formation occurred within 48 h.
3.6.1. Determination of Biofilm Formation by the Crystal Violet Assay
The crystal violet assay was performed to classify the biofilms of K. pneumoniae. After the content of each well was removed and gently washed twice with a sterile saline solution of 0.9% to remove planktonic and loosely-bound cells, 200 µL of methanol was added for 20 min to fix the biofilm. Methanol was then removed, and the plate was incubated to dry at 35 °C (±2 °C) for 24 h. It was stained with 1% (wt./vol) crystal violet solution for 5 min at room temperature, and subsequently, the excess stain was washed off twice with sterile saline solution (0.9%). The biofilms were resuspended in 200 µL of acetic acid 33% and measured by absorbance at a wavelength of 570 nm (OD570) in SpectraCount (BS10001; Packard BioScience Company, Downers Grove, IL, USA) to determine the biofilm biomass.
The OD defines the adhesion capacity of the biofilm. However, the cut-off value (ODc) should be established from the arithmetic mean of the absorbance of the negative controls with the addition of three times the SD. Thus, we classified the formed biofilms as no biofilm production (OD ≤ ODc), weak biofilm production (ODc < OD ≤ 2ODc), moderate biofilm production (2ODc < OD ≤ 4ODc), and strong biofilm production (4ODc < OD) [62].
3.6.2. Determination of Biofilm Viability by the Resazurin Assay
In the biofilm formation, the culture medium was then removed and 200 µL of AgNPs (2 × 1011 particles/mL) combined with different Imipenem concentrations (16 µg/mL to 0.25 µg/mL) were added to the wells for 24 h at 35 °C (± 2 °C). The Resazurin colorimetric assay was used to determine the viability of the biofilm of K. pneumoniae. Biofilms were washed with sterile phosphate-buffered saline (PBS) to remove planktonic and loosely bound cells after the treatment with AgNPs combined with Imipenem. Then, 200 µL of sterile PBS was used for resuspension along with the addition of 4µL of Resazurin (6.75 mg/mL) for 6 h at 35 °C (±2 °C) in a bacteriological oven. The RFU reading was performed on the Synergy HTX spectrophotometer (BioTek Instruments, USA) with excitation at 528 nm and emission at 645 nm. At least three independent replications were performed for each sample. Statistical analysis was performed by applying the Z-factor, signal window (SW), and the percent coefficient of variation (%CV) equations in RFU using Excel software (Microsoft Office Professional Plus 2016). The percentage of the viability of the grown biofilm relative to the positive control (PC) was determined according to Equation (1), in which the absorption value of the material control (MC) group was subtracted. The normality and lognormality tests were performed; data distribution analysis (QQ plot) with an evaluation of the Shapiro–Wilk test, followed by the application of the analysis of variance, one-way ANOVA, complemented by Tukey’s test, considering a significance of p ≤ 0.05 in GraphPad Prism 8.0.1 (GraphPad Software San Diego, CA, USA).
4. Conclusions
The association of the carbapenem antibiotic Imipenem with the capped AgNPs α-LA showed a synergistic and inhibitory effect against different susceptibility profiles of K. pneumoniae in planktonic cells and biofilms. Imipenem action was not inhibited when associated with nanoparticles in a resistant clinical strain (Kp-R), a characteristic observed by the bacterial response with an increasing antibiotic concentration to the detriment of AgNPs fixed concentration. As a result, a synergistic combination of antimicrobial drugs and AgNPs has offered an excellent solution to this health problem, addressing the need for new technologies to act against MDR microorganisms.
Author Contributions
Conceptualization, design of the work, acquisition, analysis, and interpretation of data for the work, writing (original draft), I.F.; contribution to the methodology, T.S.V.; conceptualization, acquisition, performed the experiments, analysis, and interpretation of data for the work, L.J.R.; conceptualization, design of the work, analysis, interpretation of data for the work, validation, supervision, writing (original draft), M.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo State Research Support Foundation (FAPESP), grant number 2018/23898-6, the National Council for Scientific and Technological Development (CNPq) grant number 427602/2018-10 and Coordination for the Improvement of Higher Education Personnel (CAPES) scholarship to I.F., grant number 88887.185627/2018-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The clinical K. pneumoniae strains were kind gifts from the Laboratório Oswaldo Cruz (Kp-S and Kp-I) and the Hospital de Clínicas Sul (Kp-R), located in São José dos Campos, SP, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, S.; Alam, M.; Hasan, G.M.; Hassan, M.I. Potential Therapeutic Targets of Klebsiella pneumoniae: A Multi-Omics Review Perspective. Brief. Funct. Genom. 2022, 21, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae Infection Biology: Living to Counteract Host Defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population Genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Ripabelli, G.; Tamburro, M.; Guerrizio, G.; Fanelli, I.; Flocco, R.; Scutellà, M.; Sammarco, M.L. Tracking Multidrug-Resistant Klebsiella pneumoniae from an Italian Hospital: Molecular Epidemiology and Surveillance by PFGE, RAPD and PCR-Based Resistance Genes Prevalence. Curr. Microbiol. 2018, 75, 977–987. [Google Scholar] [CrossRef]
- Tacconelli, E.; Magrini, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; Infection Control Africa Network: Cape Town, South Africa, 2017. [Google Scholar]
- Food & Drug Administration FDA Approves Antibiotic to Treat Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-antibiotic-treat-hospital-acquired-bacterial-pneumonia-and-ventilator-associated (accessed on 25 June 2022).
- Pourali, S. Imipenem, Cilastatin, Relebactam (Recarbrio). Available online: https://www.proquest.com/docview/2506724594/5B4D5A3631884ED7PQ/1 (accessed on 25 June 2022).
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial_resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef]
- World Health Organization Global Action Plan on Antimicrobial Resistance. Available online: http://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf;jsessionid=0ECC74DD9DDB74AAD1865B9E6348EC3F?sequence=1 (accessed on 25 June 2022).
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased Antimicrobial Resistance during the COVID-19 Pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and COVID-19: Intersections and Implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef]
- Lima, R.; Del Fiol, F.S.; Balcão, V.M. Prospects for the Use of New Technologies to Combat Multidrug-Resistant Bacteria. Front. Pharmacol. 2019, 10, 692. [Google Scholar] [CrossRef]
- Tohamy, H.G.; Lebda, M.A.; Sadek, K.M.; Elfeky, M.S.; El-Sayed, Y.S.; Samak, D.H.; Hamed, H.S.; Abouzed, T.K. Biochemical, Molecular and Cytological Impacts of Alpha-Lipoic Acid and Ginkgo Biloba in Ameliorating Testicular Dysfunctions Induced by Silver Nanoparticles in Rats. Environ. Sci. Pollut. Res. 2022, 29, 38198–38211. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, B.; Fahrer, J. The Disulfide Compound α-Lipoic Acid and Its Derivatives: A Novel Class of Anticancer Agents Targeting Mitochondria. Cancer Lett. 2016, 371, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cotton, G.C.; Gee, C.; Jude, A.; Duncan, W.J.; Abdelmoneim, D.; Coates, D.E. Efficacy and Safety of Alpha Lipoic Acid-Capped Silver Nanoparticles for Oral Applications. RSC Adv. 2019, 9, 6973–6985. [Google Scholar] [CrossRef] [PubMed]
- Hajtuch, J.; Santos-Martinez, M.J.; Wojcik, M.; Tomczyk, E.; Jaskiewicz, M.; Kamysz, W.; Narajczyk, M.; Inkielewicz-Stepniak, I. Lipoic Acid-Coated Silver Nanoparticles: Biosafety Potential on the Vascular Microenvironment and Antibacterial Properties. Front. Pharmacol. 2022, 12, 733743. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; Omole, M.; Alarcon, E.I.; McTiernan, C.D. Lipoic Acid Capped Silver Nanoparticles: A Facile Route to Covalent Protein Capping and Oxidative Stability within Biological Systems. RSC Adv. 2020, 10, 32953–32958. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Shi, C.; Sun, Y.; Zhang, X.; Zheng, Z.; Yang, M.; Ben, H.; Song, K.; Cao, Y.; Chen, Y.; Liu, X.; et al. Antimicrobial Effect of Lipoic Acid against Cronobacter Sakazakii. Food Contr. 2016, 59, 352–358. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Z.; Qi, C.; He, M.; Yu, T.; Shi, L. Construction of Chitosan/Ag Nanocomposite Sponges and Their Properties. Int. J. Biol. Macromol. 2021, 192, 272–277. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and Hemostatic Thiol-Modified Chitosan-Immobilized AgNPs Composite Sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Mačák, L.; Čižmárová, E.; Mára, V. Influence of Reagents on the Synthesis Process and Shape of Silver Nanoparticles. Materials 2022, 15, 6829. [Google Scholar] [CrossRef] [PubMed]
- Eeparuksapun, K.T.; Rasongchan, N.P.; Hawonsuwan, A.T. Alpha-Lipoic Acid Functionalized Silver Nanoparticles for Colorimetric Detection of Copper Ion. Anal. Sci. 2019, 35, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-Phase Bactericidal Mechanism of Silver Nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e0168098. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.; El-Kemary, M.; Khalifa, S.; El-Seedi, H. Spectral Studies of Silver Nanoparticles Biosynthesized by Origanum majorana. Green Process. Synth. 2018, 7, 100–105. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 8, ISBN 9780470011140. [Google Scholar]
- Le, T.T.; Pistidda, C.; Puszkiel, J.; Milanese, C.; Garroni, S.; Emmler, T.; Capurso, G.; Gizer, G.; Klassen, T.; Dornheim, M. Efficient Synthesis of Alkali Borohydrides from Mechanochemical Reduction of Borates Using Magnesium-Aluminum-Basedwaste. Metals 2019, 9, 1061. [Google Scholar] [CrossRef]
- Jensen, J.O. Vibrational Frequencies and Structural Determination of Aluminum Tetrahydroborate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 1565–1578. [Google Scholar] [CrossRef]
- Dolinina, E.S.; Akimsheva, E.Y.; Parfenyuk, E.V. Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug. Pharmaceutics 2020, 12, 1–16. [Google Scholar] [CrossRef]
- BrCAST—Brazilian Committee on Antimicrobial Susceptibility Testing/EUCAST—European Committee on Antimicrobial Susceptibility Testing Tabela Pontos de Corte Clinicos-BrCAST. Available online: http://brcast.org.br/documentos/ (accessed on 4 April 2022).
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 1562388363.
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic Antibacterial Effects of β-Lactam Antibiotic Combined with Silver Nanoparticles. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of Silver Nanoparticles in Combination with Antibiotics on the Resistant Bacteria Acinetobacter baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malawong, S.; Thammawithan, S.; Sirithongsuk, P.; Daduang, S.; Klaynongsruang, S.; Wong, P.T.; Patramanon, R. Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics 2021, 10, 839. [Google Scholar] [CrossRef]
- Chakansin, C.; Yostaworakul, J.; Warin, C.; Kulthong, K.; Boonrungsiman, S. Resazurin Rapid Screening for Antibacterial Activities of Organic and Inorganic Nanoparticles: Potential, Limitations and Precautions. Anal. Biochem. 2022, 637, 114449. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic Reduction of Resazurin; Location within the Cell for Cytotoxicity Assays. Biotechnol. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Rodloff, A.C.; Goldstein, E.J.C.; Torres, A. Two Decades of Imipenem Therapy. J. Antimicrob. Chemother. 2006, 58, 916–929. [Google Scholar] [CrossRef]
- Papich, M.G. Saunders Handbook of Veterinary Drugs: Small and Large Animal, 4th. ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 393–394. ISBN 1888873272. [Google Scholar]
- Hellinger, W.C.; Brewer, N.S. Imipenem. Mayo Clin. Proc. 1991, 66, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Oleksy-Wawrzyniak, M.; Junka, A.; Brożyna, M.; Paweł, M.; Kwiek, B.; Nowak, M.; Mączyńska, B.; Bartoszewicz, M. The In Vitro Ability of Klebsiella pneumoniae to Form Biofilm and the Potential of Various Compounds to Eradicate It from Urinary Catheters. Pathogens 2022, 11, 42. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. Multiplexing Spheroid Volume, Resazurin and Acid Phosphatase Viability Assays for High-Throughput Screening of Tumour Spheroids and Stem Cell Neurospheres. PLoS ONE 2014, 9, e103817. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Oldenburg, K.R. Z-Factor. In Encyclopedia of Cancer; Springer: Berlin, Germany, 2009; pp. 3227–3228. ISBN 978-3-540-47648-1. [Google Scholar]
- Penesyan, A.; Nagy, S.S.; Kjelleberg, S.; Gillings, M.R.; Paulsen, I.T. Rapid Microevolution of Biofilm Cells in Response to Antibiotics. NPJ Biofilms Microbiomes 2019, 5, 34. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside Antibiotics Induce Bacterial Biofilm Formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as Intermicrobiol Signaling Agents Instead of Weapons. Proc. Natl. Acad. Sci. USA 2006, 103, 19484–19489. [Google Scholar] [CrossRef]
- do Canto Canabarro, M.; Meneghetti, K.L.; Geimba, M.P.; Corção, G. Biofilm Formation and Antibiotic Susceptibility of Staphylococcus and Bacillus Species Isolated from Human Allogeneic Skin. Braz. J. Microbiol. 2022, 53, 153–160. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.N.; Kim, J.H. Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- ISO 20776-1; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the in Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobi. International Organization for Standardization: Geneva, Switzerland, 2019.
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).