The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio
Abstract
:1. Introduction
2. Results
2.1. GC-MS Analysis
2.2. Minimum Inhibitory Concentration (MIC) of Linalool against S. pyogenes
2.3. Non-Antibacterial Anti-Biofilm Activity of Linalool against S. pyogenes
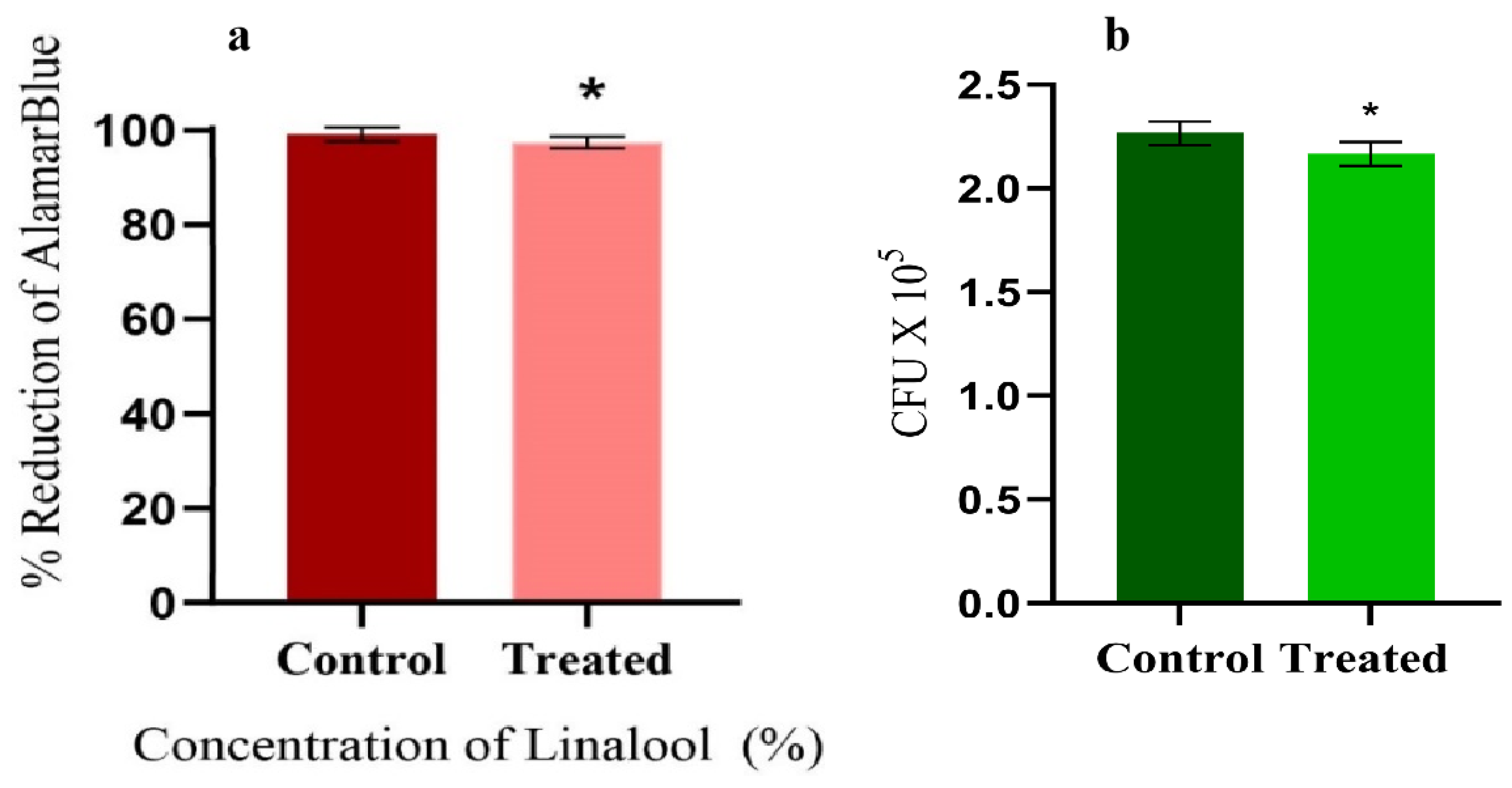
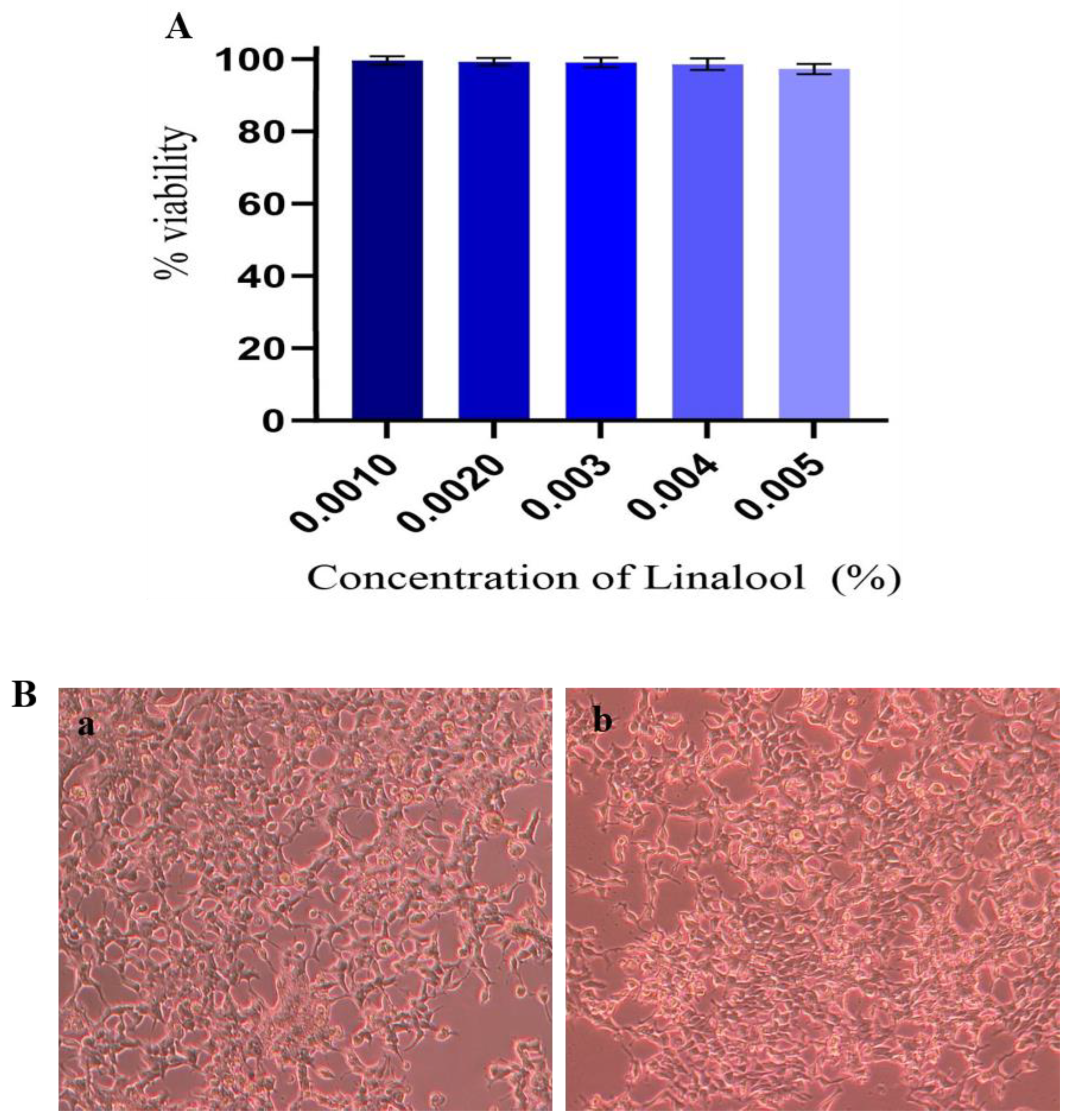
2.4. Effect of Linalool on Growth and Cell Viability
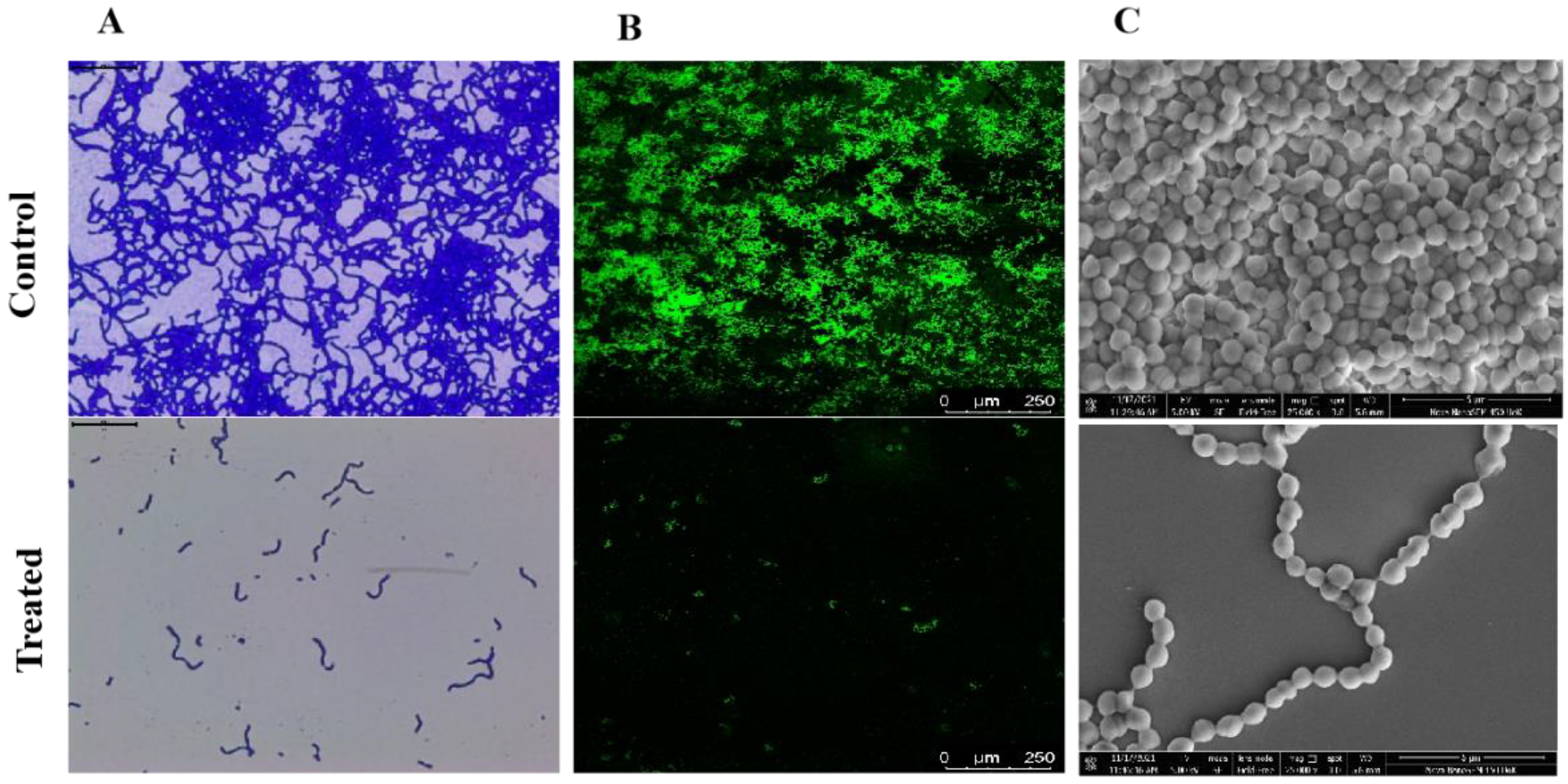
2.5. Microscopic Observation Confirms the Anti-Biofilm Potential of Linalool
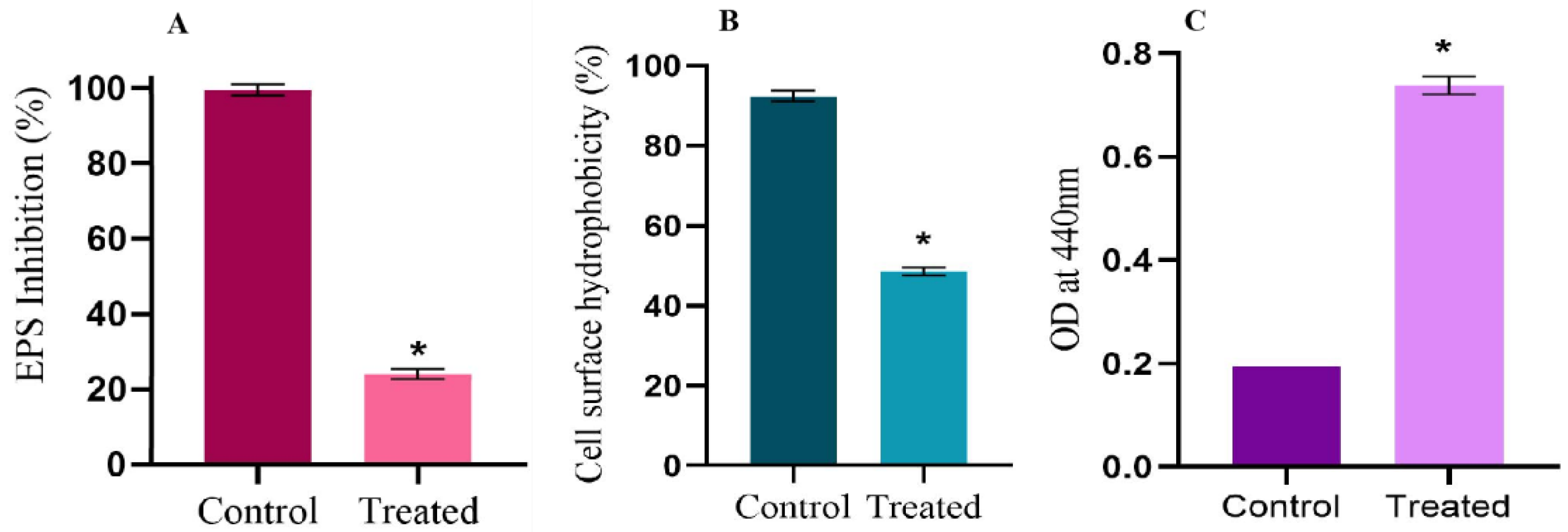
2.6. Linalool Mitigates EPS and Cell Surface Hydrophobicity
2.7. Linalool Enhances Extracellular Cysteine Protease Production
2.8. Dynamics in the Expression of Candidate Virulence Genes by Linalool
2.9. Cytotoxicity Analysis in HEK Cells
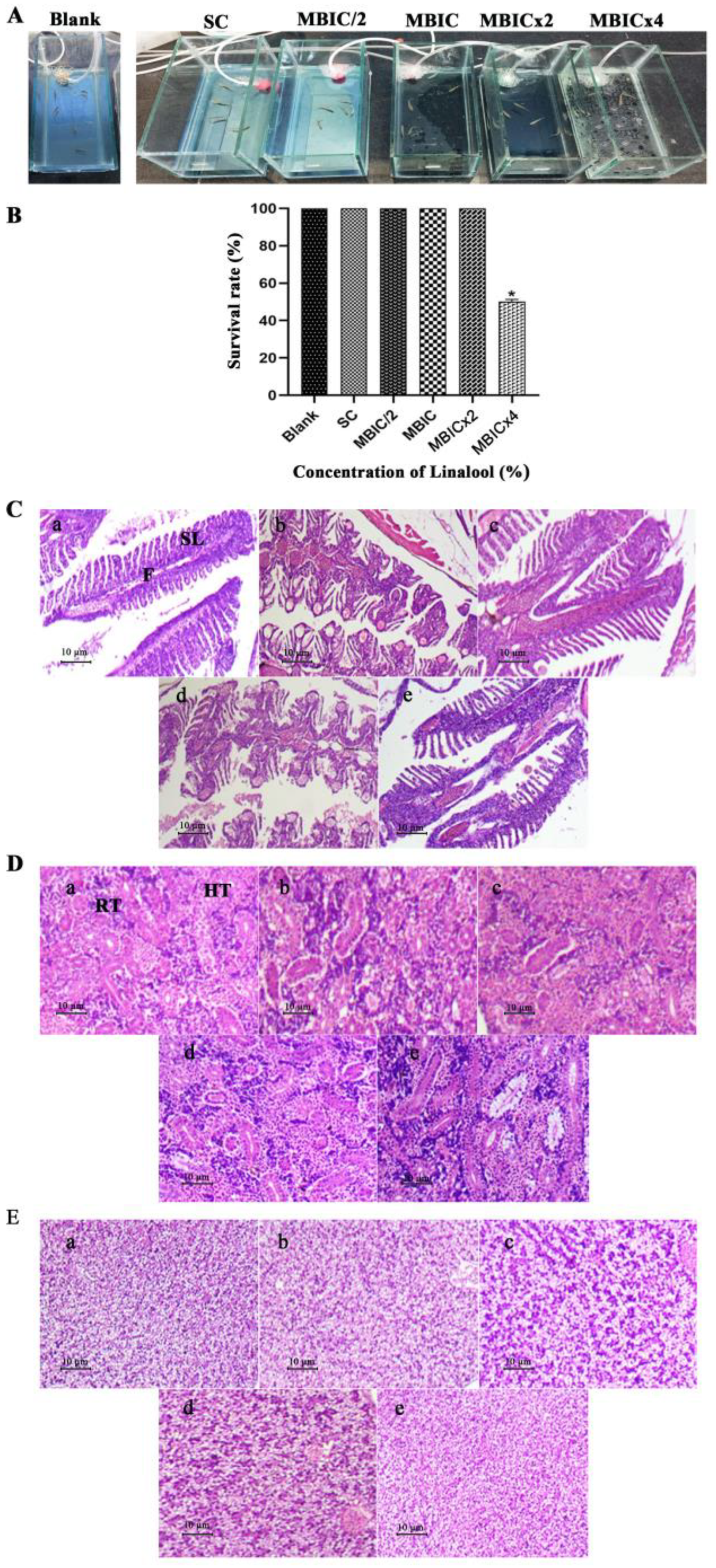
2.10. In Vivo Efficacy of Linalool in Danio Rerio
3. Discussion
4. Material and Methods
4.1. Bacterial Strain and Growth Condition
4.2. Extraction of Essential Oil Using Hydro Distillation
4.3. Phytochemical Analysis: GC-MS
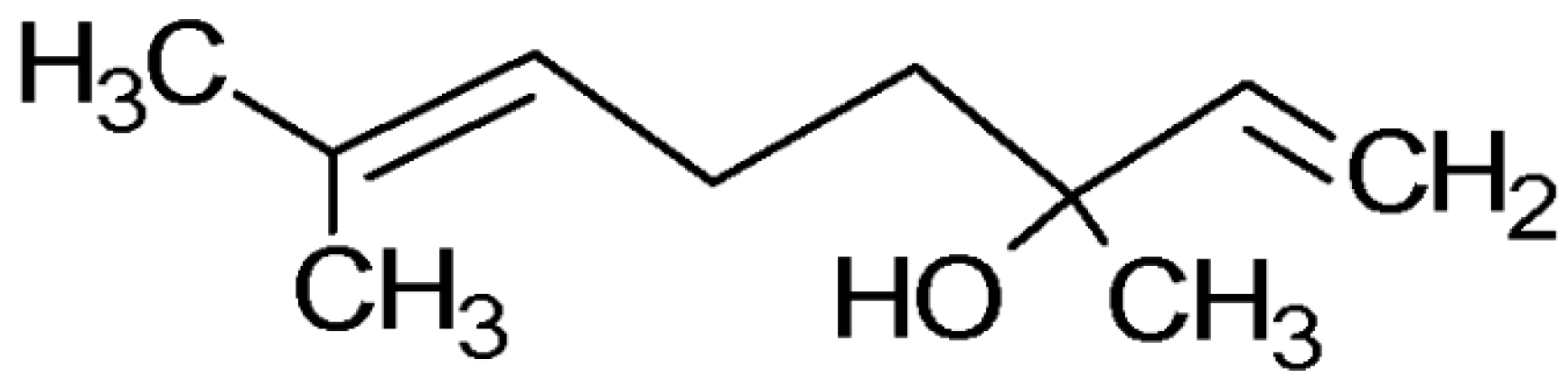
4.4. Linalool
4.5. Determination of Minimum Inhibitory Concentrations
4.6. Determination of Minimum Biofilm Inhibition Concentration (MBIC)
4.7. Colony-Forming Unit (CFU) Analyses
4.8. Cell Viability Assay
4.9. In Situ Visualization of Biofilm Inhibition
4.10. Cytotoxic Assay
4.11. Microbial Adhesion to Hydrocarbon (MATH) Assay
4.12. Extracellular Polymeric Substance (EPS) Quantification
4.13. Secreted Protease Quantification
4.14. Real-Time PCR Analysis
4.15. Histopathology Analysis Using Danio Rerio
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, L.K.; Eccersley, L.R.; Sriskandan, S. Current views of haemolytic streptococcal pathogenesis. Curr. Opin. Infect. Dis. 2014, 27, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Eleftheriadis, N.; Zwinderman, M.R.; Dömling, A.S.; Dekker, F.J.; Boersma, Y.L. Identification of potential antivirulence agents by substitution-oriented screening for inhibitors of Streptococcus pyogenes sortase A. Eur. J. Med. Chem. 2019, 161, 93–100. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- VanEpps, J.S.; Younger, J.G. Implantable device-related infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Johani, K.; Gosbell, I.B.; Jacombs, A.S.; Almatroudi, A.; Whiteley, G.S.; Deva, A.K.; Jensen, S.; Vickery, K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in bioflms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015, 91, 35–44. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, A.M. Health care associated infections—An overview. Infect. and Drug Resist. 2018, 11, 2321. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, S.A.; Bhaskar, J.P.; Krishnan, V.; Sethupathy, S.; Pandipriya, S.; Aruni, W.; Pandian, S.K. Inhibition of biofilm and biofilm-associated virulence factor production in methicillin-resistant Staphylococcus aureus by docosanol. J. Biotechnol. 2020, 317, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Gupta, P.; Pruthi, P.A.; Pruthi, V. Role of Exopolysaccharides in Bioflm Formation; American Chemical Society: Washington, DC, USA, 2019; pp. 17–57. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Murugan, R.; Pandian, S.K. Essential oils from commercial and wild Patchouli modulate Group A Streptococcal biofilms. Ind. Crops Prod. 2015, 69, 180–186. [Google Scholar] [CrossRef]
- Budzyńska, A.; Wickowska-Szakiel, M.; Sadowska, B.; Kalemba, D.; Różalska, B. Antibiofilm activity of selected plant essential oils and their major components. Pol. J. Microbiol. 2011, 60, 35. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.; Dan, M.; George, V.; Sethuraman, M.G. Studies on chemical composition of essential oils from leaf and inflorescence of Hedychium larsenii M. Dan & Sathish. J. Essent. Oil Res. 2013, 25, 33–38. [Google Scholar]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-listeria activities of linalool and its mechanism revealed by comparative transcriptome analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; p. 804. [Google Scholar]
- Adrian, J.; Hill, H.T.; Heideman, W.; Richard, E.P. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, T.; Köller, T.; Kreikemeyer, B. Streptococcus pyogenes biofilms—Formation, biology, and clinical relevance. Front. Cell. Infect. Microbiol. 2015, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Beier, R.C.; Byrd, J.A., II; Kubena, L.F.; Hume, M.E.; McReynolds, J.L.; Anderson, R.C.; Nisbet, D.J. Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: Effects on poultry. Poult. Sci. 2014, 93, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial activity and mechanism of linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Subramenium, G.A.; Vijayakumar, K.; Pandian, S.K. Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. J. Med. Microbiol. 2015, 64, 879–890. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Manigandan, V.; Jeyapragash, D.; Bharathidasan, V.; Anandharaj, B.; Sathya, M. Eucalyptol inhibits biofilm formation of Streptococcus pyogenes and its mediated virulence factors. J. Med. Microbiol. 2020, 69, 1308–1318. [Google Scholar] [CrossRef]
- Green, A.E.; Rowlands, R.S.; Cooper, R.A.; Maddocks, S.E. The effect of the flavonol morin on adhesion and aggregation of Streptococcus pyogenes. FEMS Microbiol. Lett. 2012, 333, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Wijesundara, N.M.; Rupasinghe, H.V. Essential oils from Origanum vulgare and Salvia officinalis exhibit antibacterial and anti-biofilm activities against Streptococcus pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Valliammai, A.; Selvaraj, A.; Sangeetha, M.; Sethupathy, S.; Pandian, S.K. 5-Dodecanolide inhibits biofilm formation and virulence of Streptococcus pyogenes by suppressing core regulons of virulence. Life Sci. 2020, 262, 118554. [Google Scholar] [CrossRef] [PubMed]
- Ashwinkumar Subramenium, G.; Viszwapriya, D.; Iyer, P.M.; Balamurugan, K.; Karutha Pandian, S. covR mediated antibiofilm activity of 3-furancarboxaldehyde increases the virulence of Group A Streptococcus. PLoS ONE 2015, 10, e0127210. [Google Scholar] [CrossRef] [Green Version]
- Shafreen, R.M.B.; Selvaraj, C.; Singh, S.K.; Pandian, S.K. In silico and in vitro studies of cinnamaldehyde and their derivatives against LuxS in Streptococcus pyogenes: Effects on biofilm and virulence genes. J. Mol. Recognit. 2014, 27, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Nandu, T.G.; Subramenium, G.A.; Shiburaj, S.; Viszwapriya, D.; Iyer, P.M.; Balamurugan, K.; Rameshkumar, K.B.; Pandian, S.K. Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors. J. Med. Microbiol. 2018, 67, 1391–1401. [Google Scholar] [CrossRef]
- Courtney, H.S.; Ofek, I.; Penfound, T.; Nizet, V.; Pence, M.A.; Kreikemeyer, B.; Podbielski, A.; Hasty, D.L.; Dale, J.B. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS ONE 2009, 4, e4166. [Google Scholar] [CrossRef]
- Honda-Ogawa, M.; Ogawa, T.; Terao, Y.; Sumitomo, T.; Nakata, M.; Ikebe, K.; Maeda, Y.; Kawabata, S. Cysteine proteinase from Streptococcus pyogenes enables evasion of innate immunity via degradation of complement factors. J. Biol. Chem. 2013, 288, 15854–15864. [Google Scholar] [CrossRef] [Green Version]
- Krzysciak, W.; Pluskwa, K.K.; Jurczak, A.; Koscielniak, D. The pathogenicity of the Streptococcus genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1361–1376. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.N.; Barnett, T.C.; Nizet, V.; Walker, M.J. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 2011, 9, 724–736. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; McDowell, E.J.; Chaussee, M.S. Inter-and intra serotypic variation in the Streptococcus pyogenes Rgg regulon. FEMS Microbiol. Lett. 2008, 284, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.L.; Connolly, K.L.; Doern, C.D.; Holder, R.C.; Reid, S.D. Loss of the group A Streptococcus regulator Srv decreases biofilm formation in vivo in an otitis media model of infection. Infect. Immun. 2010, 78, 4800–4808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, B.; Liu, M.; Voyich, J.M.; Prater, C.I.; Kala, S.V.; DeLeo, F.R.; Musser, J.M. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect. Immun. 2003, 71, 5962–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, C.S.; Montanez, G.E.; Woods, C.R.; Vincent, R.M.; Eichenbaum, Z. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 2003, 71, 1042–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, M.R.; Virtaneva, K.; Porcella, S.F.; Barry, W.T.; Gowen, B.B.; Johnson, C.R.; Wright, F.A.; Musser, J.M. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am. J. Pathol. 2005, 166, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Marouni, M.J.; Sela, S. The luxS gene of Streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infect. Immun. 2003, 71, 5633–5639. [Google Scholar] [CrossRef] [Green Version]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2019, 33, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Prasath, K.G.; Alexpandi, R.; Parasuraman, R.; Pavithra, M.; Ravi, A.V.; Pandian, S.K. Anti-inflammatory potential of myristic acid and palmitic acid synergism against systemic candidiasis in Danio rerio (Zebrafish). Biomed. Pharmacother. 2021, 133, 111043. [Google Scholar] [CrossRef]
- Holden, J.A.; Layfield, L.L.; Matthews, J.L. The Zebrafish: Atlas of Macroscopic and Microscopic Anatomy; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; de Souza, G.C.; Dos Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; de Oliveira Carvalho, H.; Silva, M.L. Effects of Bothrops alternatus venom in Zebrafish: A histopathological study. Inflammopharmacology 2018, 26, 273–284. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef] [Green Version]
- Kannappan, A.; Santhakumari, S.; Srinivasan, R.; Pandian, S.K.; Ravi, A.V. Hemidesmus indicus, a traditional medicinal plant, targets the adherence of multidrug-resistant pathogens to form biofilms. Biocatal. Agric. Biotechnol. 2019, 21, 101338. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Mcgaw, M.; Marchant, R.; Funston, S.; Dunlop, P.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 1015–1019. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, S.; Poornima, B.; Pandian, S.K. Inhibitory efficacy of cyclo (l-leucyl-l-prolyl) from mangrove rhizosphere bacterium–Bacillus amyloliquefaciens (MMS-50) toward cariogenic properties of Streptococcus mutans. Res. Microbiol. 2014, 165, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Viszwapriya, D.; Subramenium, G.A.; Prithika, U.; Balamurugan, K.; Pandian, S.K. Betulin inhibits virulence and biofilm of Streptococcus pyogenes by suppressing ropB core regulon, sagA, and dltA. FEMS Pathog. Dis. 2016, 74, ftw088. [Google Scholar] [CrossRef] [Green Version]
- Hollands, A.; Aziz, R.K.; Kansal, R.; Kotb, M.; Nizet, V.; Walker, M.J. A naturally occurring mutation in ropB suppresses SpeB expression and reduces M1T1 group A streptococcal systemic virulence. PLoS ONE 2008, 3, e4102. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.T.; So, J.S. A rapid method for RNA preparation from Gram-positive bacteria. J. Microbiol. Methods 2003, 52, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Cai, M.; Lv, C.; Zhao, Y.; Wei, D.; Zhu, H. The heme transporter HtsABC of group A Streptococcus contributes to virulence and innate immune evasion in murine skin infections. Front. Microbiol. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Hirose, Y.; Yamaguchi, M.; Takemoto, N.; Miyoshi-Akiyama, T.; Sumitomo, T.; Nakata, M.; Ikebe, T.; Hanada, T.; Yamaguchi, T.; Kawahara, R.; et al. Genetic Characterization of Streptococcus pyogenes emm89 Strains Isolated in Japan from 2011 to 2019. Infect. Microbes Dis. 2020, 2, 160. [Google Scholar] [CrossRef]


| Peak No. | R. Time | Area% | Name of the Metabolites | RRI (Calculated) | RRI (Reference) |
|---|---|---|---|---|---|
| 1 | 5.221 | 1.02 | α-Pinene | 932 | 932 |
| 2 | 5.639 | 0.22 | Camphene | 946 | 946 |
| 3 | 6.383 | 3.72 | β-Pinene | 976 | 974 |
| 4 | 6.654 | 0.20 | Myrcene | 987 | 988 |
| 5 | 7.794 | 11.77 | Cymene | 1023 | 1022 |
| 6 | 7.952 | 1.00 | Limonene | 1027 | 1024 |
| 7 | 8.069 | 14.48 | Eucalyptol, 1.8-Cineole | 1025 | 1026 |
| 8 | 8.933 | 0.24 | 1.4-cyclohexadiene,1-methyl-4-(1- methylethyl)- | 1055 | 1054 |
| 9 | 9.390 | 2.87 | linalool oxide B | 1068 | 1067 |
| 10 | 9.981 | 2.81 | Trans-linalool oxide | 1085 | 1084 |
| 11 | 10.592 | 52.11 | Linalool | 1102 | 1095 |
| 12 | 11.224 | 0.25 | α-fenchol | 1117 | 1114 |
| 13 | 12.071 | 0.15 | Pinocarveol | 1138 | 1135 |
| 14 | 13.456 | 0.32 | Linalool oxide trans-pyranoid | 1172 | 1173 |
| 15 | 13.692 | 1.38 | Terpinen-4-ol | 1177 | 1174 |
| 16 | 14.298 | 2.95 | α-Terpineol | 1192 | 1186 |
| 17 | 18.069 | 0.29 | Isobornyl acetate | 1280 | 1283 |
| 18 | 18.348 | 0.20 | Thymol | 1287 | 1289 |
| 19 | 18.683 | 0.26 | Carvacrol, ethyl ether | 1295 | 1297 |
| 20 | 26.337 | 0.30 | Selina-3,7(11)-diene | 1540 | 1545 |
| 21 | 27.730 | 0.29 | α-selinene | 1512 | 1520 |
| 22 | 33.230 | 0.31 | Pogostol | 1651 | 1651 |
| Gene | Function | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| mga | Virulence factor regulation and Biofilm formation. | GATCCGTTACTACAAGGG | GTTACTTGTCTGCCTCCT |
| ompA | Outer membrane protein involved in stress response | GTGCTTCCTGGCTATGAACC | GCAGCGGGTTGGTTATTGTA |
| covR | Repressor gene of TCS of covRS. Stress response, biofilm formation, and regulate 15% of genes. | TGCGCGTGATTCTATTATGG | GGCGGAAAATAGCACGAATA |
| sagA | Streptolysin S production | AAACAACTCAAGTTGCTCCTG | TGGCGTATAACTTCCGCTAC |
| covS | TCS, control of virulence sensor, Regulates biofilms | GAGTGAGCGCGATATCACAA | GCAAGCCAGGAGATGATTCT |
| hlyX | Hemolysin production | GCGCAATACCCAAAATCAGA | CGATTTCACCGACGATTTCT |
| slo | Streptolysin O synthesis | GCCAATGTTTCAACAGCTATTG | CGGAGCTGCACTAAAGGCCGC |
| col370 | Involved in adhesion | AACCCAGATACTGCACCACA | GCGAGCTGATTACCACCTTG |
| dltA | D-alanylation of LTA | GCATTTGGACATCGACTCCT | GTTTTCGAGCCGTAGAAACG |
| htsA | Heme-transporter gene | ATTGTAGCCACTTCGGTTGC | AAACCCACACGCTTAACAGC |
| srv | Regulation of virulence factor and Biofilm formation | CGGCATTGTGAAACAGAGTG | TCTGACTCGATGCGAACATT |
| speB | Production of extracellular cysteine protease | CTAGGATACTCTACCAGCG | CAGTAGCAACACATCCTG |
| hasA | Production of Hyaluronic acid capsule. | AGCGTGCTGCTCAATCATTA | AACATCGATCATCCCCAATG |
| ropB | Transcriptional factor. Regulates virulence and stress. | TGATATGGATACGGCAAAACA | TTGACCAAGGCAAAAAGGTT |
| luxS | Virulence factor regulation | CTTTTGGCTGTCGAACAGGT | TCCAGGAACATCTTCCCAAG |
| spy125 | Synthesizes minor pilin subunits. | AGAGATTAGCGACGCAACAG | ATGGCCATATGTCTCCACCA |
| srtB | Production of Class C sortase, involved in aggregation | GCTGGTTTTGGTTTGTGGGA | CCCCGGGATATTTAACCAACC |
| ciaH | Stress response TCS | GGCGGTCTTACAGAATCGTC | CATGTTGCGAACCTCGTCTA |
| gyrA | Gyrase production (House-keeping gene in the present study) | CAACGCACGTAAGGAAGAAA | CGCTTGTCAAAACGACGTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praseetha, S.; Sukumaran, S.T.; Dan, M.; Augustus, A.R.; Pandian, S.K.; Sugathan, S. The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio. Antibiotics 2023, 12, 545. https://doi.org/10.3390/antibiotics12030545
Praseetha S, Sukumaran ST, Dan M, Augustus AR, Pandian SK, Sugathan S. The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio. Antibiotics. 2023; 12(3):545. https://doi.org/10.3390/antibiotics12030545
Chicago/Turabian StylePraseetha, Sarath, Swapna Thacheril Sukumaran, Mathew Dan, Akshaya Rani Augustus, Shunmugiah Karutha Pandian, and Shiburaj Sugathan. 2023. "The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio" Antibiotics 12, no. 3: 545. https://doi.org/10.3390/antibiotics12030545






