Spread of blaCTX-M-9 and Other Clinically Relevant Resistance Genes, Such as mcr-9 and qnrA1, Driven by IncHI2-ST1 Plasmids in Clinical Isolates of Monophasic Salmonella enterica Serovar Typhimurium ST34
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates and Antimicrobial Susceptibility Testing
2.2. Whole Genome Sequencing and Bioinformatics Analysis
2.3. Conjugation Experiments
2.4. Nucleotide Sequence Accession Numbers
3. Results and Discussion
3.1. General Characteristics of the Isolates
3.2. Resistance Properties and Genetic Basis of Antimicrobial Drug Resistance
3.3. The IncHI2-ST1 Plasmids Harbor Multiple Resistance Genes
3.4. Chromosomal Regions Encoding Resistance to Antibiotics and Heavy Metals
3.4.1. The Genomic Island SGI-4
3.4.2. The RR Resistance Regions and the Genetic Bases of the Monophasic Phenotype
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- Foley, S.L.; Lynne, A.M. Food animal-associated Salmonella challenges: Pathogenicity and antimicrobial resistance. J. Anim. Sci. 2008, 86, E173–E187. [Google Scholar] [CrossRef] [PubMed]
- WHO. Critically Important Antimicrobials for Human Medicine; WHO: Geneva, Switzerland, 2016.
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 7209. [Google Scholar]
- Canton, R.; Gonzalez-Alba, J.M.; Galan, J.C. CTX-M enzymes: Origin and diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peirano, G.; Pitout, J.D.D. Extended-spectrum ß-lactamase-producing Enterobacteriaceae: Update on molecular epidemiology and treatment options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic support and diversity of acquired extended-spectrum beta-lactamases in Gram-negative rods. Infect. Genet. Evol. 2012, 12, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Bennett, P.M.; Walsh, T.R. ISCR elements: Novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 2006, 70, 296–316. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on monitoring and assessment of the public health risk of “Salmonella Typhimurium-like” strains. EFSA J. 2010, 8, 1826. [Google Scholar] [CrossRef]
- Sun, H.; Wan, Y.; Du, P.; Bai, L. The epidemiology of monophasic Salmonella typhimurium. Foodborne Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef]
- Switt, A.I.; Soyer, Y.; Warnick, L.D.; Wiedmann, M. Emergence, distribution, and molecular and phenotypic characteristics of Salmonella enterica serotype 4,5,12:i. Foodborne Pathog. Dis. 2009, 6, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Trachsel, J.M.; Bearson, B.L.; Brunelle, B.W.; Bearson, S.M.D. Relationship and distribution of Salmonella enterica serovar I 4,[5],12:i:- strain sequences in the NCBI Pathogen Detection database. BMC Genom. 2022, 23, 268. [Google Scholar] [CrossRef] [PubMed]
- Branchu, P.; Charity, O.J.; Bawn, M.; Thilliez, G.; Dallman, T.J.; Petrovska, L.; Kingsley, R.A. SGI-4 in monophasic Salmonella typhimurium ST34 is a novel ICE that enhances resistance to copper. Front. Microbiol. 2019, 10, 1118. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.G.; Landgraff, C.; Robertson, J.; Pollari, F.; Parker, S.; Nadon, C.; Gannon, V.P.J.; Johnson, R.; Nash, J. Distribution of heavy metal resistance elements in Canadian Salmonella 4,[5],12:i:- populations and association with the monophasic genotypes and phenotype. PLoS ONE 2020, 15, e0236436. [Google Scholar] [CrossRef]
- Mourao, J.; Rebelo, A.; Ribeiro, S.; Peixe, L.; Novais, C.; Antunes, P. Tolerance to arsenic contaminant among multidrug-resistant and copper-tolerant Salmonella successful clones is associated with diverse ars operons and genetic contexts. Environ. Microbiol. 2020, 22, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, L.; Mather, A.E.; AbuOun, M.; Branchu, P.; Harris, S.R.; Connor, T.; Hopkins, K.L.; Underwood, A.; Lettini, A.A.; Page, A.; et al. Microevolution of monophasic Salmonella typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016, 22, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, C.; Bertrand, S.; Mattheus, W.; Dierick, K.; Jasson, V.; Rosseel, T.; Van Borm, S.; Mahillon, J.; Wattiau, P. Extensive genetic variability linked to IS26 insertions in the fljB promoter region of atypical monophasic variants of Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2015, 81, 3169–3175. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P.; Malorny, B.; Rodicio, M.R.; Stephan, R.; Hachler, H.; Guerra, B.; Lucarelli, C. Horizontal acquisition of a multidrug-resistance module (R-type ASSuT) is responsible for the monophasic phenotype in a widespread clone of Salmonella serovar 4,[5],12:i. Front. Microbiol. 2016, 7, 680. [Google Scholar] [CrossRef] [Green Version]
- Lucarelli, C.; Dionisi, A.M.; Filetici, E.; Owczarek, S.; Luzzi, I.; Villa, L. Nucleotide sequence of the chromosomal region conferring multidrug resistance (R-type ASSuT) in Salmonella Typhimurium and monophasic Salmonella Typhimurium strains. J. Antimicrob. Chemother. 2012, 67, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Ingle, D.J.; Ambrose, R.L.; Baines, S.L.; Duchene, S.; Goncalves da Silva, A.; Lee, D.Y.J.; Jones, M.; Valcanis, M.; Taiaroa, G.; Ballard, S.A.; et al. Evolutionary dynamics of multidrug resistant Salmonella enterica serovar 4,[5],12:i:- in Australia. Nat. Commun. 2021, 12, 4786. [Google Scholar] [CrossRef]
- Vazquez, X.; Garcia, V.; Fernandez, J.; Bances, M.; de Toro, M.; Ladero, V.; Rodicio, R.; Rodicio, M.R. Colistin resistance in monophasic isolates of Salmonella enterica ST34 collected from meat-derived products in Spain, with or without CMY-2 co-production. Front. Microbiol. 2021, 12, 735364. [Google Scholar] [CrossRef]
- Vielva, L.; de Toro, M.; Lanza, V.F.; de la Cruz, F. PLACNETw: A web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics 2017, 33, 3796–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, A.; Carattoli, A. Plasmid double locus sequence typing for IncHI2 plasmids, a subtyping scheme for the characterization of IncHI2 plasmids carrying extended-spectrum ß-lactamase and quinolone resistance genes. J. Antimicrob. Chemother. 2010, 65, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.E.; Levine, J.G. Studies of temperature-sensitive transfer and maintenance of H incompatibility group plasmids. J. Gen. Microbiol. 1980, 116, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmour, M.W.; Thomson, N.R.; Sanders, M.; Parkhill, J.; Taylor, D.E. The complete nucleotide sequence of the resistance plasmid R478: Defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid 2004, 52, 182–202. [Google Scholar] [CrossRef]
- Sabate, M.; Navarro, F.; Miro, E.; Campoy, S.; Mirelis, B.; Barbe, J.; Prats, G. Novel complex sul1-type integron in Escherichia coli carrying blaCTX-M-9. Antimicrob. Agents Chemother. 2002, 46, 2656–2661. [Google Scholar] [CrossRef] [Green Version]
- Antunes, P.; Mourao, J.; Alves, T.; Campos, J.; Novais, C.; Novais, A.; Peixe, L. Salmonella enterica serotype Bovismorbificans, a new host for CTX-M-9. Int. J. Antimicrob. Agents 2013, 41, 91–93. [Google Scholar] [CrossRef]
- Novais, A.; Canton, R.; Valverde, A.; Machado, E.; Galan, J.C.; Peixe, L.; Carattoli, A.; Baquero, F.; Coque, T.M. Dissemination and persistence of blaCTX-M-9 are linked to class 1 integrons containing CR1 associated with defective transposon derivatives from Tn402 located in early antibiotic resistance plasmids of IncHI2, IncP1-alpha, and IncFI groups. Antimicrob. Agents Chemother. 2006, 50, 2741–2750. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011, 9, 2322. [Google Scholar] [CrossRef] [Green Version]
- De Toro, M.; Garcia, P.; Rodriguez, I.; Rojo-Bezares, B.; Helmuth, R.; Saenz, Y.; Rodicio, M.R.; Guerra, B.; Torres, C. Characterisation of plasmids implicated in the mobilisation of extended-spectrum and AmpC ß-lactamase genes in clinical Salmonella enterica isolates and temporal stability of the resistance genotype. Int. J. Antimicrob. Agents 2013, 42, 167–172. [Google Scholar] [CrossRef]
- Herrera-Leon, S.; Gonzalez-Sanz, R.; Rodriguez, I.; Rodicio, M.R.; Echeita, M.A. Spread of a multiresistant CTX-M-9-producing Salmonella enterica serotype Virchow phage type 19 in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Riano, I.; Garcia-Campello, M.; Saenz, Y.; Alvarez, P.; Vinue, L.; Lantero, M.; Moreno, M.A.; Zarazaga, M.; Torres, C. Occurrence of extended-spectrum ß-lactamase-producing Salmonella enterica in northern Spain with evidence of CTX-M-9 clonal spread among animals and humans. Clin. Microbiol. Infect 2009, 15, 292–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riano, I.; Moreno, M.A.; Teshager, T.; Saenz, Y.; Dominguez, L.; Torres, C. Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 2006, 58, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tran, J.H.; Jacoby, G.A.; Zhang, Y.; Wang, F.; Hooper, D.C. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 2003, 47, 2242–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Hickman, A.B.; Varani, A.M.; Siguier, P.; Chandler, M.; Dekker, J.P.; Dyda, F. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 2015, 6, e00762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieffer, N.; Royer, G.; Decousser, J.W.; Bourrel, A.S.; Palmieri, M.; Ortiz De La Rosa, J.M.; Jacquier, H.; Denamur, E.; Nordmann, P.; Poirel, L. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and Its origin. Antimicrob. Agents Chemother. 2019, 63, e00965-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyson, G.H.; Li, C.; Hsu, C.H.; Ayers, S.; Borenstein, S.; Mukherjee, S.; Tran, T.T.; McDermott, P.F.; Zhao, S. The mcr-9 gene of Salmonella and Escherichia coli is not associated with colistin resistance in the United States. Antimicrob. Agents Chemother. 2020, 64, e00573-20. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio 2019, 10, e00853-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dai, X.; Zeng, J.; Gao, Y.; Zhang, Z.; Zhang, L. Characterization of the global distribution and diversified plasmid reservoirs of the colistin resistance gene mcr-9. Sci. Rep. 2020, 10, 8113. [Google Scholar] [CrossRef]
- Lumbreras-Iglesias, P.; de Toro, M.; Vazquez, X.; Garcia-Carus, E.; Rodicio, M.R.; Fernandez, J. High-risk international clones ST66, ST171 and ST78 of Enterobacter cloacae complex causing blood stream infections in Spain and carrying blaOXA-48 with or without mcr-9. J. Infect Public Health 2023, 16, 272–279. [Google Scholar] [CrossRef]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J. Bacteriol. 2002, 184, 4259–4269. [Google Scholar] [CrossRef] [Green Version]
- Mastrorilli, E.; Pietrucci, D.; Barco, L.; Ammendola, S.; Petrin, S.; Longo, A.; Mantovani, C.; Battistoni, A.; Ricci, A.; Desideri, A.; et al. A comparative genomic analysis provides novel insights into the ecological success of the monophasic Salmonella serovar 4,[5],12:i. Front. Microbiol. 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.; Simon, M. Phase variation: Genetic analysis of switching mutants. Cell 1980, 19, 845–854. [Google Scholar] [CrossRef] [PubMed]
- McClelland, M.; Sanderson, K.E.; Spieth, J.; Clifton, S.W.; Latreille, P.; Courtney, L.; Porwollik, S.; Ali, J.; Dante, M.; Du, F.; et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 2001, 413, 852–856. [Google Scholar] [CrossRef] [Green Version]
- Martínez, N.; Mendoza, M.C.; Guerra, B.; Gonzalez-Hevia, M.A.; Rodicio, M.R. Genetic basis of antimicrobial drug resistance in clinical isolates of Salmonella enterica serotype Hadar from a Spanish region. Microb. Drug Resist. 2005, 11, 185–193. [Google Scholar] [CrossRef]
- Arlet, G.; Philippon, A.A. Construction by polymerase chain reaction and use of intragenic DNA probes for three main types of transferable betalactamases (TEM, SHV, CARB). FEMS Microbiol. Lett. 1991, 66, 19–25. [Google Scholar]

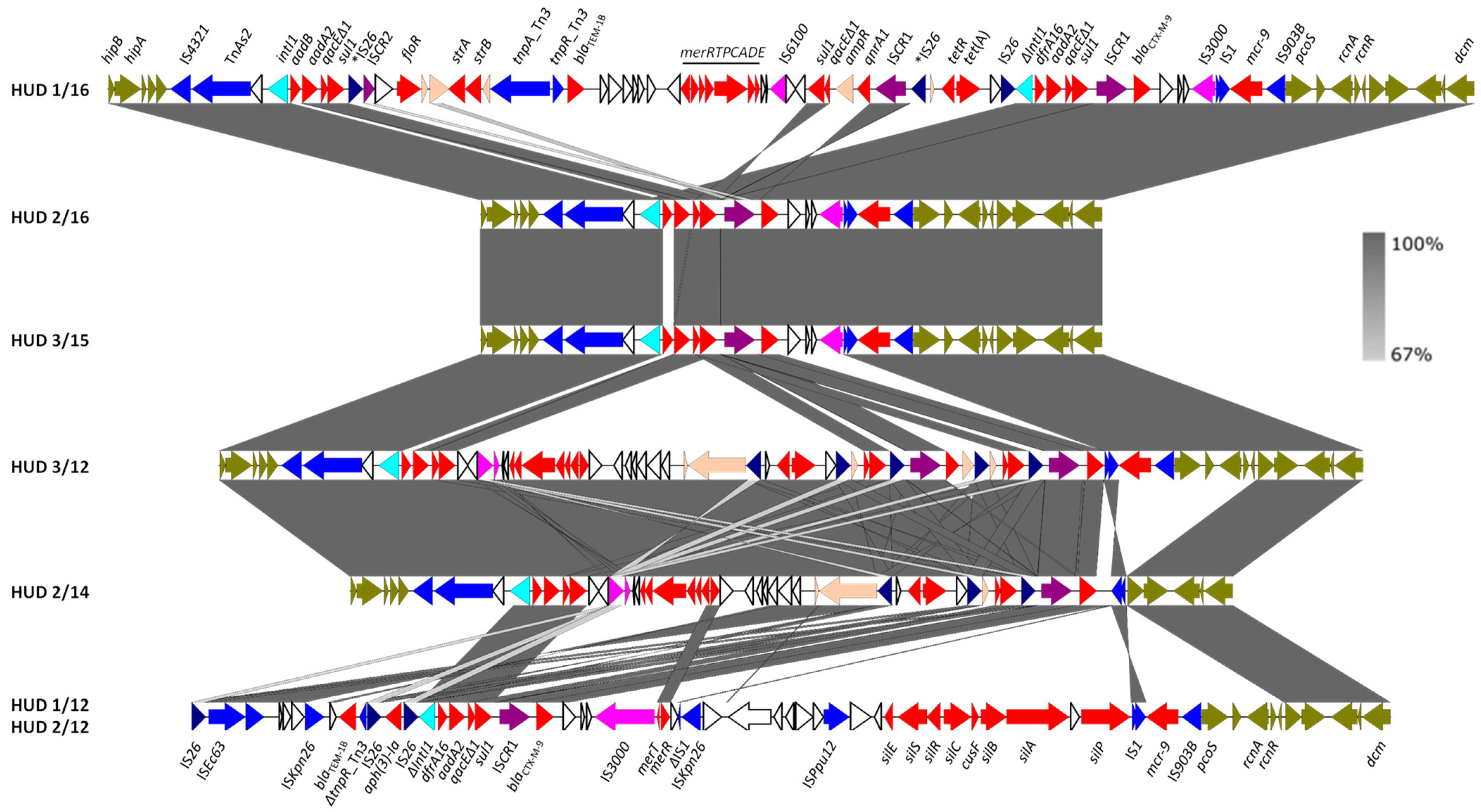
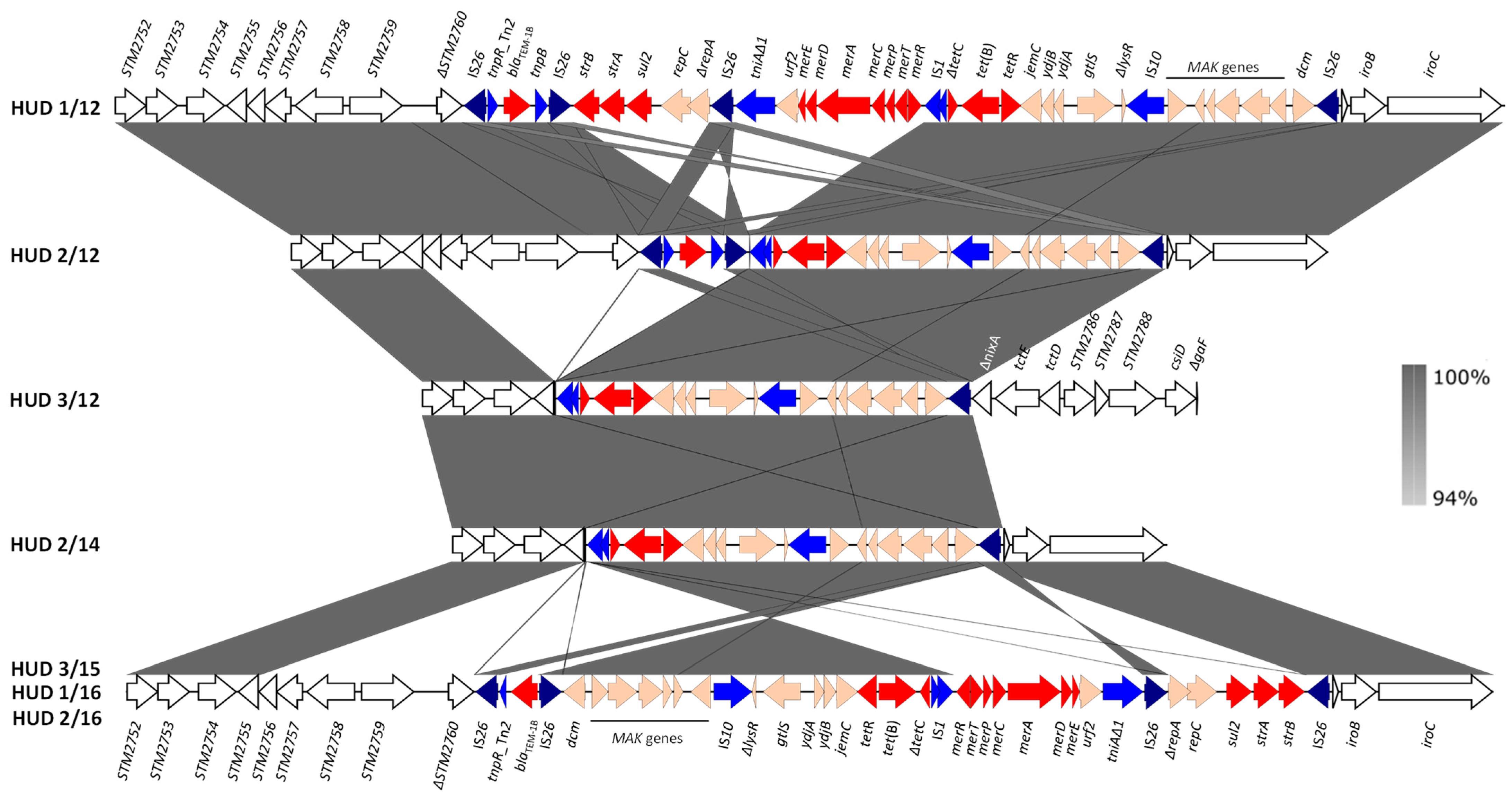
| Isolate a | Patient Sex/Age b | Resistance Phenotype c/ Antibiotic Resistance Genes d | Chromosome Plasmids e (Size bp) |
|---|---|---|---|
| HUD 1/12 | M/5 | AMP, CTX, KAN, STR, SUL, TET, TMP/ blaTEM-1B, strA, strB, sul2, tet(B) blaTEM-1B, blaCTX-M-9, aph(3′)-Ia, aadA2b, sul1, dfrA16, mcr-9 - - | Chr (4,970,905) IncHI2-ST1 (349,612) unk * (4072) ColE * (3830) |
| HUD 2/12 | F/10 | AMP, CTX, KAN, STR, SUL, TET, TMP/ blaTEM-1B, tet(B) blaTEM-1B, blaCTX-M-9, aph(3′)-1a, aadA2b, sul1, dfrA16, mcr-9 - | Chr (4,993,648) IncHI2-ST1 (301,830) ColE * (3820) |
| HUD 3/12 | M/9 | AMP, CTX, CIP, STR, SUL, TET, TMP/ tet(B) blaCTX-M-9, qnrA1, aadA2b, sul1, tet(A), dfrA16, mcr-9 | Chr (4,845,756) IncHI2-ST1 (270,777) |
| HUD 2/14 | F/78 | AMP, CTX, STR, SUL, TET, TMP/ tet(B) blaCTX-M-9, aadA2b, sul1, tet(A), dfrA16 | Chr (4,862,133) IncHI2-ST1 (263,144) |
| HUD 3/15 | M/3 | AMP, CTX, STR, SUL, TET/ blaTEM-1B, strA, strB, sul2, tet(B) blaCTX-M-9, aadB-aadA2b, sul1, mcr-9 | Chr (4,974,577) IncHI2-ST1 f (308,586) |
| HUD 1/16 | F/9 | AMP, CTX, CHL, CIP, STR, SUL, TET, TMP/ blaTEM-1B, strA, strB, sul2, tet(B) blaTEM-1B, blaCTX-M-9, floR, qnrA1, aadB-aadA2b, strA, strB, sul1, tet(A), dfrA16, mcr-9 | Chr (4,986,724) IncHI2-ST1 * (287,136) |
| HUD 2/16 | M/8 | AMP, CTX, STR, SUL, TET, TMP/ blaTEM-1B, strA, strB, sul2, tet(B) blaCTX-M-9, aadA2b, sul1, dfrA16, mcr-9 | Chr (4,962,945) IncHI2-ST1 (255,829) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, X.; Fernández, J.; Alkorta, M.; de Toro, M.; Rodicio, M.R.; Rodicio, R. Spread of blaCTX-M-9 and Other Clinically Relevant Resistance Genes, Such as mcr-9 and qnrA1, Driven by IncHI2-ST1 Plasmids in Clinical Isolates of Monophasic Salmonella enterica Serovar Typhimurium ST34. Antibiotics 2023, 12, 547. https://doi.org/10.3390/antibiotics12030547
Vázquez X, Fernández J, Alkorta M, de Toro M, Rodicio MR, Rodicio R. Spread of blaCTX-M-9 and Other Clinically Relevant Resistance Genes, Such as mcr-9 and qnrA1, Driven by IncHI2-ST1 Plasmids in Clinical Isolates of Monophasic Salmonella enterica Serovar Typhimurium ST34. Antibiotics. 2023; 12(3):547. https://doi.org/10.3390/antibiotics12030547
Chicago/Turabian StyleVázquez, Xenia, Javier Fernández, Miriam Alkorta, María de Toro, M. Rosario Rodicio, and Rosaura Rodicio. 2023. "Spread of blaCTX-M-9 and Other Clinically Relevant Resistance Genes, Such as mcr-9 and qnrA1, Driven by IncHI2-ST1 Plasmids in Clinical Isolates of Monophasic Salmonella enterica Serovar Typhimurium ST34" Antibiotics 12, no. 3: 547. https://doi.org/10.3390/antibiotics12030547






