All chemical reagents were purchased from commercial sources (Combi-Blocks (San Diego, CA, USA), Chem-Impex (Wood Dale, IL, USA), and Sigma Aldrich (St. Louis, MO, USA)) and used without further purification. The solvents were commercial and used as obtained. The reactions were performed using oven-dried glassware under an atmosphere of nitrogen and in anhydrous conditions (as required). Room temperature refers to the ambient temperature. Yields refer to chromatographically and spectroscopically pure compounds unless otherwise stated. The reactions were monitored by thin-layer chromatography (TLC) plates that were pre-coated with Merck silica gel 60 F254. Visualization was accomplished with UV light, and a ninhydrin staining solution in n-butanol. Flash chromatography and silica pipette plugs were performed under positive air pressure using Silica Gel 60 of 230–400 mesh (40–63 μm) and also using Grace Davison LC60A 6-μm for reverse phase chromatography. Infrared spectra were recorded using a Cary 630 ATR spectrophotometer. High-resolution mass spectrometry was performed by the Bioanalytical Mass Spectrometry facility, UNSW. Proton and Carbon NMR spectra were recorded in the solvents that were specified using a Bruker DPX 300 or a Bruker Avance 400 or 600 MHz spectrometer as designated. Chemical shifts (δ) are quoted in parts per million (ppm), to the nearest 0.01 ppm and internally referenced relative to the solvent nuclei. 1HNMR spectroscopic data are reported as follows (chemical shift in ppm; multiplicity in br, broad; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; sext, sextet; sept, septet; m, multiplet; or as a combination of these (e.g., dd, dt, etc.)); coupling constant (J) in hertz, integration, proton count, and assignment.
4.2.10. General Procedure for Synthesis of 20a–20d
Following the general procedure H, the compounds 20a–20d were synthesized from 19a–19d.
Analytical data:
The analytical data for intermediate up to
6a,
6b, and final compounds
10a,
12a were already mentioned in our previous publication [
20].
methyl 2-methoxy-1-naphthoate (6).
The title compound 6 was prepared from compound 5 (3.0 g, 14.8 mmol) according to the general procedure C. The product 6 was obtained as an off-white solid (2.56 g, 80%); 1H NMR (400 MHz, Chloroform-d) δ 7.94–7.87 (m, 1H), 7.76 (ddt, J = 0.9, 8.6, 25.1 Hz, 2H), 7.50 (ddd, J = 1.4, 6.8, 8.4 Hz, 1H), 7.37 (ddd, J = 1.2, 6.8, 8.1 Hz, 1H), 7.29 (d, J = 9.1 Hz, 1H), 4.04 (s, 3H), 3.97 (s, 3H);13C NMR (100 MHz, Chloroform-d) 168.7, 154.6, 131.8, 131.1, 128.7, 128.6, 128.2, 127.8, 124.3, 123.9, 113.2, 56.9, 52.6; HRMS (ESI): m/z calcd for C13H12O3 [M + Na]+: 239.0679; found: 239.0679.
2-methoxy-1-naphthoic acid (7c’).
The title compound 7c’ was prepared from compound 6 (2.0 g, 9.25 mmol) according to the general procedure C. The product 7c’ was obtained as an off-white solid (1.6 g, 90%); 1H NMR (400 MHz, DMSO-d6) δ 13.19 (s, 1H), 8.02 (d, J = 9.0 Hz, 1H), 7.95–7.88 (m, 1H), 7.69 (dd, J = 1.0, 8.5 Hz, 1H), 7.58–7.47 (m, 2H), 7.40 (ddd, J = 1.2, 6.7, 8.1 Hz, 1H), 3.93 (s, 3H); 13C NMR (100 MHz, DMSO-d6) 168.5, 153.1, 130.8, 129.9, 128.1, 128.0, 127.5, 124.0, 123.4, 118.7, 113.8, 56.5; HRMS (ESI): m/z calcd for C12H10O3 [M + Na]+: 225.0522; found: 225.0521.
methyl 3-methoxy-2-naphthoate (9).
The title compound 9 was prepared from compound 8 (3.0 g, 14.8 mmol) according to the general procedure C. The product 9 was obtained as an off-white solid (2.7 g, 85%); 1H NMR (400 MHz, Chloroform-d) δ 8.36 (s, 1H), 7.87 (ddd, J = 0.6, 1.3, 8.2 Hz, 1H), 7.82–7.71 (m, 1H), 7.57 (ddd, J = 1.3, 6.9, 8.3 Hz, 1H), 7.43 (ddd, J = 1.2, 6.9, 8.2 Hz, 1H), 7.26 (s, 1H), 4.06 (s, 3H), 4.01 (s, 3H).;13C NMR δ C (101 MHz, Chloroform-d) 166.8, 155.9, 136.2, 132.9, 128.8, 128.6, 127.7, 126.6, 124.5, 121.8, 106.9, 56.1, 52.4; HRMS (ESI): m/z calcd for C13H12O3 [M + Na]+: 1008.4593; found: 239.0679.
3-methoxy-2-naphthoic acid (7d’).
The title compound 7d’ was prepared from compound 9 (2.0 g, 9.25 mmol) according to the general procedure C. The product 7d’ was obtained as an off-white solid (1.56 g, 89%); 1H NMR (400 MHz, DMSO-d6) δ 12.81 (s, 1H), 8.16 (s, 1H), 7.91–7.84 (m, 1H), 7.79 (dd, J = 0.9, 8.3 Hz, 1H), 7.49 (ddd, J = 1.4, 6.9, 8.2 Hz, 1H), 7.40–7.29 (m, 2H), 3.86 (s, 3H);13C NMR (100 MHz, DMSO-d6) 167.4, 154.8, 135.3, 130.9, 128.4, 128.0, 127.1, 126.5, 124.3, 123.6, 106.8, 55.7; HRMS (ESI): m/z calcd for C12H10O3 [M + Na]+: 225.0522; found: 225.0522.
tert-butyl (S)-(2-(2-(2-amino-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (6a).
The title compound 6a was prepared from compound 5 (4.0 g, 8.65 mmol) and 4a (2.8 g, 8.65 mmol) according to the general procedure E. The product 6a was obtained as a grey solid (3.0 g, 65%); 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H), 8.35 (d, J = 8.0 Hz, 1H), 8.10 (t, J = 5.8 Hz, 1H), 7.69 (dd, J = 5.1, 15.9 Hz, 2H), 7.31 (d, J = 8.1 Hz, 1H), 7.23 (dd, J = 2.4, 8.8 Hz, 1H), 7.17 (d, J = 2.5 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 6.76 (t, J = 5.6 Hz, 1H), 6.62 (d, J = 8.9 Hz, 1H), 6.48 (s, 2H), 4.64–4.55 (m, 1H), 3.21–2.94 (m, 6H), 1.37 (s, 9H); 13C NMR(75 MHz, DMSO-d6) 172.4, 167.9, 156.1, 149.3, 136.5, 134.6, 131.1, 127.7, 123.9, 121.3, 118.9, 118.7, 118.6, 116.1, 111.7, 111.1, 105.2, 78.1, 54.5, 28.7, 27.8; HRMS (ESI): m/z calcd for C25H30BrN5O4 [M + Na]+: 566.1373; found: 566.1369.
tert-butyl (S)-(3-(2-(2-amino-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (6b).
The title compound 6b was prepared from compound 5 (4.0 g, 8.65 mmol) and 4b (3.1 g, 8.65 mmol) according to the general procedure E. The product 6b was obtained as a grey solid (2.8 g, 58%); 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H), 8.36 (s, 1H), 8.02 (s, 1H), 7.73–7.64 (m, 2H), 7.30 (d, J = 7.7 Hz, 1H), 7.23 (d, J = 9.1 Hz, 1H), 7.17 (s, 1H), 7.05 (s, 1H), 6.98 (s, 1H), 6.75 (s, 1H), 6.62 (d, J = 10.2 Hz, 1H), 6.47 (s, 2H), 4.63–4.54 (m, 1H), 3.23–3.14 (m, 2H), 3.10–3.06 (m, 2H), 2.93–2.88 (m, 2H), 1.51–1.46 (m, 2H), 1.37 (s, 9H).;13C (100 MHz, DMSO-d6) 172.1, 167.9, 155.0, 149.3, 136.5, 131.0, 127.7, 123.9, 121.3, 118.9, 118.7, 118.6, 116.1, 111.7, 111.1, 105.3, 77.9, 54.6, 37.8, 36.7, 30.0, 28.7, 27.9; HRMS (ESI): m/z calcd for C26H32BrN5O4 [M + H]+: 558.1710; found: 558.1711.
The synthesis and analytical data for
tert-butyl (S)-(2-(2-(2-(2-naphthamido)-5-bromobenzamido)-3-(1
H-indol-3-yl)propanamido)ethyl)carbamate (
10a) were published already [
20].
tert-butyl (S)-(2-(2-(5-bromo-2-(2-(naphthalen-1-yl)acetamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10b).
The title compound 10b was prepared from compound 6a (0.3 g, 0.55 mmol) and 7b (0.66 mmol; 7b in situ preparation from 7b’ using procedure F) according to the general procedure G. The product 10b was obtained as an off-white solid (270 mg, 70%); 1H NMR (400 MHz, DMSO-d6) δ 10.95 (s, 1H), 10.82 (s, 1H), 8.88 (d, J = 8.0 Hz, 1H), 8.24–8.15 (m, 2H), 8.04–7.96 (m, 1H), 7.94–7.86 (m, 1H), 7.86–7.78 (m, 2H), 7.69 (d, J = 7.6 Hz, 1H), 7.60 (dd, J = 2.4, 8.9 Hz, 1H), 7.54–7.30 (m, 5H), 7.15 (d, J = 2.3 Hz, 1H), 7.07 (ddd, J = 1.4, 7.0, 8.1 Hz, 1H), 7.00 (ddd, J = 1.2, 7.0, 8.0 Hz, 1H), 6.79 (t, J = 5.6 Hz, 1H), 4.70–4.55 (m, 1H), 4.16–4.05 (m, 2H), 3.25 (dd, J = 4.5, 14.7 Hz, 1H), 3.16–2.96 (m, 5H), 1.35 (s, 9H).;13C NMR (100 MHz, DMSO-d6) 171.7, 169.7, 167.0, 156.1, 138.0, 136.5, 134.6, 133.8 132.3, 131.7, 131.2, 128. 9, 128.7, 127.7, 126.7, 126.2, 126.0, 124.4, 124.1, 124.0, 122.7, 121.4, 118.9, 118.7, 114.8, 111.38, 110.9, 78.1, 60.2, 54.9, 42.2, 28.6, 27.8, 21.2; HRMS (ESI): m/z calcd for C37H38BrN5O5 [M + Na]+: 734.1949; found: 734.1950.
tert-butyl (S)-(2-(2-(5-bromo-2-(2-methoxy-1-naphthamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10c).
The title compound 10c was prepared from compound 6a (0.3 g, 0.55 mmol) and 7c (0.66 mmol; 7c in situ preparation from 7c’ using procedure F) according to the general procedure G. The product 10c was obtained as an off-white solid (288 mg, 72%); 1H NMR (400 MHz, DMSO-d6) δ 11.54 (s, 1H), 10.79 (s, 1H), 9.01 (d, J = 7.9 Hz, 1H), 8.61 (d, J = 9.0 Hz, 1H), 8.11 (t, J = 5.6 Hz, 1H), 8.05 (d, J = 8.9 Hz, 1H), 8.00 (d, J = 2.4 Hz, 1H), 7.95–7.88 (m, 1H), 7.79 (ddd, J = 1.8, 8.7, 12.5 Hz, 2H), 7.64 (d, J = 7.8 Hz, 1H), 7.54–7.43 (m, 2H), 7.39 (ddd, J = 1.3, 6.8, 8.1 Hz, 1H), 7.29 (dt, J = 0.9, 8.1 Hz, 1H), 7.16 (s, 1H), 7.03 (ddd, J = 1.3, 7.0, 8.2 Hz, 1H), 6.95 (ddd, J = 1.1, 7.0, 7.9 Hz, 1H), 6.71 (t, J = 5.7 Hz, 1H), 4.59–4.50 (m, 1H), 3.78 (s, 3H), 3.26–3.17 (m, 1H), 3.15–2.85 (m, 5H), 1.33 (s, 9H), 1.23 (s, 2H);13C NMR (101 MHz, DMSO-d6) 171.3, 167.2, 165.4, 156.0, 153.8, 138.6, 136.5, 134.7, 131.9, 131.5, 131.0, 128.66, 128.6, 128.0, 127.6, 124.5, 124.0, 123.0, 122.8, 121.3, 120.5, 120.0, 118.8, 118.7, 115.0, 114.2, 111.7, 110.8, 77.9, 56.8, 54.9, 39.4, 38.9, 29.8, 27.7; HRMS (ESI): m/z calcd for C37H38BrN5O6 [M + Na]+: 750.1898; found: 750.1904.
tert-butyl (S)-(2-(2-(5-bromo-2-(3-methoxy-2-naphthamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10d).
The title compound 10f was prepared from compound 6a (0.3 g, 0.55 mmol) and 7f (0.66 mmol; 7f in situ preparation from 7f’ using procedure F) according to the general procedure G. The product 10f was obtained as an off-white solid (260 mg, 65%); 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 10.78 (s, 1H), 8.97 (d, J = 8.1 Hz, 1H), 8.65 (d, J = 9.0 Hz, 1H), 8.57 (s, 1H), 8.22 (t, J = 5.6 Hz, 1H), 8.03–7.97 (m, 1H), 7.88 (dd, J = 1.0, 8.4 Hz, 1H), 7.83 (d, J = 2.4 Hz, 1H), 7.75–7.63 (m, 2H), 7.58 (ddd, J = 1.3, 6.8, 8.3 Hz, 1H), 7.47 (s, 1H), 7.45–7.38 (m, 1H), 7.27–7.13 (m, 2H), 7.06–6.91 (m, 2H), 6.77 (t, J = 6.0 Hz, 1H), 4.75–4.62 (m, 1H), 3.88 (s, 3H), 3.27 (dd, J = 4.2, 14.9 Hz, 1H), 3.03–2.96 (m, 2H), 3.22–3.03 (m, 3H), 1.34 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.8, 167.0, 163.5, 156.1, 154.5, 137.9, 136.5, 136.1, 134.5, 133.3, 131.5, 129.3, 128.9, 127.9, 127.7, 127.7, 126.8, 125.2, 124.9, 124.0, 123.7, 123.5, 121.3, 118.8, 118.6, 114.8, 111.7, 110.9, 107.3, 78.1, 55.9, 55.0, 28.6, 27.9; HRMS (ESI): m/z calcd for C37H38BrN5O6 [M + Na]+: 750.1896; found: 750.1901.
tert-butyl (S)-(2-(2-(5-bromo-2-(quinoline-2-carboxamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10e).
The title compound 10e was prepared from compound 6a (0.3 g, 0.55 mmol) and 7e (0.66 mmol; 7e in situ preparation from 7e’ using procedure F) according to the general procedure G. The product 10e was obtained as an off-white solid (294 mg, 77%); 1H NMR (400 MHz, DMSO-d6) δ 12.96 (s, 1H), 10.76 (s, 1H), 9.01 (d, J = 8.0 Hz, 1H), 8.70 (d, J = 8.9 Hz, 1H), 8.63 (d, J = 8.5 Hz, 1H), 8.25 (t, J = 7.9 Hz, 2H), 8.15–8.05 (m, 2H), 8.00 (d, J = 2.5 Hz, 1H), 7.89 (ddd, J = 1.5, 6.8, 8.5 Hz, 1H), 7.82–7.69 (m, 3H), 7.27–7.20 (m, 2H), 7.03–6.89 (m, 2H), 6.74 (t, J = 5.9 Hz, 1H), 4.79 (dt, J = 7.1, 13.0 Hz, 1H), 3.30–3.25 (m, 1H), 3.24–3.09 (m, 3H), 3.04–2.94 (m, 2H), 1.32 (s, 9H);13C NMR δ C (101 MHz, DMSO-d6) 171.2, 166.6, 162.7, 155.6, 149.4, 145.8, 138.3, 137.5, 136.0, 134.5, 131.3, 130.7, 129.4, 129.0, 128.5, 128.1, 127.3, 123.6, 123.5, 121.9, 120.9, 118.6, 118.4, 118.2, 114.6, 111.3, 110.5, 77.6, 54.6, 28.2, 27.4; HRMS (ESI): m/z calcd for C35H35BrN6O5 [M + Na]+: 721.1745; found: 721.1747.
tert-butyl (S)-(2-(2-(5-bromo-2-(1H-indole-2-carboxamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10f).
The title compound 10i was prepared from compound 6a (0.3 g, 0.55 mmol) and 7f (0.66 mmol; 7f in situ preparation from 7f’ using procedure F) according to the general procedure G. The product 10f was obtained as an off-white solid (294 mg, 77%); 1H NMR (400 MHz, DMSO-d6) δ 12.26 (s, 1H), 11.88 (s, 1H), 10.80 (s, 1H), 9.14 (d, J = 8.0 Hz, 1H), 8.56 (s, 1H), 8.30 (t, J = 5.5 Hz, 1H), 8.08 (d, J = 2.4 Hz, 1H), 7.73 (dd, J = 2.0, 6.5 Hz, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.49–7.42 (m, 1H), 7.30–7.16 (m, 3H), 7.12–6.95 (m, 3H), 6.89 (s, 1H), 6.80 (t, J = 5.9 Hz, 1H), 4.81–4.71 (m, 1H), 3.28 (d, J = 4.5 Hz, 1H), 3.22–3.11 (m, 3H), 3.08–3.00 (m, 2H), 1.34 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.3, 167.8, 165.0, 156.1, 138.9, 136.5, 135.3, 134.9, 132.6, 132.0, 129.5, 129.1, 128.6, 128.4, 128.1, 127.6, 127.5, 124.0, 123.6, 122.9, 122.8, 121.3, 118.9, 118.7, 115.0, 111.8, 110.9, 77.9, 55.2, 37.8, 36.8, 29.9, 28.6, 27.7; HRMS (ESI): m/z calcd for C35H35BrN6O5 [M + Na]+: 709.1745; found: 709.1749.
tert-butyl (S)-(2-(2-(5-bromo-2-(thiophene-2-carboxamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10g).
The title compound 10g was prepared from compound 6a (0.3 g, 0.55 mmol) and 7g (0.66 mmol; 7g in situ preparation from 7g’ using procedure F) according to the general procedure G. The product 10g was obtained as an off-white solid (248 mg, 69%); 1H NMR (400 MHz, DMSO-d6) δ 12.10 (s, 1H), 10.78 (s, 1H), 9.11 (d, J = 8.3 Hz, 1H), 8.40 (d, J = 8.9 Hz, 1H), 8.24 (t, J = 5.6 Hz, 1H), 8.03 (d, J = 2.4 Hz, 1H), 7.89 (dd, J = 1.1, 5.0 Hz, 1H), 7.76–7.66 (m, 2H), 7.51 (dd, J = 1.2, 3.8 Hz, 1H), 7.31–7.23 (m, 1H), 7.23–7.15 (m, 2H), 7.07–6.93 (m, 2H), 6.78 (t, J = 5.6 Hz, 1H), 4.76–4.68 (m, 1H), 3.32–3.26 (m, 2H), 3.13 (td, J = 6.0, 10.2 Hz, 3H), 3.06–2.97 (m, 2H), 1.34 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.5, 167.7, 159.7, 156.1, 138.6, 136.5, 135.3, 133.0, 131.5, 129.1, 128.8, 127.6, 124.0, 122.4, 121.3, 118.9, 118.6, 114.9, 111.8, 110.9, 78.1, 55.0, 28.6, 27.8; HRMS (ESI): m/z calcd for C30H32BrN5O5S [M + Na]+: 676.1200; found: 676.1198.
tert-butyl (S)-(2-(2-(5-bromo-2-(thiophene-3-carboxamido)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10h).
The title compound 10h was prepared from compound 11a (0.3 g, 0.55 mmol) and 7h (0.66 mmol; 7h in situ preparation from 7h’ using procedure F) according to the general procedure G. The product 10l was obtained as an off-white solid (252 mg, 70%); 1H NMR (400 MHz, DMSO-d6) δ 11.89 (s, 1H), 10.78 (s, 1H), 9.09 (d, J = 8.3 Hz, 1H), 8.44 (d, J = 8.9 Hz, 1H), 8.23 (t, J = 5.6 Hz, 1H), 8.06 (dd, J = 1.5, 3.0 Hz, 1H), 8.00 (s, 1H), 7.75–7.65 (m, 3H), 7.39 (dd, J = 1.4, 5.1 Hz, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.18 (d, J = 2.3 Hz, 1H), 7.08–6.93 (m, 2H), 6.79 (t, J = 5.7 Hz, 1H), 4.76–4.67 (m, 1H), 3.30–3.25 (m, 1H), 3.18–3.07 (m, 3H), 3.05–2.98 (m, 2H), 1.35 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.5, 167.7, 160.6, 156.1, 138.8, 138.0, 136.5, 135.2, 131.5, 130.5, 128.5, 127.6, 126.3, 124.0, 122.6, 122.5, 121.3, 118.9, 118.6, 114.8, 111.8, 110.9, 78.1, 55.0, 28.6, 27.7; HRMS (ESI): m/z calcd for C30H32BrN5O5S [M + Na]+: 676.1200; found: 676.1205.
tert-butyl (S)-(2-(2-(2-([1,1′-biphenyl]-2-carboxamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10i).
The title compound 10i was prepared from compound 11a (0.3 g, 0.55 mmol) and 7i (0.66 mmol; 7i in situ preparation from 7i’ using procedure F) according to the general procedure G. The product 10i was obtained as an off-white solid (271 mg, 68%); 1H NMR (400 MHz, DMSO-d6) δ 11.28 (s, 1H), 10.81 (s, 1H), 8.89 (d, J = 7.9 Hz, 1H), 8.21 (d, J = 8.8 Hz, 1H), 8.14 (t, J = 5.7 Hz, 1H), 7.91 (s, 1H), 7.73–7.34 (m, 7H), 7.35–7.29 (m, 3H), 7.29–7.13 (m, 4H), 7.06 (t, J = 7.6 Hz, 1H), 7.02–6.94 (m, 1H), 6.75 (t, J = 5.8 Hz, 1H), 4.60–4.50 (m, 1H), 3.27–3.18 (m, 1H), 3.18–2.99 (m, 3H), 2.95 (t, J = 6.4 Hz, 2H), 1.35 (s, 9H);13C NMR (101 MHz, DMSO-d6) 171.5, 167.7, 167.0, 156.0, 140.1, 140.0, 138.3, 136.7, 136.5, 134.9, 131.3, 130.88, 130.8, 128.7, 128.6, 127.9, 127.7, 127.6, 124.0, 123.2, 122.6, 121.3, 118.8, 118.7, 115.1, 111.8, 110.8, 78.1, 55.0, 28.6, 27.7; HRMS (ESI): m/z calcd for C38H38BrN5O5 [M + Na]+: 746.1949; found: 746.1953.
tert-butyl (S)-(2-(2-(2-([1,1′-biphenyl]-3-carboxamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10j).
The title compound 10j was prepared from compound 11a (0.3 g, 0.55 mmol) and 7j (0.66 mmol; 7j in situ preparation from 7j’ using procedure F) according to the general procedure G. The product 10j was obtained as an off-white solid (258 mg, 65%); 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 10.79 (s, 1H), 9.11 (d, J = 8.1 Hz, 1H), 8.52 (d, J = 9.0 Hz, 1H), 8.22 (t, J = 5.9 Hz, 1H), 8.11 (s, 1H), 8.01 (d, J = 2.3 Hz, 1H), 7.91 (dt, J = 1.4, 7.6 Hz, 1H), 7.82–7.65 (m, 5H), 7.62 (t, J = 7.8 Hz, 1H), 7.55–7.46 (m, 2H), 7.46–7.38 (m, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.19 (s, 1H), 7.06–6.98 (m, 1H), 6.98–6.90 (m, 1H), 6.80–6.72 (m, 1H), 4.75–4.65 (m, 1H), 3.31–3.25 (m, 1H), 3.19–3.02 (m, 3H), 3.02–2.93 (m, 2H), 1.33 (s, 9H);13C δ C (101 MHz, DMSO-d6) 171.5, 167.7, 164.9, 156.1, 141.2, 139.7, 138.8, 136.5, 135.5, 135.2, 131.6, 130.8, 130.0, 129.6, 129.3, 128.4, 127.6, 127.2, 126.2, 125.8, 124.0, 123.0, 122.7, 121.3, 118.8, 118.6, 115.11, 111.8, 110.9, 78.1, 55.1, 28.6, 27.7; HRMS (ESI): m/z calcd for C38H38BrN5O5 [M + Na]+: 746.1949; found: 746.1953.
tert-butyl (S)-(2-(2-(2-([1,1′-biphenyl]-4-carboxamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (10k).
The title compound 10k was prepared from compound 6a (0.3 g, 0.55 mmol) and 7k (0.66 mmol; 7k in situ preparation from 7k’ using procedure F) according to the general procedure G. The product 10k was obtained as an off-white solid (282 mg, 71%); 1H NMR (400 MHz, DMSO-d6) δ 12.13 (s, 1H), 10.80 (s, 1H), 9.14 (d, J = 8.1 Hz, 1H), 8.56 (d, J = 9.0 Hz, 1H), 8.27 (t, J = 5.8 Hz, 1H), 8.03 (s, 1H), 7.92–7.84 (m, 2H), 7.82 (d, J = 8.6 Hz, 2H), 7.78–7.67 (m, 4H), 7.51 (dd, J = 6.7, 8.3 Hz, 2H), 7.47–7.39 (m, 1H), 7.31–7.24 (m, 1H), 7.20 (d, J = 2.4 Hz, 1H), 7.06–6.93 (m, 2H), 6.80 (t, J = 5.8 Hz, 1H), 4.77–4.66 (m, 1H), 3.31–3.26 (m, 1H), 3.20–3.09 (m, 3H), 3.02 (q, J = 6.5 Hz, 2H), 1.32 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.15, 167.8, 164.6, 156.1, 144.1, 139.3, 138.9, 136.5, 135.3, 132.9, 131.1, 129.1, 128.3, 127.6, 127.2, 127.1, 127.0, 123.6, 122.2, 122.1, 120.9, 118.5, 118.2, 114.5, 111.3, 110.5, 77.7, 54.7, 28.2, 27.3; HRMS (ESI): m/z calcd for C38H38BrN5O5 [M + Na]+: 746.1949; found: 746.1954.
tert-butyl (S)-(3-(2-(2-(2-naphthamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (11a).
The title compound 11a was prepared from compound 6b (0.3 g, 0.54 mmol) and 7a (0.66 mmol; 7a in situ preparation from 7a’ using procedure F) according to the general procedure G. The product 11a was obtained as an off-white solid (230 mg, 60%); 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 10.80 (s, 1H), 9.17 (d, J = 8.0 Hz, 1H), 8.56 (d, J = 8.9 Hz, 1H), 8.43 (d, J = 2.0 Hz, 1H), 8.18 (t, J = 5.8 Hz, 1H), 8.09–7.98 (m, 4H), 7.84 (dd, J = 2.0, 8.7 Hz, 1H), 7.76 (dd, J = 2.3, 9.0 Hz, 1H), 7.73–7.58 (m, 3H), 7.30–7.23 (m, 1H), 7.21 (s, 1H), 7.05–6.93 (m, 2H), 6.72 (t, J = 5.8 Hz, 1H), 4.77–4.68 (m, 1H), 3.30–3.25 (m, 1H), 3.21–3.04 (m, 3H), 2.90 (q, J = 6.2, 6.7 Hz, 2H), 1.50 (p, J = 6.8 Hz, 2H), 1.32 (s, 9H); 13C NMR (100 MHz, DMSO-d6) 171.3, 167.8, 165.0, 156.0, 138.9, 136.5, 135.2, 134.9, 132.6, 132.0, 131.5, 129.5, 129.1, 128.6, 128.4, 128.1, 127.6, 127.5, 124.0, 123.6, 122.9, 122.8, 121.3, 118.9, 118.7, 115.0, 111.8, 110.9, 77.9, 55.2, 37.8, 36.8, 29.9, 28.6, 27.7; HRMS (ESI): m/z calcd for C37H38BrN5O5 [M + Na]+: 734.1949; found: 734.1952.
tert-butyl (S)-(3-(2-(5-bromo-2-(2-(naphthalen-1-yl)acetamido)benzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (11b).
The title compound 11b was prepared from compound 6b (0.3 g, 0.54 mmol) and 7b (0.66 mmol; 7b in situ preparation from 7b’ using procedure F) according to the general procedure G. The product 11b was obtained as an off-white solid (263 mg, 67%); 1H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H), 10.82 (s, 1H), 8.90 (d, J = 8.0 Hz, 1H), 8.19 (d, J = 8.9 Hz, 1H), 8.10 (t, J = 5.8 Hz, 1H), 8.05–7.97 (m, 1H), 7.95–7.87 (m, 1H), 7.87–7.77 (m, 2H), 7.69 (d, J = 7.5 Hz, 1H), 7.60 (dd, J = 2.4, 8.9 Hz, 1H), 7.54–7.30 (m, 5H), 7.16 (s, 1H), 7.11–7.04 (m, 1H), 7.04–6.96 (m, 1H), 6.76 (t, J = 5.8 Hz, 1H), 4.81–4.48 (m, 1H), 4.10 (d, J = 8.5 Hz, 2H), 3.29–3.20 (m, 1H), 3.14–3.00 (m, 3H), 2.97–2.87 (m, 2H), 1.50 (p, J = 6.3 Hz, 2H), 1.35 (s, 9H);13C NMR δ (101 MHz, DMSO-d6) 171.5, 169.7, 167.0, 156.1, 137.9, 136.5, 134.6, 133.8, 132.3, 131.8, 131.2, 128.9, 128.7, 128.0, 127.7, 126.7, 126.2, 126.0, 124.4, 124.0, 1202.8, 121.4, 118.9, 118.7, 114.8, 111.8, 110.9, 77.9, 60.2, 54.9, 42.2, 37.8, 36.8, 29.5, 28.7; HRMS (ESI): m/z calcd for C38H40BrN5O5 [M + Na]+: 748.2105; found: 748.2105.
tert-butyl (S)-(3-(2-(5-bromo-2-(2-methoxy-1-naphthamido)benzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (11c).
The title compound 11c was prepared from compound 6b (0.3 g, 0.54 mmol) and 7c (0.66 mmol; 7c in situ preparation from 7c’ using procedure F) according to the general procedure G. The product 11c was obtained as an off-white solid (280 mg, 70%); 1H NMR (400 MHz, DMSO-d6) δ 11.53 (s, 1H), 10.79 (s, 1H), 9.02 (d, J = 8.0 Hz, 1H), 8.60 (d, J = 9.0 Hz, 1H), 8.10–7.97 (m, 3H), 7.92 (dd, J = 1.5, 8.1 Hz, 1H), 7.83–7.73 (m, 2H), 7.63 (d, J = 7.7 Hz, 1H), 7.54–7.43 (m, 2H), 7.43–7.35 (m, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.16 (s, 1H), 7.08–6.99 (m, 1H), 6.99–6.91 (m, 1H), 4.63–4.45 (m, 1H), 3.78 (s, 3H), 3.20 (dd, J = 4.4, 14.3 Hz, 1H), 3.09 (dd, J = 10.1, 14.6 Hz, 1H), 3.05–2.89 (m, 2H), 2.87–2.66 (m, 2H), 1.38 (s, 1H), 1.43–1.37 (m, 2H), 1.35 (s, 9H);13C NMR (101 MHz, DMSO-d6) 171.6, 170.8, 167.2, 165.4, 156.0, 153.8, 138.6, 136.5, 135.2, 131.9, 131.5, 131.0, 128.66, 128.6, 128.0, 127.6, 124.5, 124.0, 122.9, 122.7, 121.3, 120.4, 118.8, 118.6, 115.0, 114.2, 111.7, 110.8, 78.1, 60.2, 56.8, 54.9, 37.3, 36.2, 28.6, 27.7, 21.2; HRMS (ESI): m/z calcd for C38H40BrN5O6 [M + Na]+: 764.2057; found: 764.2054.
tert-butyl (S)-(3-(2-(5-bromo-2-(3-methoxy-2-naphthamido)benzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (11d).
The title compound 11d was prepared from compound 6b (0.3 g, 0.54 mmol) and 7d (0.66 mmol; 7d in situ preparation from 7d’ using procedure F) according to the general procedure G. The product 11d was obtained as an off-white solid (270 mg, 65%); 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 10.79 (s, 1H), 8.99 (d, J = 8.2 Hz, 1H), 8.65 (d, J = 9.1 Hz, 1H), 8.57 (s, 1H), 8.13 (t, J = 5.8 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 8.3 Hz, 1H), 7.82 (d, J = 2.5 Hz, 1H), 7.75–7.67 (m, 2H), 7.58 (t, J = 7.6 Hz, 1H), 7.46 (s, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.26–7.16 (m, 2H), 7.05–6.92 (m, 2H), 6.78–6.70 (m, 1H), 4.69 (td, J = 4.4, 8.6, 9.2 Hz, 1H), 3.88 (s, 3H), 3.30–3.21 (m, 1H), 3.18–3.01 (m, 3H), 2.93–2.84 (m, 2H), 1.48 (p, J = 7.4 Hz, 2H), 1.35 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.6, 167.0, 163.5, 156.0, 154.5, 137.9, 136.5, 136.1,134.5, 133.3, 131.5, 129.3, 128.9, 127.9, 127.6, 125.2, 124.9, 124.0, 123.7, 123.5, 121.3, 118.8, 118.6, 114.8, 111.1, 110.9, 107.3, 77.9, 65.3, 55.9, 55.1, 37.4, 36.3, 29.9, 28.7, 27.9; HRMS (ESI): m/z calcd for C38H40BrN5O6 [M + Na]+: 764.2054; found: 764.2059.
tert-butyl (S)-(3-(2-(5-bromo-2-(quinoline-2-carboxamido)benzamido)-3-(1H-indol-3-yl)propanamido)propyl)carbamate (11e).
The title compound 11e was prepared from compound 11e (0.3 g, 0.54 mmol) and 7e (0.66 mmol; 7e in situ preparation from 7e’ using procedure F) according to the general procedure G. The product 11e was obtained as an off-white solid (265 mg, 69%); 1H NMR (400 MHz, DMSO-d6) δ 12.95 (s, 1H), 10.76 (s, 1H), 9.03 (d, J = 7.9 Hz, 1H), 8.70 (d, J = 9.0 Hz, 1H), 8.63 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 8.5 Hz, 1H), 8.17 (t, J = 5.8 Hz, 1H), 8.10 (dd, J = 8.2, 14.7 Hz, 2H), 7.99 (s, 1H), 7.90 (t, J = 7.2 Hz, 1H), 7.80–7.70 (m, 3H), 7.28–7.20 (m, 2H), 6.97 (dt, J = 6.8, 20.7 Hz, 2H), 6.68 (t, J = 5.5 Hz, 1H), 4.78 (td, J = 5.2, 9.7 Hz, 1H), 3.30–3.24 (m, 1H), 3.24–3.14 (m, 1H), 3.13–3.02 (m, 2H), 2.89–2.80 (m, 2H), 1.46 (q, J = 7.0 Hz, 2H), 1.33 (s, 9H);13C NMR (100 MHz, DMSO-d6) 171.5, 167.1, 163.1, 156.0, 149.8, 146.2, 138.7, 137.9, 136.5, 135.0, 131.7, 131.1, 129.8, 128.9, 128.5, 127.7, 124.0, 123.9, 122.3, 121.3, 119.0, 118.9, 118.9, 118.6, 115.1, 111.7, 110.9, 77.9, 55.1, 37.2, 36.7, 29.9, 28.6, 27.8; HRMS (ESI): m/z calcd for C36H37BrN6O5 [M + Na]+: 735.1901; found: 735.1905.
The analytical data for
12a were already published [
20].
(S)-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-5-bromo-2-(2-(naphthalen-1-yl)acetamido)benzamide (12b).
The title compound 12b was prepared from compound 10c (0.1 g, 0.14 mmol) according to the general procedure H. The product 12b was obtained as gummy solid (0.053 g, 64%); 1H NMR (400 MHz, DMSO-d6) δ 10.86 (s, 2H), 8.91 (d, J = 8.0 Hz, 1H), 8.29 (t, J = 5.8 Hz, 1H), 8.18 (d, J = 9.0 Hz, 1H), 8.03–7.96 (m, 1H), 7.91 (dt, J = 3.0, 8.6 Hz, 1H), 7.88–7.77 (m, 5H), 7.66 (d, J = 7.8 Hz, 1H), 7.61 (dd, J = 2.4, 8.9 Hz, 1H), 7.55–7.46 (m, 2H), 7.43 (t, J = 7.6 Hz, 1H), 7.39–7.32 (m, 2H), 7.14 (d, J = 2.4 Hz, 1H), 7.08 (t, J = 7.0 Hz, 1H), 7.00 (t, J = 7.4 Hz, 1H), 4.70–4.60 (m, 1H), 4.18–3.99 (m, 2H), 3.32–3.27 (m, 2H), 3.19–3.03 (m, 2H), 2.87–2.78 (m, 2H);13C NMR δ C (101 MHz, DMSO-d6) 172.3, 169.8, 167.1, 137.8, 136.5, 134.7, 133.8, 132.3, 132.3, 131.7, 131.2, 128.9, 128.7, 128.0, 127.7, 126.7, 126.2, 124.4, 124.3, 124.1, 122.9, 121.4, 118.9, 118.7, 114.9, 111.8, 110.7, 54.8, 46.1, 42.2, 38.4, 36.6, 27.6; HRMS (ESI): m/z calcd for C32H30BrN5O3 [M + H]+: 612.1605; found: 612.1606.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-2-methoxy-1-naphthamide (12c).
The title compound 12c was prepared from compound 10c (0.1 g, 0.137 mmol) according to the general procedure H. The product 12c was obtained as off-white solid (0.051 g, 60%); 1H NMR (600 MHz, DMSO-d6) δ 10.82 (s, 1H), 9.13 (d, J = 8.1 Hz, 1H), 8.58 (d, J = 8.9 Hz, 1H), 8.28 (t, J = 5.6 Hz, 1H), 8.06 (d, J = 9.2 Hz, 1H), 7.99 (s, 1H), 7.92 (dd, J = 1.3, 8.2 Hz, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.77 (dd, J = 2.4, 8.9 Hz, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.54–7.45 (m, 2H), 7.43–7.36 (m, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.17 (s, 1H), 7.06–7.00 (m, 1H), 6.98–6.91 (m, 1H), 4.60–4.54 (m, 1H), 3.79 (s, 3H), 3.24–3.19 (m, 1H), 3.12–3.06 (m, 3H), 2.57 (t, J = 6.8 Hz, 2H);13C NMR (150 MHz, DMSO-d6) 171.7, 167.2, 166.4, 165.4, 153.8, 138.5, 136.4, 135.1, 131.9, 131.5, 131.0, 128.6, 128.60, 128.0, 127.6, 124.5, 124.0, 123.2, 122.8, 121.3, 120.4, 118.8, 118.7, 115.1, 114.3, 111.8, 110.8, 56.8, 55.0, 27.2; HRMS (ESI): m/z calcd for C32H30BrN5O4 [M + H]+: 628.1554; found: 628.1557.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-3-methoxy-2-naphthamide (12d).
The title compound 12d was prepared from compound 10d (0.1 g, 0.137 mmol) according to the general procedure H. The product 12d was obtained as off-white solid (0.054 g, 63%); 1H NMR (600 MHz, DMSO-d6) δ 10.80 (s, 1H), 9.09 (s, 1H), 8.63 (d, J = 8.9 Hz, 1H), 8.57 (s, 1H), 8.43 (s, 2H), 8.00 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 2.5 Hz, 1H), 7.73–7.67 (m, 2H), 7.58 (ddd, J = 1.4, 6.8, 8.2 Hz, 1H), 7.48 (s, 1H), 7.43 (ddd, J = 1.4, 6.8, 8.1 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 7.03–6.92 (m, 2H), 4.75–4.68 (m, 1H), 3.89 (s, 3H), 3.30–3.07 (m, 6H), 2.67 (td, J = 2.6, 6.4 Hz, 2H); HRMS (ESI): m/z calcd for C32H30BrN5O4 [M + H]+: 628.1554; found: 628.1555.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)quinoline-2-carboxamide (12e).
The title compound 12e was prepared from compound 10h (0.1 g, 0.14 mmol) according to the general procedure H. The product 12e was obtained as gummy liquid (0.055 g, 64%); 1H NMR (600 MHz, DMSO-d6) δ 12.93 (s, 1H), 10.76 (s, 1H), 9.05 (d, J = 7.8 Hz, 1H), 8.70 (d, J = 8.9 Hz, 1H), 8.65 (d, J = 8.5 Hz, 1H), 8.37 (t, J = 5.8 Hz, 1H), 8.26 (d, J = 8.5 Hz, 1H), 8.13 (dd, J = 1.6, 8.2 Hz, 1H), 8.12–8.05 (m, 1H), 8.00 (d, J = 2.5 Hz, 1H), 7.94–7.87 (m, 1H), 7.82–7.75 (m, 3H), 7.75 (s, 2H), 7.70 (d, J = 7.8 Hz, 1H), 7.28–7.18 (m, 2H), 6.98 (ddd, J = 1.3, 6.9, 8.2 Hz, 1H), 6.92 (td, J = 1.1, 7.0, 7.5 Hz, 1H), 4.87–4.79 (m, 1H), 3.31–3.17 (m, 3H), 2.83 (t, J = 6.8 Hz, 2H);13C NMR (150 MHz, DMSO-d6) 1172.2, 167.1, 163.1, 158.1, 149.8, 146.2, 138.8, 137.9, 136.5, 135.1, 131.8, 131.2, 129.8, 128.6, 127.7, 124.0, 123.9, 122.4, 121.3, 119.0, 118.8, 118.6, 115.1, 111.7, 110.7, 54.9, 38.4, 36.6, 27.5; HRMS (ESI): m/z calcd for C30H27BrN6O3 [M + H]+: 599.1401; found: 599.1403.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-1H-indole-2-carboxamide (12f).
The title compound 12f was prepared from compound 10f (0.1 g, 0.15 mmol) according to the general procedure H. The product 12f was obtained as gummy liquid (0.057 g, 67%); 1H NMR (400 MHz, DMSO-d6) δ 12.21 (s, 1H), 11.90 (s, 1H), 10.82 (s, 1H), 9.17 (d, J = 8.1 Hz, 1H), 8.56 (d, J = 8.9 Hz, 1H), 8.41 (t, J = 5.8 Hz, 1H), 8.08 (s, 1H), 7.82 (s, 2H), 7.80–7.60 (m, 4H), 7.47 (d, J = 8.3 Hz, 1H), 7.33–7.16 (m, 3H), 7.15–6.95 (m, 3H), 6.89 (s, 1H), 4.86–4.76 (m, 1H), 3.37 (dd, J = 4.3, 8.5 Hz, 3H), 3.23–3.12 (m, 1H), 2.86 (t, J = 6.8 Hz, 2H);13C NMR (100 MHz, DMSO-d6) 172.1, 167.8, 159.6, 158.3, 139.0, 137.6, 136.5, 135.5, 131.8, 131.6, 127.6, 127.3, 124.6, 124.0, 122.3, 122.2, 121.7, 121.4, 120.7, 118.7, 114.6, 113.0, 111.8, 110.7, 103.3, 54.9, 39.9, 37.1, 27.6; HRMS (ESI): m/z calcd for C29H27BrN6O3 [M + H]+: 587.1401; found: 587.1407.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)thiophene-2-carboxamide (12g).
The title compound 12g was prepared from compound 10g (0.1 g, 0.15 mmol) according to the general procedure H. The product 12g was obtained as off-white solid (0.058 g, 69%); 1H NMR (400 MHz, DMSO-d6) δ 12.01 (s, 1H), 10.81 (s, 1H), 9.14 (d, J = 8.0 Hz, 1H), 8.45–8.31 (m, 2H), 8.01 (s, 1H), 7.91 (dd, J = 1.2, 5.0 Hz, 1H), 7.83 (s, 2H), 7.73 (dd, J = 2.3, 8.9 Hz, 1H), 7.67 (d, J = 7.5 Hz, 1H), 7.52 (dd, J = 1.2, 3.8 Hz, 1H), 7.27 (d, J = 7.9 Hz, 1H), 7.24–7.15 (m, 2H), 7.07–6.93 (m, 2H), 4.80–4.70 (m, 1H), 3.39–3.33 (m, 5H), 3.20–3.09 (m, 1H), 2.85 (t, J = 6.9 Hz, 2H);13C NMR (100 MHz, DMSO-d6) 1172.1, 167.7, 159.7, 139.8, 138.5, 136.5, 135.3, 133.0, 131.5, 129.1, 128.9, 127.6, 124.0, 122.7, 121.4, 118.8, 118.7, 115.0, 111.8, 110.7, 54.9, 339.9, 38.8, 27.5; HRMS (ESI): m/z calcd for C25H24BrN5O3S [M + H]+: 554.0856; found: 554.0860.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)thiophene-3-carboxamide (12h).
The title compound 12h was prepared from compound 10h (0.1 g, 0.15 mmol) according to the general procedure H. The product 12h was obtained as off-white solid (0.055 g, 67%); 1H NMR (400 MHz, DMSO-d6) δ 11.83 (s, 1H), 10.81 (s, 1H), 9.12 (d, J = 8.1 Hz, 1H), 8.42 (d, J = 8.9 Hz, 1H), 8.35 (t, J = 5.7 Hz, 1H), 8.06 (dd, J = 1.4, 3.0 Hz, 1H), 7.99 (d, J = 2.3 Hz, 1H), 7.82 (s, 2H), 7.76–7.57 (m, 4H), 7.38 (s, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.18 (s, 1H), 7.06–6.93 (m, 2H), 4.80–4.70 (m, 1H), 3.51–3.35 (m, 2H), 3.25–3.04 (m, 2H), 2.84 (t, J = 6.8 Hz, 2H);13C NMR (100 MHz, DMSO-d6) 172.1, 167.7, 160.7, 138.7, 138.0, 136.5, 135.3, 131.5, 130.5, 128.5, 127.6, 126.3, 124.0, 122.7, 121.4, 118.8, 114.9, 111.8, 110.7, 54.9, 39.9, 38.8, 27.5;.; HRMS (ESI): m/z calcd for C25H24BrN5O3S [M + H]+: 554.0856; found: 554.0859.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-[1,1′-biphenyl]-2-carboxamide (12i).
The title compound 12i was prepared from compound 10m (0.1 g, 0.14 mmol) according to the general procedure H. The product 12i was obtained as gummy solid (0.058 g, 67%); 1H NMR (600 MHz, DMSO-d6) δ 11.22 (s, 1H), 10.85 (d, J = 2.5 Hz, 1H), 8.93 (d, J = 7.8 Hz, 1H), 8.25 (t, J = 5.7 Hz, 1H), 8.16 (d, J = 8.9 Hz, 1H), 7.89 (d, J = 2.3 Hz, 1H), 7.85–7.71 (m, 3H), 7.70–7.42 (m, 7H), 7.37–7.19 (m, 7H), 7.16 (d, J = 2.5 Hz, 1H), 7.06 (ddd, J = 1.4, 6.9, 8.2 Hz, 1H), 6.98 (td, J = 1.2, 7.0, 7.5 Hz, 1H), 4.62–4.54 (m, 1H), 3.31–3.20 (m, 3H), 3.10 (dd, J = 9.8, 14.8 Hz, 1H), 2.77 (t, J = 6.9 Hz, 2H).; 13C NMR (150 MHz, DMSO-d6) 172.1, 167.8, 167.1, 158.5, 158.3, 140.1, 140.0, 138.2, 136.7, 136.5, 131.3, 130.9, 130.8, 128.7, 128.0, 127.8, 127.6, 124.0, 123.5, 122.8, 121.4, 118.8, 118.7, 115.2, 111.8, 110.7, 65.3, 54.9, 39.5, 37.0, 27.5; HRMS (ESI): m/z calcd for C33H30BrN5O3 [M + H]+: 624.1605; found: 624.1607.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-[1,1′-biphenyl]-3-carboxamide (12j).
The title compound 12j was prepared from compound 10j (0.1 g, 0.14 mmol) according to the general procedure H. The product 12j was obtained as off-white solid (0.061 g, 70%); 1H NMR (400 MHz, DMSO-d6) δ 12.09 (s, 1H), 10.82 (s, 1H), 9.14 (d, J = 8.0 Hz, 1H), 8.50 (d, J = 9.0 Hz, 1H), 8.34 (q, J = 6.6, 7.4 Hz, 1H), 8.12 (s, 1H), 8.01 (s, 1H), 7.92 (dt, J = 1.4, 7.7 Hz, 1H), 7.82–7.68 (m, 6H), 7.68–7.59 (m, 2H), 7.51 (dd, J = 6.7, 8.4 Hz, 2H), 7.47–7.38 (m, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.18 (s, 1H), 7.06–6.98 (m, 1H), 6.98–6.90 (m, 1H), 4.79–4.69 (m, 1H), 3.36–3.11 (m, 4H), 2.82 (q, J = 5.7, 6.1 Hz, 2H);13C NMR (100 MHz, DMSO-d6) 1172.1, 167.7, 164.9, 141.2, 139.6, 138.7, 136.5, 135.5, 135.3, 131.6, 130.8, 130.1, 129.6, 128.4, 127.6, 127.3, 126.2, 125.8, 124.0, 118.8, 118.7, 115.2, 111.8, 110.8, 54.9, 42.2, 37.0, 27.5; HRMS (ESI): m/z calcd for C33H30BrN5O3 [M + H]+: 624.1605; found: 624.1606.
(S)-N-(2-((1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-[1,1′-biphenyl]-4-carboxamide (12k).
The title compound 12k was prepared from compound 10k (0.1 g, 0.14 mmol) according to the general procedure H. The product 12k was obtained as off-white solid (0.060 g, 70%); 1H NMR (600 MHz, DMSO-d6) δ 12.06 (s, 1H), 10.82 (s, 1H), 9.16 (d, J = 8.1 Hz, 1H), 8.54 (d, J = 8.9 Hz, 1H), 8.38 (t, J = 5.7 Hz, 1H), 8.02 (s, 1H), 7.90–7.73 (m, 11H), 7.92–7.72 (m, 11H), 7.67 (d, J = 7.8 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.19 (s, 1H), 7.00 (dt, J = 7.1, 29.3 Hz, 2H), 4.80–4.74 (m, 1H), 3.41–3.35 (m, 3H), 3.19–3.12 (m, 1H), 2.85 (t, J = 7.2 Hz, 2H);13C NMR (75 MHz, CDCl3): δ δ C (151 MHz, DMSO-d6) 1172.1, 167.8, 164.6, 158.5, 158.3, 144.1, 139.3, 138.8, 136.5, 135.3, 133.4, 131.5, 129.5, 128.8, 128.1, 127.6, 127.67, 127.6, 127.4, 124.0, 122.8, 122.7, 121.3, 118.8, 118.7, 115.0, 111.8, 110.8, 54.9, 38.8, 37.1, 27.6; HRMS (ESI): m/z calcd for C25H24BrN5O3S [M + H]+: 624.1605; found: 624.1605.
(S)-N-(2-((1-((3-aminopropyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-2-naphthamide (13a).
The title compound 13a was prepared from compound 11a (0.1 g, 0.14 mmol) according to the general procedure H. The product 13a was obtained as gummy solid (0.052 g, 62%); 1H NMR (600 MHz, DMSO-d6) δ 12.18 (s, 1H), 10.82 (s, 1H), 9.20 (d, J = 8.1 Hz, 1H), 8.53 (d, J = 8.9 Hz, 1H), 8.44 (s, 1H), 8.39 (d, J = 6.0 Hz, 1H), 8.08–7.97 (m, 4H), 7.87–7.55 (m, 9H), 7.26 (d, J = 8.1 Hz, 1H), 7.21 (s, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 4.78–4.68 (m, 1H), 3.29–3.26 (m, 1H), 3.20–3.13 (m, 3H), 2.76–2.70 (m, 2H), 1.68 (p, J = 7.3 Hz, 2H); HRMS (ESI): m/z calcd for C32H30BrN5O3 [M + H]+: 612.1605; found: 612.1606.
(S)-N-(1-((3-aminopropyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-5-bromo-2-(2-(naphthalen-1-yl)acetamido)benzamide (13b).
The title compound 13b was prepared from compound 11b (0.1 g, 0.14 mmol) according to the general procedure H. The product 13b was obtained as pale brown solid (0.060 g, 69%); 1H NMR (600 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.93 (s, 1H), 8.41 (s, 1H), 8.27 (t, J = 5.9 Hz, 1H), 8.17 (d, J = 8.9 Hz, 1H), 8.03–7.98 (m, 1H), 7.94–7.86 (m, 1H), 7.85–7.78 (m, 2H), 7.69 (d, J = 7.8 Hz, 1H), 7.60 (dd, J = 2.3, 8.9 Hz, 1H), 7.53–7.46 (m, 2H), 7.46–7.37 (m, 2H), 7.34 (d, J = 8.1 Hz, 1H), 7.16 (s, 1H), 7.10–7.04 (m, 1H), 7.04–6.98 (m, 1H), 4.64–4.59 (m, 1H), 4.10 (d, J = 15.6 Hz, 2H), 3.20–3.13 (m, 2H), 3.13–3.07 (m, 2H), 2.65 (t, J = 7.1 Hz, 2H), 1.63–1.55 (m, 2H);13C NMR (δ C (151 MHz, DMSO-d6) 171.9, 169.8, 167.06, 166.0, 137.9, 136.5, 134.5, 133.8, 132.3, 131.8, 131.2, 128.9, 128.7, 128.0, 127.7, 126.7, 126.2, 126.0, 124.4, 124.4, 124.1, 122.9, 121.3, 118.9, 118.7, 114.8, 111.8, 110.8, 54.9, 42.1, 39.5, 27.7; HRMS (ESI): m/z calcd for C33H32BrN5O3 [M + H]+: 626.1761; found: 626.1763.
(S)-N-(2-((1-((3-aminopropyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-2-methoxy-1-naphthamide (13c).
The title compound 13c was prepared from compound 11c (0.1 g, 0.14 mmol) according to the general procedure H. The product 13c was obtained as off-white solid (0.060 g, 67%); 1H NMR (600 MHz, DMSO-d6) δ 10.86 (s, 1H), 9.18 (s, 1H), 8.58 (d, J = 8.9 Hz, 1H), 8.47 (s, 1H), 8.28 (t, J = 5.8 Hz, 1H), 8.06 (d, J = 9.1 Hz, 1H), 8.01 (d, J = 2.5 Hz, 1H), 7.94–7.90 (m, 1H), 7.80 (d, J = 8.5 Hz, 1H), 7.77 (dd, J = 2.5, 8.8 Hz, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 9.1 Hz, 1H), 7.47 (ddd, J = 1.4, 6.7, 8.4 Hz, 1H), 7.40 (ddd, J = 1.2, 6.7, 8.1 Hz, 1H), 7.29 (s, 1H), 7.17 (s, 1H), 7.06–7.01 (m, 1H), 6.95 (t, J = 7.4 Hz, 1H), 4.54 (dd, J = 4.9, 10.0 Hz, 1H), 3.77 (s, 3H), 3.23–3.18 (m, 1H), 3.14–2.96 (m, 4H), 2.54 (tt, J = 3.6, 7.3 Hz, 2H), 1.50 (p, J = 7.0 Hz, 2H);13C NMR (150 MHz, DMSO-d6) 171.9, 167.0, 166.2, 163.5,154.5, 137.9, 136.5, 136.1, 134.5, 133.3, 131.5, 129.3, 128.9, 127.9, 127.7, 126.8, 125.3, 124.9, 124.0, 123.7, 123.5, 121.3, 118.8, 118.6, 114.9, 111.7, 110.8, 107.3, 56.0, 55.1, 40.5, 27.9; HRMS (ESI): m/z calcd for C33H32BrN5O4 [M + H]+: 642.1710; found: 642.1713.
(S)-N-(2-((1-((3-aminopropyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)-3-methoxy-2-naphthamide (13d).
The title compound 13d was prepared from compound 12d (0.1 g, 0.14 mmol) according to the general procedure H. The product 13d was obtained as off-white solid (0.054 g, 60%); 1H NMR (600 MHz, DMSO-d6) δ 11.85 (s, 1H), 10.85 (s, 1H), 9.15 (d, J = 8.1 Hz, 1H), 8.64 (s, 1H), 8.57 (s, 1H), 8.47–8.41 (m, 2H), 8.00 (d, J = 7.8 Hz, 1H), 7.90–7.81 (m, 2H), 7.73–7.67 (m, 2H), 7.58 (ddd, J = 1.4, 6.8, 8.2 Hz, 1H), 7.47 (s, 1H), 7.42 (ddd, J = 1.2, 6.7, 8.1 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H), 7.19 (d, J = 2.3 Hz, 1H), 7.04–6.93 (m, 2H), 4.73–4.66 (m, 1H), 3.88 (s, 3H), 3.28–3.24 (m, 1H), 3.19–3.11 (m, 3H), 2.67 (t, J = 7.2 Hz, 2H), 1.66–1.61 (m, 2H).;13C NMR δ C (151 MHz, DMSO-d6) 172.0-, 167.0, 166.3, 163.5, 154.5, 137.9, 136.5, 136.1, 134.5, 133.3, 131.5, 129.3, 128.9, 127.9, 127.6, 126.8, 125.2, 124.9, 124.1, 123.7, 123.5, 121.3, 118.8, 118.7, 114.9, 111.8, 110.8, 107.3, 56.0, 55.2, 40.4, 37.3, 28.8, 28.0.; HRMS (ESI): m/z calcd for C33H32BrN5O4 [M + H]+: 642.1710; found: 642.1713.
(S)-N-(2-((1-((3-aminopropyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-4-bromophenyl)quinoline-2-carboxamide (13e).
The title compound 13e was prepared from compound 11e (0.1 g, 0.14 mmol) according to the general procedure H. The product 13e was obtained as gummy solid (0.054 g, 63%); 1H NMR (600 MHz, DMSO-d6) δ 12.93 (s, 1H), 10.77 (s, 1H), 9.07 (d, J = 7.8 Hz, 1H), 8.70 (d, J = 8.9 Hz, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.39 (t, J = 5.9 Hz, 1H), 8.25 (d, J = 8.4 Hz, 1H), 8.13 (dd, J = 1.5, 8.2 Hz, 1H), 8.10–8.04 (m, 1H), 8.01 (s, 1H), 7.91 (ddd, J = 1.5, 6.9, 8.4 Hz, 1H), 7.82–7.74 (m, 2H), 7.71 (d, J = 7.8 Hz, 1H), 7.66 (s, 2H), 7.28–7.21 (m, 2H), 7.04–6.92 (m, 2H), 4.79 (ddd, J = 5.2, 7.8, 9.8 Hz, 1H), 3.30–3.21 (m, 2H), 3.17 (q, J = 6.6 Hz, 2H), 2.78–2.63 (m, 2H), 1.67 (p, J = 7.0 Hz, 2H);13C NMR δ C (151 MHz, DMSO-d6) 172.2, 167.2, 163.1, 149.8, 146.2, 138.8, 137.9, 136.5, 135.0, 131.8, 131.1, 129.8, 129.5, 129.0, 128.6, 127.6, 124.0, 123.9, 122.4, 121.3, 119.0, 118.8, 118.6, 115.1, 111.8, 110.8, 55.0, 40.5, 37.1, 36.1, 27.8; HRMS (ESI): m/z calcd for C31H29BrN6O3 [M + H]+: 613.1557; found: 613.1560.
tert-butyl (S)-(2-(2-(4-amino-[1,1′-biphenyl]-3-carboxamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (15a).
The title compound 15a was prepared from compound 5a (0.25 g, 0.46 mmol) and 16a (0.084 g, 0.69 mmol) according to the general procedure I. The product 15a was obtained as an off-white solid (0.151 g, 61%); 1H NMR (300 MHz, DMSO-d6) δ 10.80 (s, 1H), 8.42 (d, J = 8.0 Hz, 1H), 8.12 (t, J = 5.9 Hz, 1H), 7.86–7.56 (m, 4H), 7.53–7.17 (m, 6H), 7.15–6.89 (m, 3H), 6.85–6.64 (m, 2H), 6.46 (s, 2H), 4.64 (dd, J = 4.9, 8.1 Hz, 1H), 3.30–3.09 (m, 4H), 3.09–2.98 (m, 2H), 1.37 (d, J = 1.5 Hz, 9H);13C NMR (75 MHz, DMSO-d6) 172.5, 169.0, 156.1, 148.9, 137.6, 136.5, 132.7, 132.5, 132.0, 131.9, 130.3, 129.2, 129.1, 127.8, 126.9, 125.9, 124.0, 121.2, 118.9, 118.6, 117.5, 111.7, 111.2, 78.1, 54.6, 39.3, 34.6, 27.9; HRMS (ESI): m/z calcd for C31H35N5O4 [M + H]+: 542.2762; found: 542.2765.
tert-butyl (S)-(2-(2-(4-amino-4′-(tert-butyl)-[1,1′-biphenyl]-3-carboxamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (15a).
The title compound 15a was prepared from compound 6a (0.25 g, 0.46 mmol) and 14a (0.122 g, 0.69 mmol) according to the general procedure I. The product 15a was obtained as an off-white solid (0.178 g, 65%); 1H NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.41 (d, J = 7.7 Hz, 1H), 8.12 (t, J = 5.9 Hz, 1H), 7.78 (s, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.69–7.51 (m, 5H), 7.49–7.41 (m, 3H), 7.30 (d, J = 8.1 Hz, 1H), 7.22 (s, 1H), 7.09–7.01 (m, 1H), 7.01–6.93 (m, 1H), 6.76 (d, J = 8.5 Hz, 2H), 4.64 (td, J = 5.0, 9.2 Hz, 1H), 3.18–2.97 (m, 6H), 1.36 (s, 9H), 1.32 (s, 9H);13C NMR (100 MHz, DMSO-d6) 172.5, 169.0, 156.1, 148.9, 137.6, 136.5, 132.7, 132.5, 132.0, 131.9, 130.3, 129.2, 129.1, 127.8, 126.9, 125.9, 124.0, 121.2, 118.9, 118.6, 117.5, 111.7, 111.2, 78.1, 54.6, 39.3, 34.6, 27.9.
tert-butyl (S)-(2-(2-(2-amino-5-(naphthalen-2-yl)benzamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (15b).
The title compound 15b was prepared from compound 6a (0.25 g, 0.46 mmol) and 14b (0.118 g, 0.69 mmol) according to the general procedure I. The product 15b was obtained as pale yellow solid (0.171 g, 63%); 1H NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.53–8.46 (m, 1H), 8.15 (s, 2H), 8.02–7.85 (m, 5H), 7.76 (d, J = 7.8 Hz, 1H), 7.65 (dd, J = 2.2, 8.6 Hz, 1H), 7.57–7.43 (m, 2H), 7.34–7.23 (m, 2H), 7.09–6.92 (m, 2H), 6.79 (dd, J = 7.1, 9.5 Hz, 2H), 6.53 (s, 2H), 4.67 (td, J = 5.1, 9.3 Hz, 1H), 3.25–2.99 (m, 6H), 1.37 (s, 9H);13C NMR (101 MHz, DMSO-d6) 172.6, 169.1, 156.1, 149.8, 137.9, 136.5, 133.9, 132.0, 130.7, 128.6, 128.3, 127.9, 127.8, 127.3, 126.7, 126.4, 125.8, 125.2, 124.0, 123.6, 121.2, 119.0, 118.6, 117.4, 114.9, 111.7, 111.3, 78.1, 65.3, 54.7, 39.3, 28.7, 27.9; HRMS (ESI): m/z calcd for C35H37N5O4 [M + Na]+: 614.2738; found: 614.2732.
tert-butyl (S)-(2-(2-(4-amino-4′-fluoro-[1,1′-biphenyl]-3-carboxamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (15c).
The title compound 15c was prepared from compound 6a (0.25 g, 0.46 mmol) and 14c (0.096 g, 0.69 mmol) according to the general procedure I. The product 15c was obtained as an off-white solid (0.167 g, 65%); 1H NMR (400 MHz, DMSO-d6) δ 10.79 (s, 1H), 8.41 (d, J = 8.3 Hz, 1H), 8.12 (t, J = 5.8 Hz, 1H), 7.93–7.55 (m, 5H), 7.73–7.61 (m, 4H), 7.44 (dd, J = 2.2, 8.5 Hz, 1H), 7.30–7.20 (m, 4H), 7.00 (dt, J = 7.4, 31.7 Hz, 3H), 6.78–6.70 (m, 2H), 6.44 (s, 2H), 4.67–4.61 (m, 1H), 3.20–3.10 (m, 4H), 3.03–2.96 (m, 2H), 1.36 (s, 9H); 13C NMR (100 MHz, DMSO-d6) 172.5, 169.1, 162.7, 160.3, 156.1, 149.5, 137.0, 136.4, 130.4, 127.9, 127.8, 127.0, 125.7, 124.0, 121.3, 118.9, 118.6, 117.2, 115.9, 115.7, 114.8, 111.7, 111.2, 79.6, 78.1, 54.6, 39.3, 28.6, 27.9; HRMS (ESI): m/z calcd for C31H34FN5O4 [M + Na]+: 582.2487; found: 582.2482.
tert-butyl (S)-(2-(2-(4-amino-4′-(trifluoromethyl)-[1,1′-biphenyl]-3-carboxamido)-3-(1H-indol-3-yl)propanamido)ethyl)carbamate (15d).
The title compound 15d was prepared from compound 5a (0.25 g, 0.46 mmol) and 15f (0.131 g, 0.69 mmol) according to the general procedure I. The product 15f was obtained as an off-white solid (0.168 g, 60%); 1H NMR (400 MHz, DMSO-d6) δ 10.80 (s, 1H), 8.47 (d, J = 8.3 Hz, 1H), 8.14 (t, J = 5.7 Hz, 1H), 7.89–7.82 (m, 3H), 7.75 (dd, J = 8.1, 19.7 Hz, 3H), 7.56 (dd, J = 2.2, 8.6 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.23 (s, 1H), 7.08–7.00 (m, 1H), 7.00–6.92 (m, 1H), 6.82–6.72 (m, 2H), 6.60 (s, 2H), 4.65 (td, J = 4.8, 9.8 Hz, 1H), 3.24–2.97 (m, 6H), 1.36 (s, 9H);13C NMR (100 MHz, DMSO-d6) 172.5, 168.9, 156.1, 150.4, 144.4, 136.5, 135.1, 130.6, 127.8, 127.7, 126.8, 126.5, 126.4, 126.0, 124.7, 124.1, 123.7, 121.3, 118.9, 118.6, 117.3, 114.9, 118.6, 117.3, 114.9, 111.7, 111.2, 78.1, 54.5, 39.3, 31.4, 28.6, 27.9; HRMS (ESI): m/z calcd for C32H34F3N5O4 [M + Na]+: 632.2455; found: 632.2451.
(S)-4-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-[1,1’-biphenyl]-3-carboxamide (16a).
The title compound 16a was prepared from compound 15a (0.1 g, 0.18 mmol) according to the general procedure H. The product 16a was obtained as off-white solid (0.054 g, 67%); 1H NMR (600 MHz, DMSO-d6) δ 10.86 (s, 1H), 8.84 (d, J = 8.1 Hz, 1H), 8.45 (q, J = 4.7, 5.2 Hz, 1H), 8.15–8.06 (m, 3H), 7.88 (s, 1H), 7.78–7.67 (m, 3H), 7.62 (dd, J = 2.2, 8.4 Hz, 1H), 7.47 (t, J = 7.7 Hz, 3H), 7.36–7.20 (m, 3H), 7.08–7.00 (m, 2H), 6.94 (t, J = 7.4 Hz, 1H), 4.73–4.69 (m, 1H), 3.42–3.31 (m, 3H), 3.25–3.17 (m, 1H), 2.91–2.83 (m, 2H);13C NMR (150 MHz, DMSO-d6) 172.2, 167.8, 139.4, 136.0, 132.0, 131.5, 130.1, 128.8, 128.7, 127.4, 126.9, 126.8, 126.1, 123.7, 123.6, 120.9, 120.8, 118.5, 118.2, 111.3, 110.7, 110.5, 54.4, 38.4, 36.6, 27.2; HRMS (ESI): m/z calcd for C26H27N5O2 [M + H]+: 442.2237; found: 442.2238.
(S)-4-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-4′-(tert-butyl)-[1,1′-biphenyl]-3-carboxamide (16a).
The title compound 16a was prepared from compound 15a (0.1 g, 0.17 mmol) according to the general procedure H. The product 16a was obtained as off-white solid (0.057 g, 69%); 1H NMR (400 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.80 (d, J = 8.1 Hz, 1H), 8.42 (t, J = 5.4 Hz, 1H), 8.06 (s, 4H), 7.87 (s, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.63–7.57 (m, 5H), 7.51–7.44 (m, 3H), 7.30 (d, J = 8.1 Hz, 1H), 7.24 (s, 1H), 7.06–6.93 (m, 4H), 4.71–4.66 (m, 1H), 3.34–3.29 (m, 2H), 3.24–3.15 (m, 2H), 2.88–2.83 (m, 2H), 1.33 (s, 9H);13C NMR (100 MHz, DMSO-d6) 172.7, 168.7, 149.5, 137.0, 136.5, 130.3, 129.3, 127.8, 127.1, 126.3, 126.0, 124.1, 121.2, 118.9, 118.7, 111.8, 111.1, 54.8, 39.3, 37.0, 27.7, 27.6; HRMS (ESI): m/z calcd for C30H35N5O2 [M + H]+: 498.2864; found: 498.2857.
(S)-2-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-5-(naphthalen-2-yl)benzamide (16b).
The title compound 16b was prepared from compound 15b (0.1 g, 0.17 mmol) according to the general procedure H. The product 16b was obtained as off-white solid (0.058 g, 70%); 1H NMR (400 MHz, DMSO-d6) δ 10.86 (s, 1H), 8.97 (d, J = 8.1 Hz, 1H), 8.50 (t, J = 5.5 Hz, 1H), 8.31–8.26 (m, 1H), 8.15 (s, 2H), 8.09 (s, 1H), 8.04–7.91 (m, 4H), 7.84–7.75 (m, 2H), 7.60–7.47 (m, 2H), 7.34–7.20 (m, 2H), 7.12 (d, J = 8.4 Hz, 1H), 7.07–6.97 (m, 1H), 6.97–6.88 (m, 1H), 4.78–4.70 (m, 1H), 3.43–3.26 (m, 4H), 2.89 (q, J = 6.1 Hz, 2H);13C NMR δ C (101 MHz, DMSO-d6) 172.6, 168.1, 137.1, 136.5, 133.8, 132.4, 130.7, 128.8, 128.5, 127.9, 127.8, 127.6, 126.8, 126.3, 125.3, 124.7, 124.1, 121.2, 120.6, 119.0, 118.6, 111.8, 111.2, 66.8, 55.0, 37.0, 27.7; HRMS (ESI): m/z calcd for C30H35N5O2 [M + H]+: 498.2394; found: 498.2390.
(S)-4-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-4′-fluoro-[1,1′-biphenyl]-3-carboxamide (16c).
The title compound 16c was prepared from compound 15c (0.1 g, 0.18 mmol) according to the general procedure H. The product 16c was obtained as off-white solid (0.053 g, 65%); 1H NMR (400 MHz, DMSO-d6) δ 10.86 (s, 1H), 8.91 (d, J = 8.4 Hz, 1H), 8.47 (t, J = 5.7 Hz, 1H), 8.14 (s, 2H), 7.85 (s, 1H), 7.81–7.65 (m, 3H), 7.62 (dd, J = 2.2, 8.4 Hz, 1H), 7.36–7.20 (m, 4H), 7.11–6.89 (m, 3H), 4.73–4.70 (m, 1H), 3.47–3.29 (m, 3H), 3.27–3.16 (m, 1H), 2.94–2.82 (m, 2H); 13C NMR (100 MHz, DMSO-d6) 172.6, 168.0, 163.2, 160.8, 136.5, 136.2, 130.4, 128.6, 128.5, 127.8, 127.3, 124.1, 121.2, 120.7, 118.9, 118.7, 116.1, 115.9, 111.8, 111.1, 66.8, 54.8, 40.6, 37.0, 27.7; HRMS (ESI): m/z calcd for C26H26FN5O2 [M + H]+: 460.2143; found: 460.2144.
(S)-4-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-4′-(trifluoromethyl)-[1,1′-biphenyl]-3-carboxamide (16d).
The title compound 16d was prepared from compound 15d (0.1 g, 0.16 mmol) according to the general procedure H. The product 16d was obtained as off-white solid (0.052 g, 62%); 1H NMR (400 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.76 (d, J = 8.2 Hz, 1H), 8.44 (t, J = 5.7 Hz, 1H), 8.11 (s, 3H), 8.02–7.88 (m, 3H), 7.86–7.57 (m, 5H), 7.46–7.20 (m, 3H), 7.12–6.88 (m, 4H), 4.72–4.68 (m, 1H), 3.42–3.29 (m, 3H), 3.21 (dd, J = 10.0, 14.6 Hz, 1H), 2.87 (h, J = 6.0 Hz, 2H);13C NMR δ C (101 MHz, DMSO-d6) 172.8, 168.5, 144.1, 136.5, 130.7, 127.89, 127.8, 127.1, 126.7, 126.3, 126.0, 126.05, 124.1, 123.6, 121.2, 118.9, 118.6, 118.68, 111.8, 111.1, 54.7, 40.6, 37.02, 27.8; HRMS (ESI): m/z calcd for C27H26F3N5O2 [M + H]+: 510.2108; found: 510.2111.
(S)-2-amino-N-(1-((2-aminoethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)-5-bromobenzamide (17).
The title compound 17 was prepared from compound 5a (0.1 g, 0.17 mmol) according to the general procedure H. The product 17 was obtained as gummy solid (0.064 g, 85%); 1H NMR (400 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.57 (d, J = 7.9 Hz, 1H), 8.39 (t, J = 5.7 Hz, 1H), 8.12 (s, 2H), 7.77 (d, J = 2.4 Hz, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.37–7.27 (m, 2H), 7.19 (d, J = 2.4 Hz, 1H), 7.05 (ddd, J = 1.4, 7.0, 8.2 Hz, 1H), 6.98 (ddd, J = 1.2, 6.9, 8.0 Hz, 1H), 6.75 (d, J = 8.8 Hz, 1H), 4.68–4.58 (m, 2H), 3.47–3.03 (m, 4H), 2.90–2.78 (m, 2H);13C NMR (100 MHz, DMSO-d6) 172.2, 167.1, 146.4, 136.0, 134.4, 130.8, 127.3, 123.6, 120.9, 119.6, 118.5, 118.2, 117.5, 111.4, 110.6, 107.1, 54.3, 38.4, 36.5, 27.2; HRMS (ESI): m/z calcd for C20H22BrN5O2 [M + H]+: 444.1030; found: 444.1037.
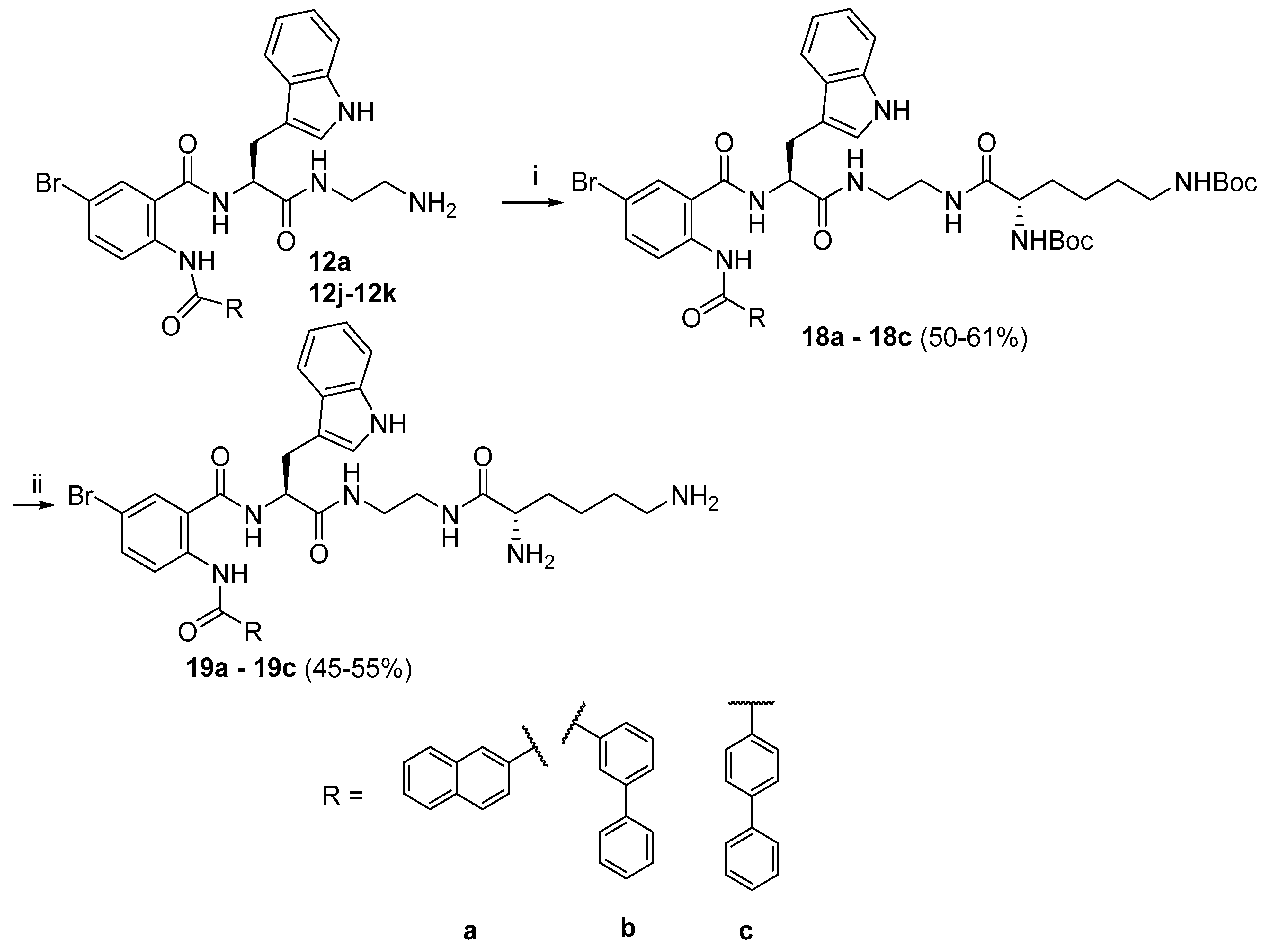
di-tert-butyl ((RS)-6-((2-((S)-2-(2-(2-naphthamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)amino)-6-oxohexane-1,5-diyl)dicarbamate (18a).
The title compound 18a was prepared from Boc-Lys(Boc)-Osu (0.142 g, 0.32 mmol) and 12a (0.2 g, 0.32 mmol) according to the general procedure J. The product 18a was obtained as an off-white solid (0.16 g, 55%); 1H NMR1H NMR (400 MHz, DMSO-d6): δ 12.19 (s, 1H), 10.80 (d, J = 1.5 Hz, 1H), 9.14 (d, J = 7.7 Hz, 1H), 8.56 (d, J = 8.9 Hz, 1H), 8.43 (s, 1H), 8.27 (bs, 1H), 8.08–7.99 (m, 4H), 7.91–7.82 (m, 2H), 7.77 (dd, J = 8.9, 2.3 Hz, 1H), 7.73–7.60 (m, 3H), 7.25 (d, J = 7.4 Hz, 1H), 7.20 (d, J = 2.3 Hz, 1H), 7.04–6.93 (m, 2H), 6.79–6.65 (m, 2H), 4.79–4.66 (m, 1H), 3.87–3.71 (m, 1H), 3.32– 3.26 (m, 1H), 3.23–3.02 (m, 5H), 2.91–2.78 (m, 2H), 1.49–1.11 (m, 24H); 13C NMR (100 MHz, DMSO-d6): δ 172.8, 171.6, 167.8, 165.1, 155.9, 138.9, 136.5, 135.3, 134.9, 132.6, 132.1, 131.6, 129.6, 129.1, 128.6, 128.4, 128.2, 127.7, 127.5, 124.0, 123.7, 123.1, 122.8, 121.4, 118.9, 118.7, 115.1, 111.8, 111.0, 78.4, 77.8, 55.1, 54.9, 39.0, 38.7, 33.5, 32.1, 29.7, 28.7, 28.6, 27.8, 23.3.; HRMS (ESI): m/z calcd for C49H58BrN7O8 [M + Na]+: 974.3422; found: 974.3420.
di-tert-butyl ((S)-6-((2-((S)-2-(2-([1,1′-biphenyl]-3-carboxamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)amino)-6-oxohexane-1,5-diyl)dicarbamate (18b).
The title compound 18b was prepared from Boc-Lys(Boc)-Osu (0.142 g, 0.32 mmol) and 12j (0.2 g, 0.32 mmol) according to the general procedure J. The product 18b was obtained as an off-white solid (0.15 g, 56%); 1H NMR (400 MHz, DMSO-d6) δ 12.13 (s, 1H), 10.79 (s, 1H), 9.12 (d, J = 8.1 Hz, 1H), 8.52 (d, J = 8.9 Hz, 1H), 8.24 (s, 1H), 8.11 (s, 1H), 8.02 (s, 1H), 7.88 (q, J = 5.8, 7.5 Hz, 2H), 7.79–7.65 (m, 5H), 7.61 (t, J = 7.7 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.42 (dd, J = 6.2, 8.5 Hz, 1H), 7.26 (d, J = 7.9 Hz, 1H), 7.19 (s, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.94 (t, J = 7.5 Hz, 1H), 6.75–6.68 (m, 2H), 4.74–4.64 (m, 1H), 3.85–3.71 (m, 1H), 3.30–2.97 (m, 6H), 2.85 (d, J = 6.5 Hz, 2H), 1.66–1.42 (m, 2H), 1.35 (s, 18H), 1.31–1.16 (m, 4H);13C NMR (100 MHz, DMSO-d6) 172.8, 171.5, 167.7,164.9, 156.0, 155.7, 141.2, 139.7, 138.7, 136.5, 135.5, 135.2, 131.6, 130.8, 130.0, 129.6, 128.4, 127.6, 126.2, 125.8, 124.0, 123.1, 122.7, 121.3, 118.9, 118.7, 115.1, 111.7, 110.9, 78.4, 77.7, 55.1, 54.8, 39.9, 38.6, 29.6, 28.7, 28.6, 27.7, 23.2; HRMS (ESI): m/z calcd for C49H58BrN7O8 [M + Na]+: 974.3422; found: 974.3420.
Di-tert-butyl ((S)-6-((2-((S)-2-(2-([1,1′-biphenyl]-4-carboxamido)-5-bromobenzamido)-3-(1H-indol-3-yl)propanamido)ethyl)amino)-6-oxohexane-1,5-diyl)dicarbamate (18c).
The title compound 18c was prepared from Boc-Lys(Boc)-Osu (0.142 g, 0.32 mmol) and 12k (0.2 g, 0.32 mmol) according to the general procedure J. The product 18c was obtained as an off-white solid (0.15 g, 50%);
1H NMR (400 MHz, DMSO-d6): δ 12.12 (s, 1H), 10.80 (d, J = 1.8 Hz, 1H), 9.14 (d, J = 8.0 Hz, 1H), 8.57 (d, J = 9.0 Hz, 1H), 8.28 (bs, 1H), 8.04 (d, J = 2.4 Hz, 1H), 7.93–7.80 (m, 5H), 7.79–7.73 (m, 3H), 7.70 (d, J = 7.7 Hz, 1H), 7.55–7.49 (m, 2H), 7.47–7.41 (m, 1H), 7.26 (d, J = 7.6 Hz, 1H), 7.20 (d, J = 2.2 Hz, 1H), 7.06–6.94 (m, 2H), 6.79–6.65 (m, 2H), 4.79–4.67 (m, 1H), 3.88–3.75 (m, 1H), 3.36– 3.27 (m, 1H), 3.21–3.08 (m, 5H), 2.90–2.79 (m, 2H), 1.49–1.13 (m, 24H); 13C NMR (100 MHz, DMSO-d6): δ 172.8, 171.5, 167.8, 164.6, 155.9, 144.1, 139.4, 138.9, 136.5, 135.3, 133.4, 131.6, 129.5, 128.7, 128.1, 127.7, 127.6, 127.4, 124.0, 122.8, 122.6, 121.3, 118.9, 118.7, 115.0, 111.8, 111.0, 78.4, 77.8, 55.1, 54.8, 39.1, 38.7, 32.1, 29.6, 28.7, 28.6, 27.8.; HRMS (ESI): m/z calcd for C49H58BrN7O8 [M + Na]+: 974.3422; found: 974.3418.
N-(4-bromo-2-(((S)-1-((2-((S)-2,6-diaminohexanamido)ethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)phenyl)-2-naphthamide (19a).
The title compound 19a was prepared from compound 18a (0.1 g, 0.10 mmol) according to the general procedure H. The product 19a was obtained as an off-white solid (0.038 g, 49%);
1H NMR (600 MHz, DMSO-d6): δ 12.17 (d, J = 6.1 Hz, 1H), 10.84 (d, J = 2.0 Hz, 1H), 9.17 (dd, J = 8.0, 1.7 Hz, 1H), 8.58 (t, J = 4.5 Hz, 1H), 8.54 (dd, J = 8.9, 1.6 Hz, 1H), 8.44 (d, J = 1.0 Hz, 1H), 8.41–8.34 (m, 1H), 8.18 (bs, 3H), 8.08–8.00 (m, 4H), 7.85 (dd, J = 8.6, 1.8 Hz, 1H), 7.84–7.76 (m, 4H), 7.73–7.61 (m, 3H), 7.26 (d, J = 7.9 Hz, 1H), 7.22 (d, J = 1.7 Hz, 1H), 7.04–6.95 (m, 2H), 4.78–4.72 (m, 1H), 3.72–3.64 (m, 1H), 3.31 (td, J = 14.1, 3.2 Hz, 1H), 3.28–3.08 (m, 5H), 2.79–2.69 (m, 2H), 1.75–1.63 (m, 2H), 1.56–1.47 (m, 2H), 1.36–1.25 (m, 2H); 13C NMR (150 MHz, DMSO-d6): δ 171.7, 169.0, 167.8, 165.1, 158.7, 138.8, 136.5, 135.3, 134.9, 132.6, 132.0, 131.6, 129.6, 129.1, 128.7, 128.4, 128.2, 127.6, 127.6, 124.1, 123.7, 123.2, 123.1, 122.9, 121.4, 118.9, 118.7, 118.4, 116.4, 115.2, 111.8, 110.9, 55.1, 52.6, 38.9, 38.8, 38.6, 30.8, 27.8, 27.0, 21.7, 21.7.; HRMS (ESI): m/z calcd for C37H40BrN7O4 [M + H]+: 726.2396; found: 726.2395.
N-(4-bromo-2-(((S)-1-((2-((S)-2,6-diaminohexanamido)ethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)phenyl)-[1,1′-biphenyl]-3-carboxamide (19b)
The title compound 19ab was prepared from compound 18b (0.1 g, 0.10 mmol) according to the general procedure H. The product 19b was obtained as an off-white solid (0.041 g, 55%);
1H NMR (600 MHz, DMSO-d6): δ 12.11 (s, 1H), 10.83 (d, J = 1.7 Hz, 1H), 9.14 (d, J = 7.9 Hz, 1H), 8.56 (bs, 1H), 8.49 (d, J = 8.9 Hz, 1H), 8.35–8.31 (m, 1H), 8.20–7.80 (bs, 5H), 8.12 (t, J = 8.9 Hz, 1H), 8.01 (d, J = 2.3 Hz, 1H), 7.94–7.90 (m, 1H), 7.79–7.70 (m, 5H), 7.68 (d, J = 7.9 Hz, 1H), 7.54–7.50 (m, 2H), 7.46–7.41 (m, 1H), 7.28 (d, J = 8.1 Hz, 1H), 7.21 (d, J = 2.2 Hz, 1H), 7.05–7.00 (m, 1H), 6.98–6.93 (m, 1H), 4.76–4.67 (m, 1H), 3.67 (t, J = 6.4 Hz, 1H), 3.39– 3.28 (m, 1H), 3.21–3.10 (m, 5H), 2.75 (t, J = 7.8 Hz, 2H), 1.75–1.63 (m, 2H), 1.57–1.48 (m, 2H), 1.35–1.27 (m, 2H); 13C NMR (150 MHz, DMSO-d6): δ 171.7, 169.1, 167.8, 165.0, 158.5, 141.3, 139.7, 138.7, 136.5, 135.5, 135.3, 131.6, 130.8, 130.1, 129.6, 128.5, 127.6, 127.3, 126.3, 125.9, 124.0, 123.3, 122.9, 121.4, 118.8, 118.7, 116.7, 115.2, 111.8, 110.9, 55.1, 52.6, 38.9, 38.7, 38.6, 30.9, 27.8, 27.0, 21.7.; HRMS (ESI): m/z calcd for C39H42BrN7O4 [M + H]+: 752.2554; found: 752.2553.
N-(4-bromo-2-(((S)-1-((2-((S)-2,6-diaminohexanamido)ethyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)phenyl)-[1,1′-biphenyl]-4-carboxamide (19c).
The title compound 19c was prepared from compound 18c (0.1 g, 0.10 mmol) according to the general procedure H. The product 19c was obtained as an off-white solid (0.031 g, 49%); 1H NMR (600 MHz, DMSO-d6): δ 12.08 (s, 1H), 10.83 (d, J = 2.1 Hz, 1H), 9.16 (d, J = 8.1 Hz, 1H), 8.58 (t, J = 5.2 Hz, 1H), 8.55 (d, J = 9.0 Hz, 1H), 8.37 (t, J = 5.2 Hz, 1H), 8.16 (s, 3H), 8.04 (d, J = 2.4 Hz, 1H), 7.89–7.86 (m, 2H), 7.85–7.82 (m, 2H), 7.81–7.72 (m, 6H), 7.69 (d, J = 7.7 Hz, 1H), 7.55–7.51 (m, 2H), 7.47–7.43 (m, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 2.3 Hz, 1H), 7.05–7.01 (m, 1H), 7.00–6.96 (m, 1H), 6.58 (bs, 1H), 4.79–4.72 (m, 1H), 3.72–3.64 (m, 1H), 3.35–3.29 (m, 1H), 3.25–3.12 (m, 5H), 2.79–2.70 (m, 2H), 1.75–1.64 (m, 2H), 1.56–1.47 (m, 2H), 1.36–1.26 (m, 2H); 13C NMR (150 MHz, DMSO-d6): δ 171.7, 169.0, 167.8, 164.6, 158.6, 144.1, 139.3, 138.9, 136.5, 135.4, 133.4, 131.6, 129.6, 128.8, 128.1, 127.7, 127.6, 127.4, 124.1, 122.8, 122.7, 121.4, 118.9, 118.7, 115.0, 111.9, 110.9, 55.1, 52.6, 38.9, 38.8, 38.6, 30.8, 27.8, 27.0, 21.7.; HRMS (ESI): m/z calcd for C39H42BrN7O4 [M + H]+: 752.2554; found: 752.2551.