Comparison of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Retail Table Eggs Produced by Laying Hens from the Cage and Non-Cage Housing Systems in Western Australia
Abstract
1. Introduction
2. Results
2.1. Description of Submissions
2.2. Descriptive Analyses
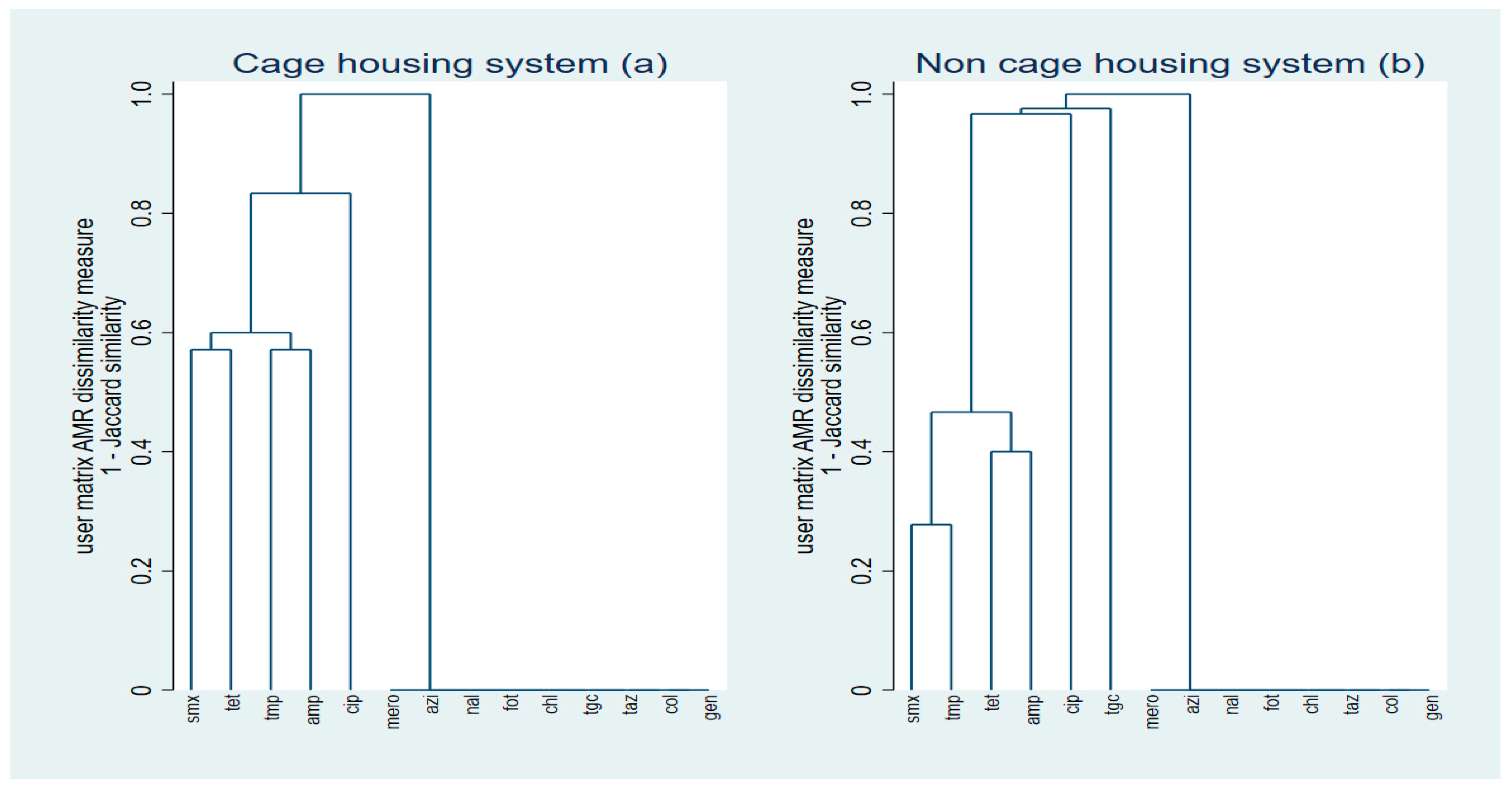
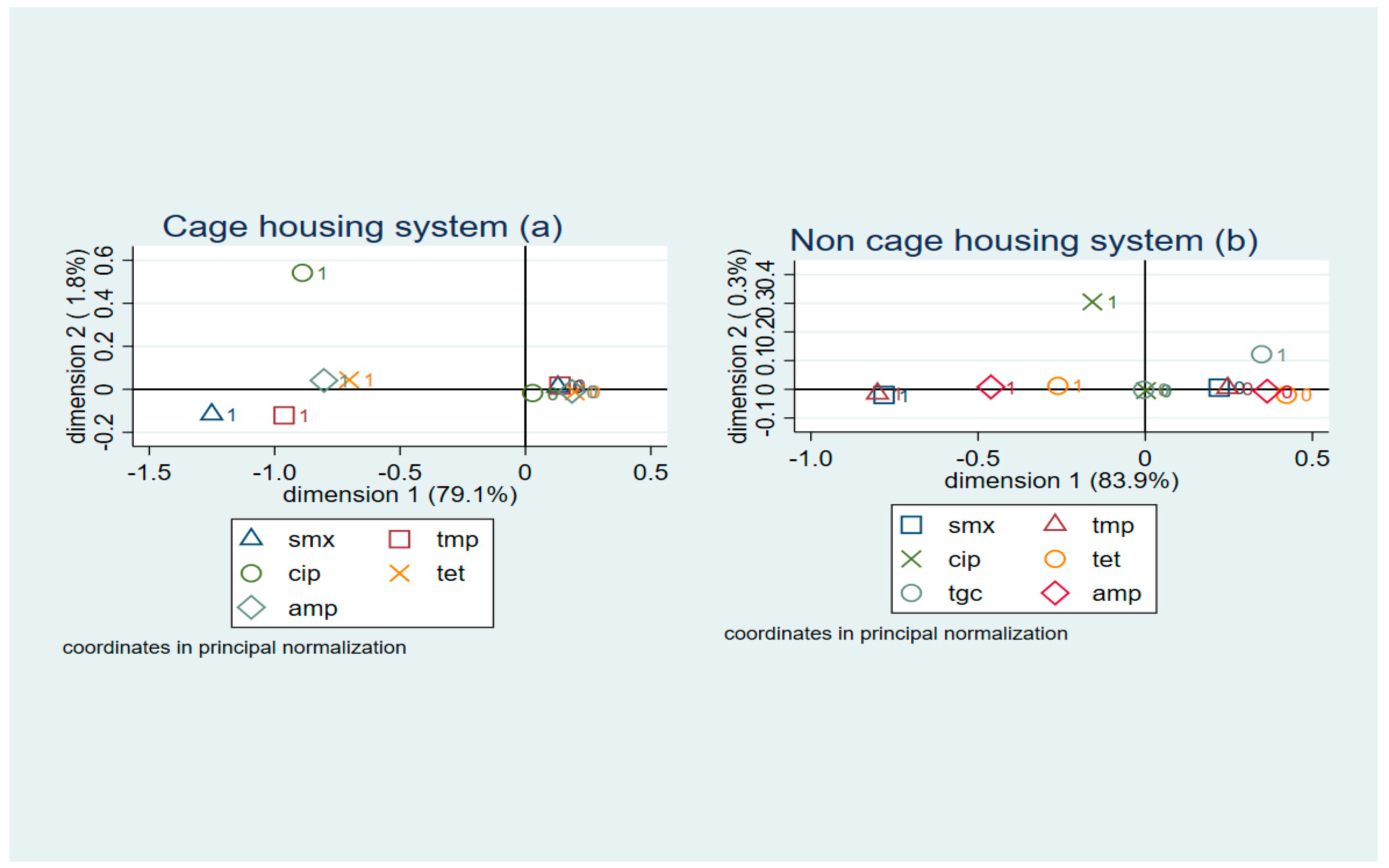
2.3. Cluster Analyses
2.4. Logistic Regression
2.5. Poisson Regression
3. Discussion
4. Materials and Methods
4.1. Study Design and Lab Methods
4.2. Data Analysis
4.2.1. Descriptive Analysis
4.2.2. Cluster Analysis
4.2.3. Logistic Regression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jabir, F.; Hague, M.T. Study on production performance of ISA Brown strain at Krishibid Firm Ltd. Trishal, Mymensingh. Bangladesh Res. Publ. J. 2010, 3, 1039–1044. [Google Scholar]
- Australian Eggs. Australian Egg Industry Overview. Available online: https://www.australianeggs.org.au/egg-industry (accessed on 23 February 2023).
- Keller, L.H.; Benson, C.E.; Krotec, K.; Eckroade, R.J. Salmonella enteritidis colonization of the reproductive tract and forming and freshly laid eggs of chickens. Infect. Immun. 1995, 63, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Agabou, A.; Lezzar, N.; Ouchenane, Z.; Khemissi, S.; Satta, D.; Sotto, A.; Lavigne, J.P.; Pantel, A. Clonal relationship between human and avian ciprofloxacin-resistant Escherichia coli isolates in North-Eastern Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 227–234. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority Panel on Biological Hazards (BIOHAZ); Europena Medicines Agency Committee for Medicinal Products for Veterinary Use (CVMP). ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA J. 2017, 15, e05017. [Google Scholar] [CrossRef]
- Vangchhia, B.; Blyton, M.D.; Collignon, P.; Kennedy, K.; Gordon, D.M. Factors affecting the presence, genetic diversity and antimicrobial sensitivity of Escherichia coli in poultry meat samples collected from Canberra, Australia. Environ. Microbiol. 2018, 20, 1350–1361. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Wegener, H.C.; Collignon, P. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Rev. Anti Infect. Ther. 2008, 6, 733–750. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Davis, M.; Leibler, J.H.; Peterson, A.E. One reservoir: Redefining the community origins of antimicrobial resistant infections. Med. Clin. N. Am. 2008, 92, 391–1407. [Google Scholar] [CrossRef]
- Shrestha, R.D.; Agunos, A.; Gow, S.P.; Deckert, A.E.; Varga, C. Associations between antimicrobial resistance in fecal Escherichia coli isolates and antimicrobial use in Canadian turkey flocks. Front. Microbiol. 2022, 13, 954123. [Google Scholar] [CrossRef]
- Adesiyun, A.; Offiah, N.; Seepersadsingh, N.; Rodrigo, S.; Lashley, V.; Musai, L. Frequency and antimicrobial resistance of enteric bacteria with spoilage potential isolated from table eggs. Food Res. Int. 2006, 39, 212–219. [Google Scholar] [CrossRef]
- Adeboye, O.A.; Kwofie, M.K.; Bukari, N. Campylobacter, Salmonella and Escherichia coli Food Contamination Risk in Free-Range Poultry Production System. Adv. Appl. Microbiol. 2020, 10, 525–542. [Google Scholar] [CrossRef]
- Snyder, H.L.; Niebuhr, S.E.; Dickson, J.S. Transfer of methicillinresistant Staphylococcus aureus from retail pork products onto food contact surfaces and the potential summer exposure. J. Food Prot. 2013, 76, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; McEntire, J.C.; Zhang, L.; Li, X.; Doyle, M. The transfer of antibiotic resistance from food to humans: Facts, implications and future directions. Rev. Sci. Tech. 2012, 31, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Australian Veterinary Association (AVA). Veterinary Use of Antibiotics Critical to Human Health. Australian Veterinary Association. 2015. Available online: https://www.ava.com.au/siteassets/resources/fighting-antimicrobial-resistance/veterinary-use-of-antibiotics-critical-to-human-health.pdf (accessed on 23 February 2023).
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Importance Ratings and Summary of Antibacterial Uses in Human and Animal Health in Australia; Australian Strategic and Technical Advisory Group on Antimicrobial Resistance (ASTAG); Department of Agriculture, Water Resources, and the Environment: Canberra, Australia, 2018. Available online: https://www.amr.gov.au/sites/default/files/2022-10/importance-ratings-and-summary-of-antibacterial-uses-in-human-and-animal-health-in-australia.pdf (accessed on 23 February 2023).
- Groves, P.; Underwood, G. Impact of antibiotic use and disease risks on Australian laying hen welfare. Anim. Prod. Sci. 2021, 61, 1037–1041. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Wang, P.; Robertson, I.; Abraham, S.; Sahibzada, S.; Habib, I. Antimicrobial resistance and genomic characterisation of Escherichia coli isolated from caged and non-caged retail table eggs in Western Australia. Int. J. Food Microbiol. 2021, 340, 109054. [Google Scholar] [CrossRef] [PubMed]
- Australian Eggs. Surveillance for Antimicrobial Resistance in Enteric Commensals and Pathogens in the Australian Commercial Egg Industry; Australian Eggs Limited: North Sydney, Australia, 2021; Available online: https://www.australianeggs.org.au/assets/research/documents/Egg-industry-AMR-survey-Final-Report2_May-2021.pdf (accessed on 23 February 2023).
- Abbott, R. Hen housing systems and egg safety. Poult. Int. 2010, 49, 32–33. [Google Scholar]
- Chousalkar, K.; Gast, R.; Martelli, F.; Pande, V. Review of Egg-Related Salmonellosis and Reduction Strategies in the United States, Australia, United Kingdom, and New Zealand. Crit. Rev. Microbiol. 2018, 44, 290–303. [Google Scholar] [CrossRef]
- Gole, V.C.; Woodhouse, R.; Caraguel, C.; Moyle, T.; Rault, J.L.; Sexton, M.; Chousalkar, K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017, 83, e03313-16. [Google Scholar] [CrossRef] [PubMed]
- Weeks, C.A.; Lambton, S.L.; Williams, A.G. Implications for welfare, productivity and sustainability of the variation in reported levels of mortality for laying hen flocks kept in different housing systems: A meta-analysis of ten studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial resistance in fecal Escherichia coli and Salmonella enterica isolates: A two-year prospective study of small poultry flocks in Ontario, Canada. BMC Vet. Res. 2019, 15, 464. [Google Scholar] [CrossRef]
- Schwaiger, K.; Schmied, E.M.; Bauer, J. Comparative analysis of antibiotic resistance characteristics of Gram-negative bacteria isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, germany. Zoonoses Public Health 2008, 55, 331–341. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). OIE Annual Report on Antimicrobial Agents Intended for Use in Animal. 2021. Available online: https://www.woah.org/app/uploads/2021/05/a-fifth-annual-report-amr.pdf (accessed on 23 February 2023).
- Vlčková, J.; Tůmová, E.; Ketta, M.; Englmaierová, M.; Chodová, D. Effect of housing system and age of laying hens on eggshell quality, microbial contamination, and penetration of microorganisms into eggs. Czech J. Anim. Sci. 2018, 63, 51–60. [Google Scholar] [CrossRef]
- De Reu, K.; Messens, W.; Heyndrickx, M.; Rodenburg, T.B.; Uyttendaele, M.; Herman, L. Bacterial contamination of table eggs and the influence of housing systems. World’s Poult. Sci. J. 2008, 64, 5–19. [Google Scholar] [CrossRef]
- Mallet, S.; Guesdon, V.; Ahmed, A.M.H.; Nys, Y. Comparison of eggshell hygiene in two housing systems: Standard and furnished cages. Br. Poult. Sci. 2006, 47, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Chousalkar, K.K. Effect of production system and flock age on egg quality and total bacterial load in commercial laying hens. J. Appl. Poult. Res. 2014, 23, 59–70. [Google Scholar] [CrossRef]
- Abraham, S.; O’Dea, M.; Sahibzada, S.; Hewson, K.; Pavic, A.; Veltman, T.; Abraham, R.; Harris, T.; Trott, D.J.; Jordan, D. Escherichia coli and Salmonella spp. isolated from Australian meat chickens remain susceptible to critically important antimicrobial agents. PLoS ONE 2019, 14, e0224281. [Google Scholar] [CrossRef]
- Kidsley, A.K.; Abraham, S.; Bell, J.M.; O’Dea, M.; Laird, T.J.; Jordan, D.; Mitchell, P.; McDevitt, C.A.; Trott, D.J. Antimicrobial Susceptibility of Escherichia coli and Salmonella spp. Isolates From Healthy Pigs in Australia: Results of a Pilot National Survey. Front. Microbiol. 2018, 9, 1207. [Google Scholar] [CrossRef]
- Barlow, R.S.; McMillan, K.E.; Duffy, L.L.; Fegan, N.; Jordan, D.; Mellor, G.E. Prevalence and Antimicrobial Resistance of Salmonella and Escherichia coli from Australian Cattle Populations at Slaughter. J. Food Prot. 2015, 78, 912–920. [Google Scholar] [CrossRef]
- Veltman, T.; Jordan, D.; McDevitt, C.A.; Bell, J.; Howden, B.P.; Valcanis, M.; O’Dea, M.; Abraham, S.; Scott, P.; Kovac, J.H.; et al. Absence of high priority critically important antimicrobial resistance in Salmonella sp. isolated from Australian commercial egg layer environments. Int. J. Food Microbiol. 2021, 340, 109042. [Google Scholar] [CrossRef]
- Podnecky, N.L.; Rhodes, K.A.; Mima, T.; Drew, H.R.; Chirakul, S.; Wuthiekanun, V.; Schupp, J.M.; Sarovich, D.S.; Currie, B.J.; Keim, P.; et al. Mechanisms of Resistance to Folate Pathway Inhibitors in Burkholderia pseudomallei: Deviation from the Norm. mBio 2017, 8, e01357-17. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- ISO 16649-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 1: Colony-Count Technique at 44 Degrees C Using Membranes and 5-Bromo-4-chloro-3-indolyl Beta-Dglucuronide. 2018. Available online: https://www.iso.org/standard/64951.html (accessed on 12 November 2020).
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 12 November 2020).
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Greenacre, M.J. Multiple and Joint Correspondence Analysis. In Correspondence Analysis in the Social Sciences; Greenacre, M.J., Blasius, J., Eds.; Academic Press: London, UK, 1994. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999. [Google Scholar]

| Antimicrobial Class | Antimicrobial A | Cage (n = 32) n (%) B [CI] C | Non-Cage (n = 68) n (%) B [CI] C | Total (n = 100) n (%) B [CI] C |
|---|---|---|---|---|
| Folate pathway inhibitors | SMX | 3 (9.4) [2–25] | 15 (22) [12.9–33.7] | 18 (18) [11–26.9] |
| TMP | 4 (12.5) [3.5–29] | 16 (23.5) [14.1–35.4] | 20 (20) [12.7–29.2] | |
| Quinolones | CIP | 1 (3.1) [0.08–16.2] | 1 (1.5) [0.04–7.92] | 2 (2) [0.2–7] |
| NAL | 0 | 0 | 0 | |
| Tetracyclines | TET | 7 (21.9) [9.28–40] | 42 (61.8) [49.2–73.3] | 49 (49) [38.9–59.2] |
| Carbapenem | MERO | 0 | 0 | 0 |
| Macrolides D | AZI | 0 | 0 | 0 |
| Third-generation cephalosporin | FOT | 0 | 0 | 0 |
| TAZ | 0 | 0 | 0 | |
| Phenicols | CHL | 0 | 0 | 0 |
| Glycylcyclines | TGC | 0 | 1 (1.5) [0.04–7.92] | 1 (1) [0–5.4] |
| Polymixins | COL | 0 | 0 | 0 |
| Beta-lactams | AMP | 6 (18.7) [7.2–36.4] | 30 (44.1) [32.1–56.7] | 36 (36) [26.6–46.2] |
| Aminoglycosides | GEN | 0 | 0 | 0 |
| Production System | Antimicrobial Resistance Pattern A | Number of Antimicrobial Classes in Pattern (Multidrug-Resistant) B | n (%) C |
|---|---|---|---|
| Cage | TET | 1 (no) | 3 (9.37) |
| TMP | 1 (no) | 1 (3.12) | |
| AMP | 1 (no) | 2 (6.25) | |
| TMP-AMP | 2 (no) | 1 (3.12) | |
| SMX-TET | 2 (no) | 1 (3.12) | |
| CIP-TET-AMP | 3 (yes) | 1 (3.12) | |
| SMX-TMP-TET-AMP | 3 (yes) | 2 (6.25) | |
| Non-cage | AMP | 1 (no) | 3 (4.41) |
| TET | 1 (no) | 14 (20.58) | |
| SMX | 1 (no) | 1 (1.47) | |
| TET-AMP | 2 (no) | 9 (13.23) | |
| TET-TGC | 2 (no) | 1 (1.47) | |
| TMP-TET-AMP | 3 (yes) | 3 (4.41) | |
| SMX-TET-AMP | 3 (yes) | 1 (1.47) | |
| CIP-TET-AMP | 3 (yes) | 1 (1.47) | |
| SMX-TMP-TET-AMP | 3 (yes) | 13 (19.11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodagari, H.R.; Varga, C.; Habib, I.; Sahibzada, S. Comparison of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Retail Table Eggs Produced by Laying Hens from the Cage and Non-Cage Housing Systems in Western Australia. Antibiotics 2023, 12, 588. https://doi.org/10.3390/antibiotics12030588
Sodagari HR, Varga C, Habib I, Sahibzada S. Comparison of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Retail Table Eggs Produced by Laying Hens from the Cage and Non-Cage Housing Systems in Western Australia. Antibiotics. 2023; 12(3):588. https://doi.org/10.3390/antibiotics12030588
Chicago/Turabian StyleSodagari, Hamid Reza, Csaba Varga, Ihab Habib, and Shafi Sahibzada. 2023. "Comparison of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Retail Table Eggs Produced by Laying Hens from the Cage and Non-Cage Housing Systems in Western Australia" Antibiotics 12, no. 3: 588. https://doi.org/10.3390/antibiotics12030588
APA StyleSodagari, H. R., Varga, C., Habib, I., & Sahibzada, S. (2023). Comparison of Antimicrobial Resistance among Commensal Escherichia coli Isolated from Retail Table Eggs Produced by Laying Hens from the Cage and Non-Cage Housing Systems in Western Australia. Antibiotics, 12(3), 588. https://doi.org/10.3390/antibiotics12030588








