Steady-State Piperacillin Concentrations in the Proximity of an Orthopedic Implant: A Microdialysis Porcine Study
Abstract
1. Introduction
2. Results
2.1. fT>MIC
2.2. Pharmacokinetic Parameters
3. Discussion
4. Materials and Methods
4.1. Microdialysis
4.2. Anesthehsia and Surgical Procedures
4.3. Drug Administration and Sampling Procedure
4.4. Quantification of Piperacillin and Benzylpenicillin
4.5. MIC Targets, Pharmacokinetic Analysis, and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Q. Recent Advances and Perspective of Nanotechnology-Based Implants for Orthopedic Applications. Front. Bioeng. Biotechnol. 2022, 10, 878257. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ouyang, Y.; Poh, C.K. Orthopaedic implant technology: Biomaterials from past to future. Ann. Acad. Med. Singap. 2011, 40, 237–244. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Cook, G.E.; Markel, D.C.; Ren, W.; Webb, L.X.; McKee, M.D.; Schemitsch, E.H. Infection in Orthopaedics. J. Orthop. Trauma 2015, 29 (Suppl. 12), S19–S23. [Google Scholar] [CrossRef]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Antti-Poika, I.; Josefsson, G.; Konttinen, Y.; Lidgren, L.; Santavirta, S.; Sanzén, L. Hip arthroplasty infection: Current concepts. Acta Orthop. Scand. 1990, 61, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Haenle, M.; Skripitz, C.; Mittelmeier, W.; Skripitz, R. Economic Impact of Infected Total Knee Arthroplasty. Sci. World J. 2012, 2012, 196515. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Dolores del Toro, M.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e731–732.e738. [Google Scholar] [CrossRef]
- Pfang, B.G.; García-Cañete, J.; García-Lasheras, J.; Blanco, A.; Auñón, Á.; Parron-Cambero, R.; Macías-Valcayo, A.; Esteban, J. Orthopedic Implant-Associated Infection by Multidrug Resistant Enterobacteriaceae. J. Clin. Med. 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Southwood, R.T.; Rice, J.L.; McDonald, P.J.; Hakendorf, P.H.; Rozenbilds, M.A. Infection in experimental hip arthroplasties. J. Bone Jt. Surg.—Ser. B 1985, 67, 229–231. [Google Scholar] [CrossRef]
- Elek, S.D. Experimental staphylococcal infections in the skin of man. Ann. N. Y. Acad. Sci. 1956, 65, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Cui, Z.; Guo, Y.; Zhang, W.; Wu, J. Clinical and microbiological features of anaerobic implant-related infection in 80 patients after orthopedic surgery. Anaerobe 2021, 71, 102413. [Google Scholar] [CrossRef] [PubMed]
- Corder, J.; Munuera, L.; Folgueira, M.D. Influence of bacterial strains on bone infection. J. Orthop. Res. 1996, 14, 663–667. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Petersen, E.K.; Hanberg, P.; Knudsen, M.; Tøstesen, S.K.; Jørgensen, A.R.; Öbrink-Hansen, K.; Søballe, K.; Stilling, M.; Bue, M. Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis. Antibiotics 2022, 11, 910. [Google Scholar] [CrossRef]
- Gin, A.; Dilay, L.; Karlowsky, J.A.; Walkty, A.; Rubinstein, E.; Zhanel, G.G. Piperacillin–tazobactam: A β-lactam/β-lactamase inhibitor combination. Expert Rev. Anti-Infect. Ther. 2007, 5, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Ribera, A.; Soldevila, L.; Rigo-Bonnin, R.; Tubau, F.; Padullés, A.; Gómez-Junyent, J.; Ariza, J.; Murillo, O. Beta-lactams in continuous infusion for Gram-negative bacilli osteoarticular infections: An easy method for clinical use. Infection 2018, 46, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Breilh, D.; Debon, R.; Duflo, F.; Bel, J.C.; Saux, M.C.; Chassard, D.; Allaouchiche, B. Penetration of Piperacillin/Tazobactam (4 g/500 mg) into Synovial Tissue. J. Chemother. 2002, 14, 54–58. [Google Scholar] [CrossRef]
- Knudsen, M.; Bue, M.; Pontoppidan, L.L.; Hvistendahl, M.A.; Søballe, K.; Stilling, M.; Hanberg, P. Evaluation of Benzylpenicillin as an Internal Standard for Measurement of Piperacillin Bone Concentrations via Microdialysis. J. Pharm. Sci. 2021, 110, 3500–3506. [Google Scholar] [CrossRef] [PubMed]
- Vittrup, S.; Stilling, M.; Hanberg, P.; Tøstesen, S.K.; Knudsen, M.B.; Kipp, J.O.; Bue, M. Concentrations of co-administered vancomycin and meropenem in the internal dead space of a cannulated screw and in cancellous bone adjacent to the screw—Evaluated by microdialysis in a porcine model. Injury 2022, 53, 2734–2740. [Google Scholar] [CrossRef]
- Tøstesen, S.K.; Stilling, M.; Hanberg, P.; Thillemann, T.M.; Falstie-Jensen, T.; Tøttrup, M.; Knudsen, M.; Petersen, E.T.; Bue, M. High Cefuroxime Concentrations and Long Elimination in an Orthopaedic Surgical Deadspace—A Microdialysis Porcine Study. Antibiotics 2022, 11, 208. [Google Scholar] [CrossRef]
- Corbett, S.A.; McCarthy, I.D.; Batten, J.; Hukkanen, M.; Polak, J.M.; Hughes, S.P. Nitric oxide mediated vasoreactivity during fracture repair. Clin. Orthop. Relat. Res. 1999, 365, 247–253. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef]
- Lauderdale, K.J.; Malone, C.L.; Boles, B.R.; Morcuende, J.; Horswill, A.R. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 2010, 28, 55–61. [Google Scholar] [CrossRef]
- Oliveira, M.; Nunes, S.F.; Carneiro, C.; Bexiga, R.; Bernardo, F.; Vilela, C.L. Time course of biofilm formation by Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet. Microbiol. 2007, 124, 187–191. [Google Scholar] [CrossRef]
- Gristina, A.G. Implant Failure and the Immuno-Incompetent Fibro-Inflammatory Zone. Clin. Orthop. Relat. Res. 1994, 298, 106–118. Available online: https://journals.lww.com/clinorthop/toc/1994/01000 (accessed on 7 March 2023). [CrossRef]
- Hayashi, Y.; Lipman, J.; Udy, A.A.; Ng, M.; McWhinney, B.; Ungerer, J.; Lust, K.; Roberts, J.A. β-Lactam therapeutic drug monitoring in the critically ill: Optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int. J. Antimicrob. Agents 2013, 41, 162–166. [Google Scholar] [CrossRef]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wallemacq, P.E.; Van Bambeke, F.; Tulkens, P.M. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. Anti Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.S.; Waldrep, T. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam when administered by continuous infusion and intermittent dosing. Clin. Ther. 2002, 24, 1090–1104. [Google Scholar] [CrossRef]
- Lister, P.D.; Prevan, A.M.; Sanders, C.C. Importance of beta-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: Studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob. Agents Chemother. 1997, 41, 721–727. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, T.S.; Mulder, P.O.M.; Zijlstra, J.G.; Uges, D.R.A.; Stegeman, C.A. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal faliure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med. 1997, 23, 873–877. [Google Scholar] [CrossRef]
- Sörgel, F.; Kinzig, M. Pharmacokinetic characteristics of piperacillin/tazobactam. Intensive Care Med. 1994, 20, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Müller, M. Microdialysis: Current applications in clinical pharmacokinetic studies and its potential role in the future. Clin. Pharm. 2005, 44, 895–913. [Google Scholar] [CrossRef]
- Kho, C.M.; Enche Ab Rahim, S.K.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2017, 54, 3506–3527. [Google Scholar] [CrossRef]
- Biorender. Available online: https://www.biorender.com (accessed on 21 February 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available online: https://mic.eucast.org/search/ (accessed on 21 February 2023).
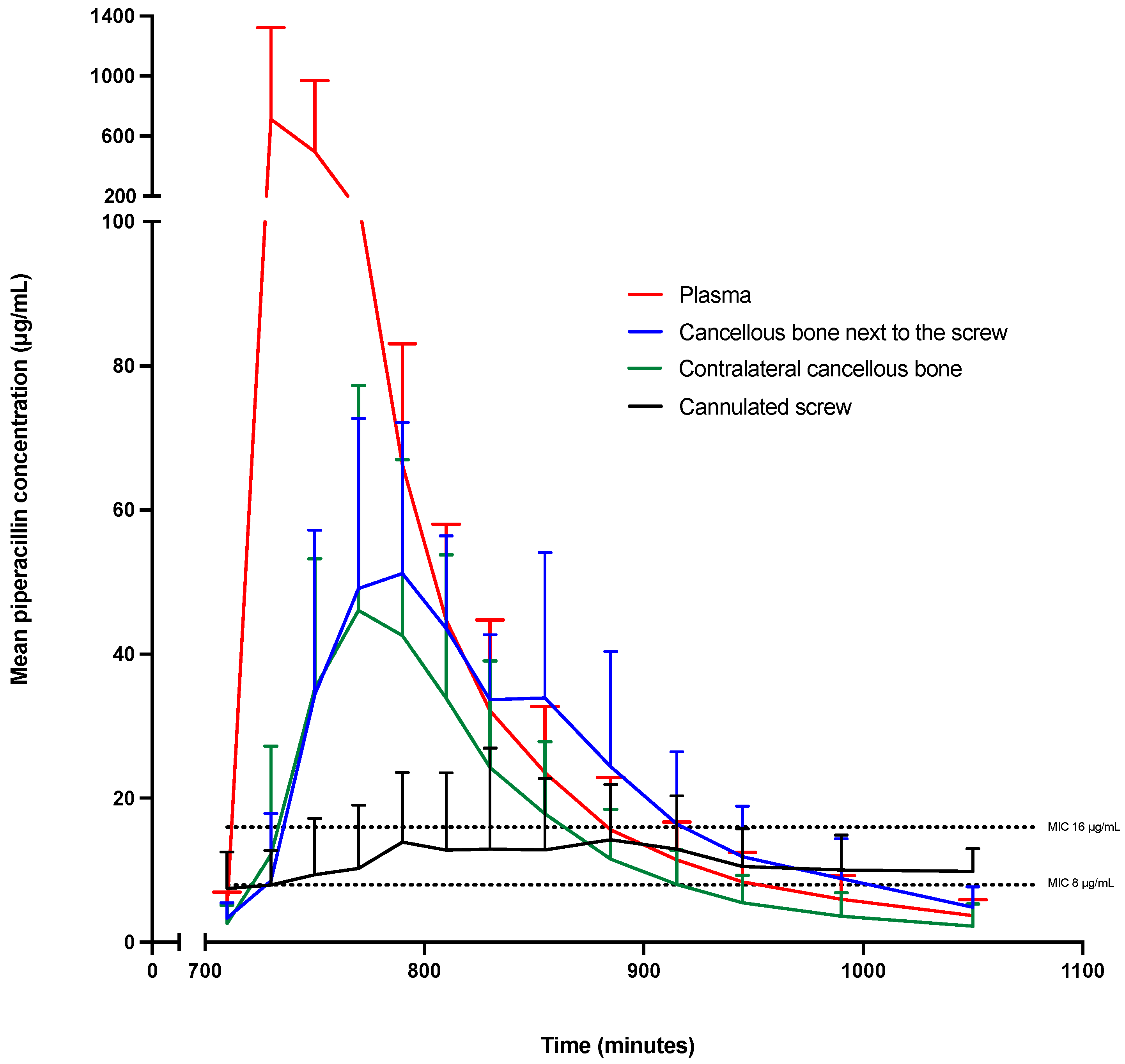
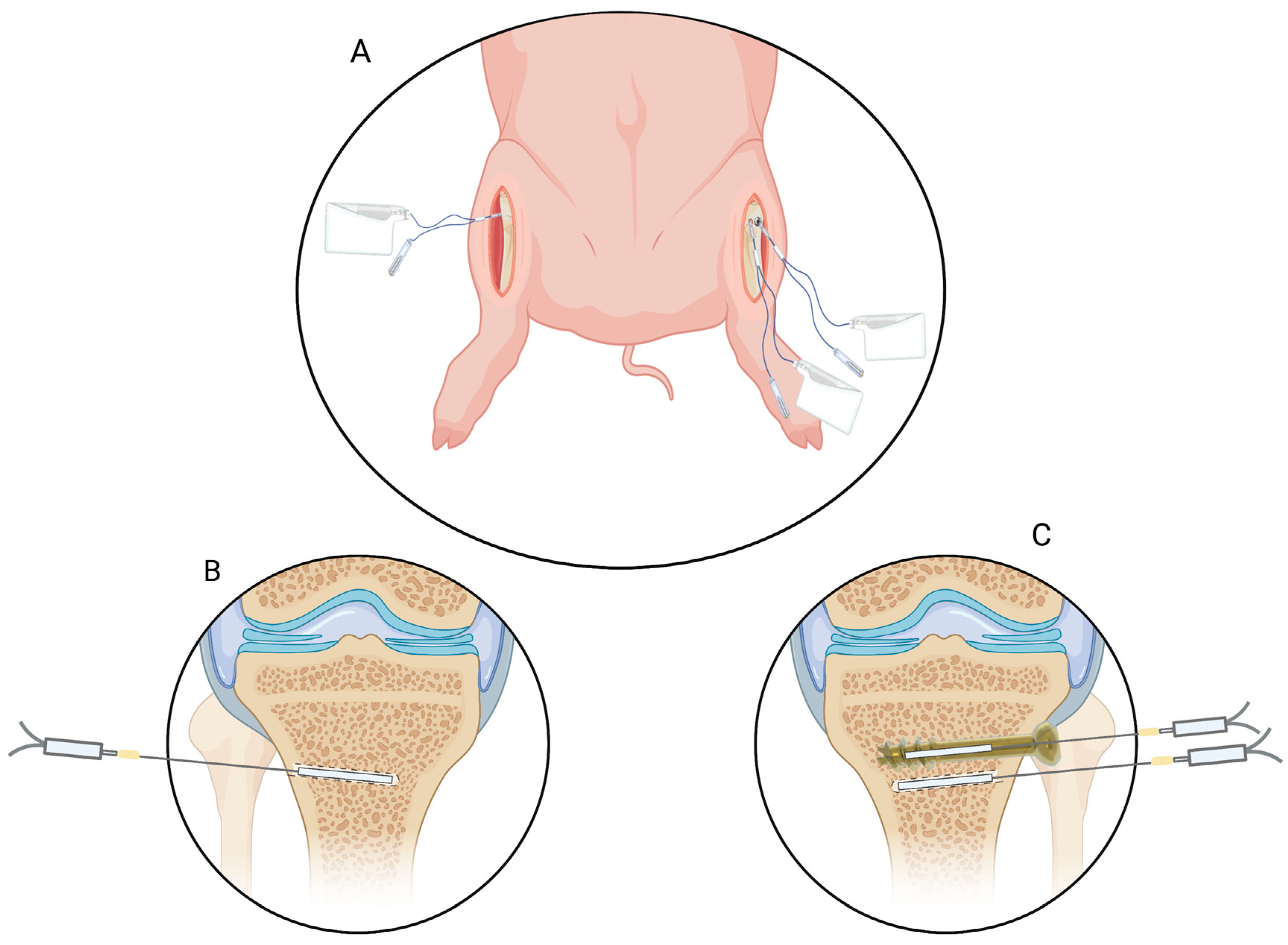
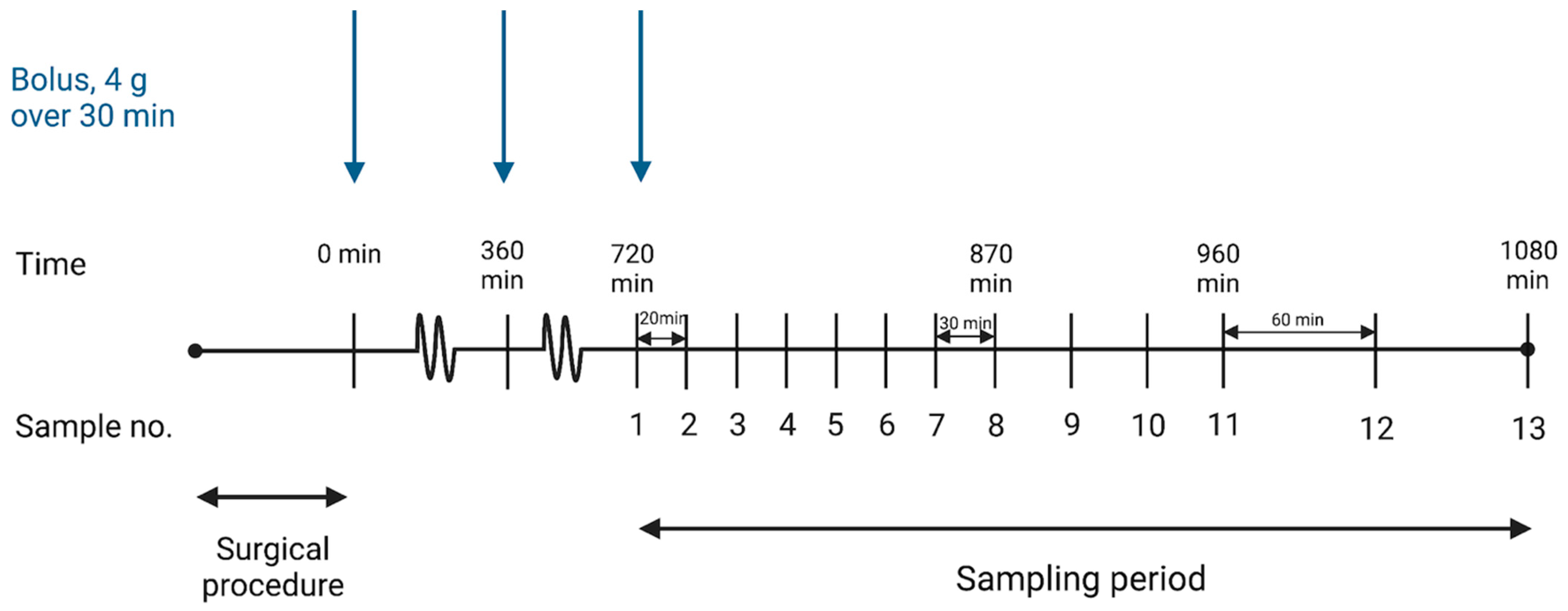
| Parameter | Minutes (95% CI) | Percentages (95% CI) |
|---|---|---|
| Low target: fT>MIC 8 µg/mL | ||
| Cannulated screw | 180 (97–263) | 55 (29–80) |
| Cancellous bone next to the screw | 243 (151–335) | 74 (46–100) * |
| Contralateral cancellous bone | 178 (95–261) | 54 (29–79) |
| Plasma | 238 (155–321) | 72 (47–97) |
| High target: fT>MIC 16 µg/mL | ||
| Cannulated screw | 53 (3–103) a | 16 (1–31) |
| Cancellous bone next to the screw | 177 (121–233) | 54 (37–71) |
| Contralateral cancellous bone | 112 (62–163) | 34 (19–49) |
| Plasma | 162 (111–212) a | 49 (34–64) |
| Parameter | Cannulated Screw | Cancellous Bone Next to the Screw | Contralateral Cancellous Bone | Plasma a |
|---|---|---|---|---|
| AUC12–18 h, min µg/mL | 4170 (1315–13,227) | 8821 (5511–14,144) | 7998 (3896–16,418) | 26,167 (14,867–46,055) |
| Cmax, µg/mL | 11 (4–31) b | 49 (32–75) | 45 (27–77) | 571 (215–1522) |
| Tmax, min c | 121 (47–203) | 54 (31–78) | 53 (38–69) | 20 (9–32) |
| T1/2, min | 807 (375–1740) | 324 (170–618) | 131 (79–217) d | 65 (39–108) |
| fAUCtissue/fAUCplasma | 0.16 (0.07–0.38) | 0.35 (0.16–0.79) | 0.31 (0.12–0.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilleøre, J.G.; Jørgensen, A.R.; Knudsen, M.B.; Hanberg, P.; Öbrink-Hansen, K.; Tøstesen, S.K.; Søballe, K.; Stilling, M.; Bue, M. Steady-State Piperacillin Concentrations in the Proximity of an Orthopedic Implant: A Microdialysis Porcine Study. Antibiotics 2023, 12, 615. https://doi.org/10.3390/antibiotics12030615
Lilleøre JG, Jørgensen AR, Knudsen MB, Hanberg P, Öbrink-Hansen K, Tøstesen SK, Søballe K, Stilling M, Bue M. Steady-State Piperacillin Concentrations in the Proximity of an Orthopedic Implant: A Microdialysis Porcine Study. Antibiotics. 2023; 12(3):615. https://doi.org/10.3390/antibiotics12030615
Chicago/Turabian StyleLilleøre, Johanne Gade, Andrea René Jørgensen, Martin Bruun Knudsen, Pelle Hanberg, Kristina Öbrink-Hansen, Sara Kousgaard Tøstesen, Kjeld Søballe, Maiken Stilling, and Mats Bue. 2023. "Steady-State Piperacillin Concentrations in the Proximity of an Orthopedic Implant: A Microdialysis Porcine Study" Antibiotics 12, no. 3: 615. https://doi.org/10.3390/antibiotics12030615
APA StyleLilleøre, J. G., Jørgensen, A. R., Knudsen, M. B., Hanberg, P., Öbrink-Hansen, K., Tøstesen, S. K., Søballe, K., Stilling, M., & Bue, M. (2023). Steady-State Piperacillin Concentrations in the Proximity of an Orthopedic Implant: A Microdialysis Porcine Study. Antibiotics, 12(3), 615. https://doi.org/10.3390/antibiotics12030615








