Population Pharmacokinetic Study of Benzylpenicillin in Critically Unwell Adults
Abstract
:1. Introduction
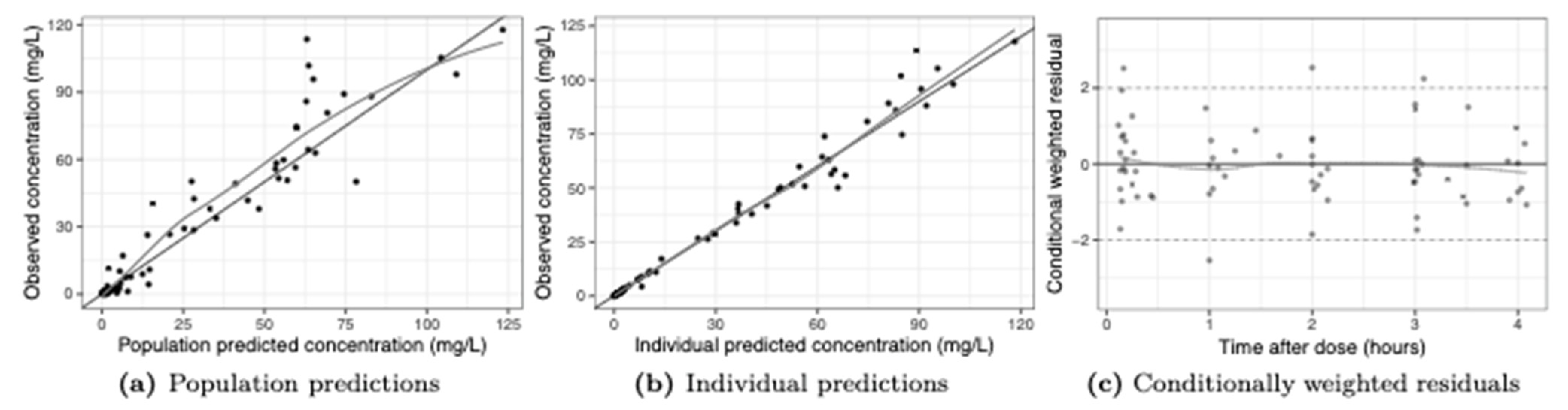
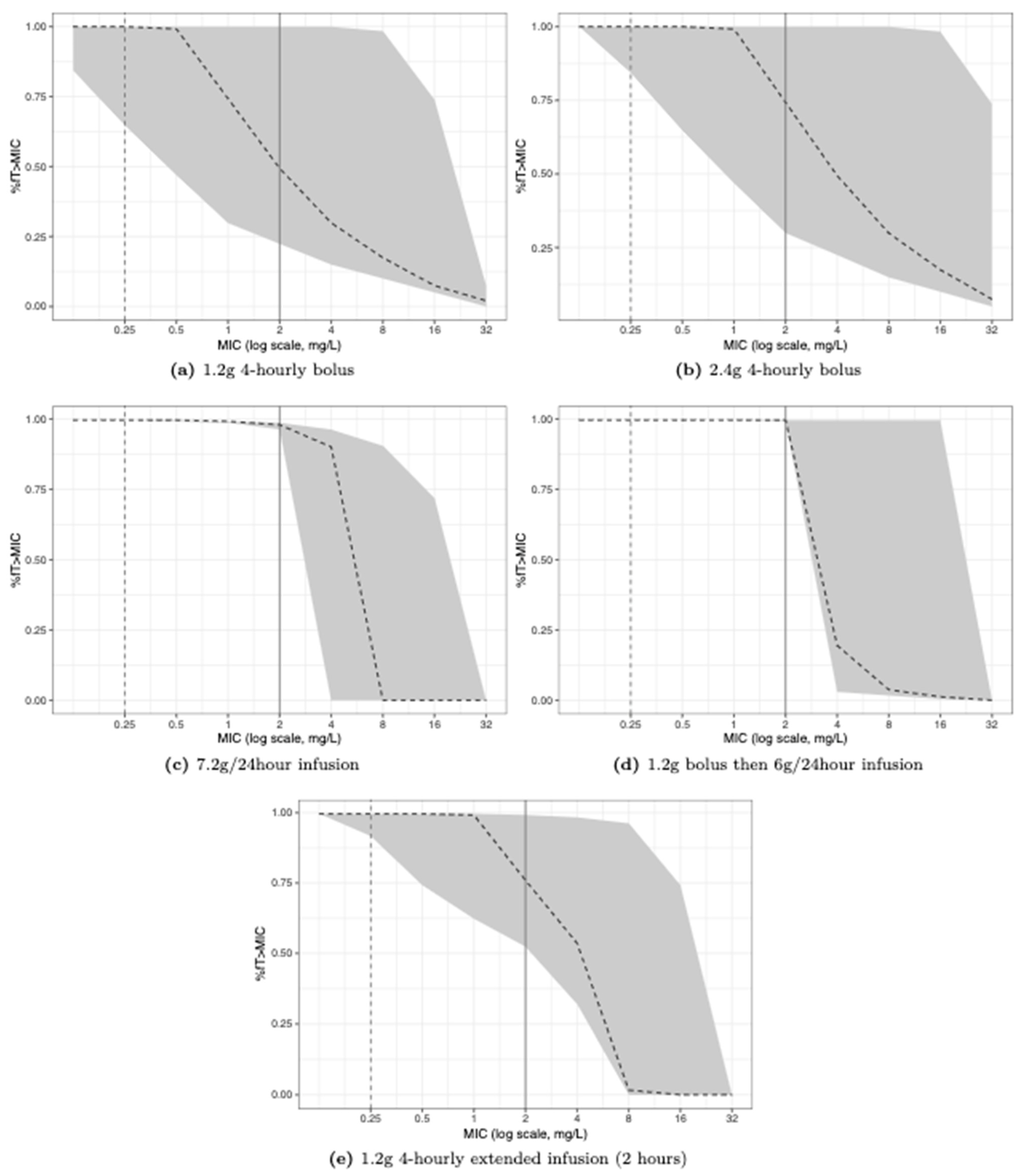
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [Green Version]
- Niederman, M.S.; Baron, R.M.; Bouadma, L.; Calandra, T.; Daneman, N.; DeWaele, J.; Kollef, M.H.; Lipman, J.; Nair, G.B. Initial antimicrobial management of sepsis. Crit. Care 2021, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Lonsdale, D.O.; Kipper, K.; Baker, E.H.; Barker, C.I.S.; Oldfield, I.; Philips, B.J.; Johnston, A.; Rhodes, A.; Sharland, M.; Standing, J.F. β-Lactam antimicrobial pharmacokinetics and target attainment in critically ill patients aged 1 day to 90 years: The ABDose study. J. Antimicrob. Chemother. 2020, 75, 3625–3634. [Google Scholar] [CrossRef]
- Craig, W.A. Antimicrobial resistance issues of the future. Diagn. Microbiol. Infect. Dis. 1996, 25, 213–217. [Google Scholar] [CrossRef]
- Andes, D.; Craig, W.A. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: Application to breakpoint determinations. Antimicrob. Agents Chemother. 1998, 42, 2375–2379. [Google Scholar] [CrossRef] [Green Version]
- Moine, P.; Vallée, E.; Azoulay-Dupuis, E.; Bourget, P.; Bédos, J.P.; Bauchet, J.; Pocidalo, J.J. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob. Agents Chemother. 1994, 38, 1953–1958. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Liebchen, U.; Paal, M.; Taubert, M.; Vogeser, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J. Intensive Care 2020, 8, 86. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: http://www.eucast.org (accessed on 25 October 2022).
- Gould, F.K.; Denning, D.W.; Elliott, T.S.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.; Spry, M.J.; Watkin, R.W.; Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2012, 67, 269–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Rizk, N.A.; Kanafani, Z.A.; Tabaja, H.Z.; Kanj, S.S. Extended infusion of beta-lactam antibiotics: Optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2017, 15, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.C.; van Hest, R.M.; Mistício, M.C.; Nunguiane, G.; Lang, C.N.; Beirão, J.C.; Mathôt, R.A.A.; Prins, J.M. Pharmacokinetics and Pharmacodynamic Target Attainment of Benzylpenicillin in an Adult Severely Ill Sub-Saharan African Patient Population. Clin. Infect. Dis. 2018, 66, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öbrink-Hansen, K.; Wiggers, H.; Bibby, B.M.; Hardlei, T.F.; Jensen, K.; Kragh Thomsen, M.; Brock, B.; Petersen, E. Penicillin G Treatment in Infective Endocarditis Patients-Does Standard Dosing Result in Therapeutic Plasma Concentrations? Basic Clin. Pharmacol. Toxicol. 2017, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Compendium, E.M. Summary of Product Characteristics: Benzylpenicillin Sodium 600 mg Powder for Injection. Available online: https://www.medicines.org.uk/emc/product/3828/smpc (accessed on 26 September 2022).
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. MIC Distributions for Benzylpenicillin. Available online: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=17&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50&page=1 (accessed on 25 October 2022).
- Kipper, K.; Barker, C.I.S.; Standing, J.F.; Sharland, M.; Johnston, A. Development of a Novel Multipenicillin Assay and Assessment of the Impact of Analyte Degradation: Lessons for Scavenged Sampling in Antimicrobial Pharmacokinetic Study Design. Antimicrob. Agents Chemother. 2018, 62, e01540-17. [Google Scholar] [CrossRef] [Green Version]
- Beal, S.; Sheine, L.B.; Boeckmann, A.; Bauer, R.J. NONMEM User’s Guides (1989–2011); Icon Development Solutions: Ellicott City, MD, USA, 2011. [Google Scholar]
- Jonsson, E.N.; Karlsson, M.O. Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 1999, 58, 51–64. [Google Scholar] [CrossRef]
- Keizer, R.J.; Karlsson, M.O.; Hooker, A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e50. [Google Scholar] [CrossRef] [PubMed]
- Lindbom, L.; Ribbing, J.; Jonsson, E.N. Perl-speaks-NONMEM (PsN)—A Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 2004, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D.O.; Baker, E.H.; Kipper, K.; Barker, C.; Philips, B.; Rhodes, A.; Sharland, M.; Standing, J.F. Scaling beta-lactam antimicrobial pharmacokinetics from early life to old age. Br. J. Clin. Pharmacol. 2019, 85, 316–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoonjans, F. Values of the Chi-Squared Distribution. Available online: https://www.medcalc.org/manual/chi-square-table.php (accessed on 14 November 2022).
- Rich, B. Linpk: Generate Concentration-Time Profiles from Linear PK Systems; R Package Version 1.1.1; 2021. Available online: https://CRAN.R-project.org/package=linpk (accessed on 3 August 2022).


| Characteristic | Median or n (Interquartile Range) | Full Range |
|---|---|---|
| No. of participants | 12 | - |
| Male: Female | 6:6 | - |
| Age | 57.7 (44.3–63.2) | 25.7–71.7 |
| Height (cm) | 172.0 (163.5–178.0) | 150.0–188.0 |
| Weight (kg) | 70.0 (65.7–90.0) | 60.0–120.0 |
| BMI (kg/m2) | 26.1 (22.1–27.9) | 19.8–39.6 |
| Ethnicity: | ||
| Asian | 2 | |
| Caribbean | 1 | |
| White British | 6 | |
| White Irish | 1 | |
| Other/Not stated | 2 | |
| Infection source: * | ||
| Soft tissue/Skin infection or abscess | 5 | |
| Lower respiratory tract infection | 7 | |
| Infective endocarditis | 1 | |
| Sepsis of unknown source | 1 | |
| Clinical outcome at 90-day review: | ||
| Died in hospital | 1 | |
| Discharged home | 10 | |
| Died at home | 1 | |
| Serum creatinine (mmol/L) | 70 (52–103.5) | 34–486 |
| Serum albumin (g/L) | 28 (24–34) | 14–43 |
| CRP (mg/L) | 133.9 (29.4–306) | 13.6–386.5 |
| APACHE II | 14 (12.5–18) | 5–23 |
| Vasopressors (no. of patients) | 5 | |
| Ventilation (no. of patients with status recorded): ** | ||
| Intubated and ventilated | 1 | |
| Non-invasive ventilation | 3 | |
| Spontaneous ventilation | 10 | |
| Not recorded | 1 |
| Mean Parameter Estimate (%RSE) | Individual Estimates (Range) | Bootstrap (n = 2000) Median (95% Interval) | |
|---|---|---|---|
| Fixed effects | |||
| θCL L/h/70 kg | 23.1 (14) | 4.1–53.1 | 24.0 (19.0–31.5) |
| θV1 L/70 kg | 15.1 (8) | 9.9–27.6 | 14.4 (9.4–16.8) |
| θQ L/h/70 kg | 11.1 (50) | 11.4 (7.0–39.4) | |
| θV2 L/70 kg | 9.8 (29) | 6.5–19.4 | 10.5 (7.4–21.3) |
| θCREAT | −0.916 (18) | −0.97 (−1.26–−0.67) | |
| Random effects | |||
| ω21 CL (%CV) | 42.0 (43) | 40.0 (21.9–56.9) | |
| ω22 V1 (%CV) | 22.6 (42) | 22.8 (12.2–53.3) | |
| ω23 V2 (%CV) | 20.5 (60) | 20.2 (7.7–33.6) | |
| Residual error | |||
| σ21 (proportional) | 0.021 (47) | 0.014 (0.006–0.032) | |
| σ22 (additive) | 0.006 (67) | 0.006 (0.003–0.031) | |
| Derived parameters | |||
| T1/2 | 1.11 h | ||
| Dosing Schedule | Sampling Interval 1 | Sampling Interval 2 |
|---|---|---|
| 4-hourly | 0.1, 2, 3.5, 4 | 0.2, 1, 3, 4 |
| 6-hourly | 0.1, 2, 3, 6 | 0.1, 1, 5, 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.V.; Kipper, K.; Baker, E.H.; Barker, C.I.S.; Oldfield, I.; Philips, B.J.; Johnston, A.; Lipman, J.; Rhodes, A.; Basarab, M.; et al. Population Pharmacokinetic Study of Benzylpenicillin in Critically Unwell Adults. Antibiotics 2023, 12, 643. https://doi.org/10.3390/antibiotics12040643
Shah RV, Kipper K, Baker EH, Barker CIS, Oldfield I, Philips BJ, Johnston A, Lipman J, Rhodes A, Basarab M, et al. Population Pharmacokinetic Study of Benzylpenicillin in Critically Unwell Adults. Antibiotics. 2023; 12(4):643. https://doi.org/10.3390/antibiotics12040643
Chicago/Turabian StyleShah, Reya V., Karin Kipper, Emma H. Baker, Charlotte I. S. Barker, Isobel Oldfield, Barbara J. Philips, Atholl Johnston, Jeffrey Lipman, Andrew Rhodes, Marina Basarab, and et al. 2023. "Population Pharmacokinetic Study of Benzylpenicillin in Critically Unwell Adults" Antibiotics 12, no. 4: 643. https://doi.org/10.3390/antibiotics12040643
APA StyleShah, R. V., Kipper, K., Baker, E. H., Barker, C. I. S., Oldfield, I., Philips, B. J., Johnston, A., Lipman, J., Rhodes, A., Basarab, M., Sharland, M., Almahdi, S., Wake, R. M., Standing, J. F., & Lonsdale, D. O. (2023). Population Pharmacokinetic Study of Benzylpenicillin in Critically Unwell Adults. Antibiotics, 12(4), 643. https://doi.org/10.3390/antibiotics12040643








