Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope?
Abstract
:1. Introduction
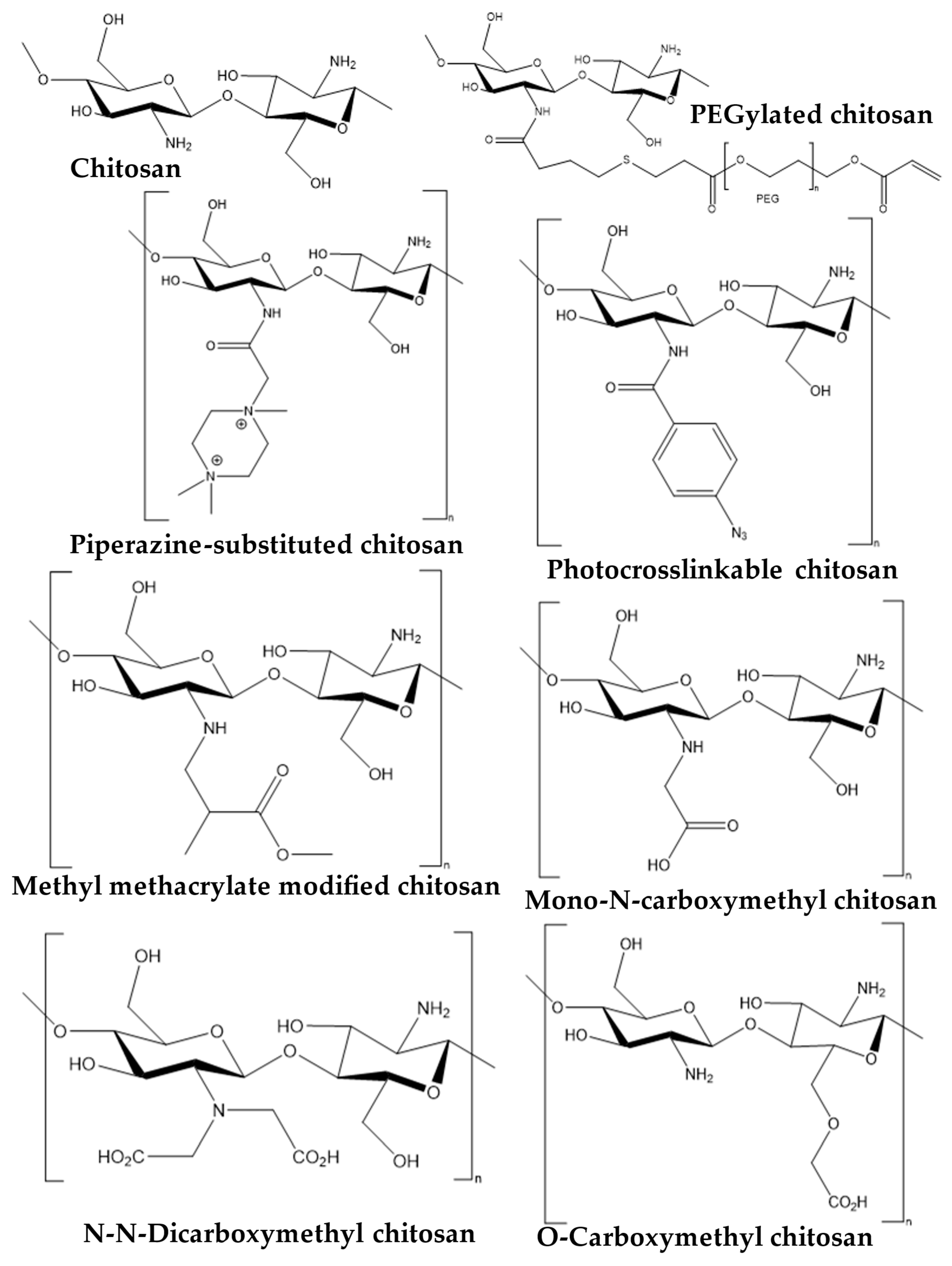
2. Snapshot of Antipathogenic Applications of Chitosan Composites and Its Derivatives
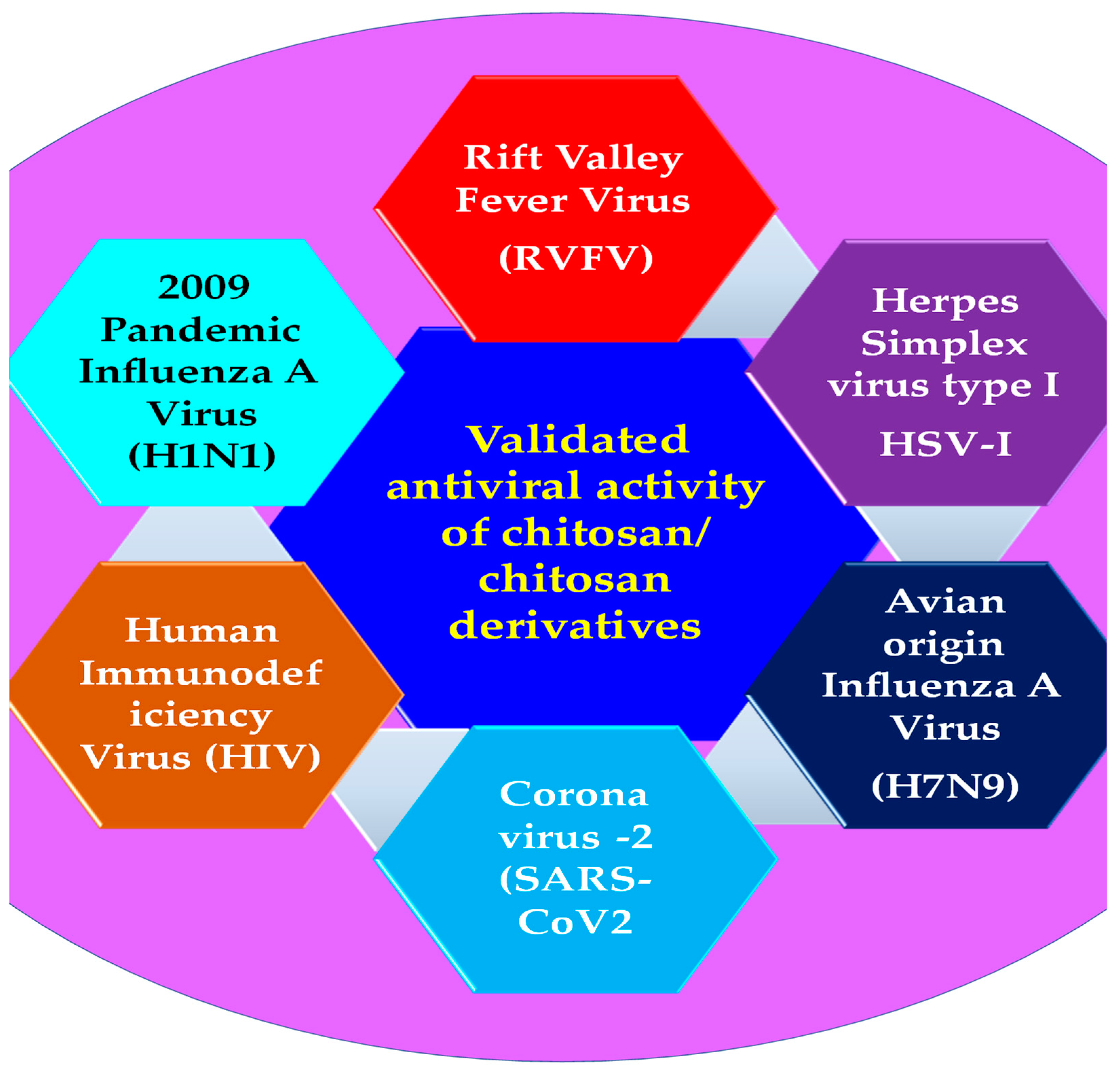
3. Antiviral Applications of Chitosan Composites and Derivatives
4. Anti-COVID-19 Applications of Chitosan Composites and Derivatives
5. Future Perspective and Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fonte, P.; Araújo, F.; Silva, C.; Pereira, C.; Reis, S.; Santos, H.A.; Sarmento, B. Polymer-Based Nanoparticles for Oral Insulin Delivery: Revisited Approaches. Biotechnol. Adv. 2015, 33, 1342–1354. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is Nanotechnology a Boon for Oral Drug Delivery? Drug Discov. Today 2014, 19, 1530–1546. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N. Current Views on Fungal Chitin/Chitosan, Human Chitinases, Food Preservation, Glucans, Pectins and Inulin: A Tribute to Henri Braconnot, Precursor of the Carbohydrate polymers science, on the chitin bicentennial. Carbohydrate 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Deacetylation Modification Techniques of Chitin and Chitosan. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 117–133. ISBN 9780081002308. [Google Scholar]
- Xia, Z.; Wu, S.; Chen, J. Preparation of Water Soluble Chitosan by Hydrolysis Using Hydrogen Peroxide. Int. J. Biol. Macromol. 2013, 59, 242–245. [Google Scholar] [CrossRef]
- Lu, S.; Song, X.; Cao, D.; Chen, Y.; Yao, K. Preparation of Water-Soluble Chitosan. J. Appl. Polym. Sci. 2004, 91, 3497–3503. [Google Scholar] [CrossRef]
- Ilium, L. Chitosan and Its Use as a Pharmaceutical Excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A Special Report on the Chitosan-Based Hemostatic Dressing: Experience in Current Combat Operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, F.; Teranishi, K.; Okada, T.; Parreño-Sacdalan, D.; Hupalo, D.; McNamee, G.; Carr, W.; Burris, D.; McCarron, R. Comparison of Combat Gauze and TraumaStat in Two Severe Groin Injury Models. J. Surg. Res. 2011, 169, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-Biofilm Properties of Chitosan-Coated Surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan Coupling Makes Microbial Biofilms Susceptible to Antibiotics. Sci. Rep. 2013, 3, 3364. [Google Scholar] [CrossRef] [Green Version]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for Gene Delivery: Methods for Improvement and Applications. Adv. Colloid Interface Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan—As a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Kurakula, M. Prospection of Recent Chitosan Biomedical Trends: Evidence from Patent Analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and PH-Responsive Chitosan-Based Injectable Hydrogels for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110862. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan Based Biocomposite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The Growing Role of Nanotechnology in Combating Infectious Disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Bowman, K.; Leong, K.W. Chitosan Nanoparticles for Oral Drug and Gene Delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Farrera-Rebollo, R.R.; Andraca-Adame, J.A.; Arzate-Vázquez, I.; Mendez-Mendez, J.V.; Moreno-Ruiz, L.A. Physical and Structural Characterisation of Zein and Chitosan Edible Films Using Nanotechnology Tools. Int. J. Biol. Macromol. 2013, 61, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. The Safety of Chitosan as a Pharmaceutical Excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2017, 50, 33–44. [Google Scholar] [CrossRef]
- Sharland, M.; Saroey, P.; Berezin, E.N. The Global Threat of Antimicrobial Resistance—The Need for Standardized Surveillance Tools to Define Burden and Develop Interventions. J. Pediatr. 2015, 91, 410–412. [Google Scholar] [CrossRef] [Green Version]
- Bertesteanu, S.; Chifiriuc, M.C.; Grumezescu, A.M.; Printza, A.G.; Marie-Paule, T.; Grumezescu, V.; Mihaela, V.; Lazar, V.; Grigore, R. Biomedical Applications of Synthetic, Biodegradable Polymers for the Development of Anti-Infective Strategies. Curr. Med. Chem. 2014, 21, 3383–3390. [Google Scholar] [CrossRef]
- O’Rourke, A.; Beyhan, S.; Choi, Y.; Morales, P.; Chan, A.P.; Espinoza, J.L.; Dupont, C.L.; Meyer, K.J.; Spoering, A.; Lewis, K.; et al. Mechanism-of-Action Classification of Antibiotics by Global Transcriptome Profiling. Antimicrob. Agents Chemother. 2020, 64, e01207-19. [Google Scholar] [CrossRef] [Green Version]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological Effects of Chitosan and Its Derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Garg, P.; Kumar, S.; Pandey, S.; Seonwoo, H.; Choung, P.-H.; Koh, J.; Chung, J.H. Triphenylamine Coupled Chitosan with High Buffering Capacity and Low Viscosity for Enhanced Transfection in Mammalian Cells, in Vitro and in Vivo. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 6053–6065. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Danin-Poleg, Y.; Kashi, Y.; Bianco-Peled, H. Modified Chitosan: A Step toward Improving the Properties of Antibacterial Food Packages. Food Packag. Shelf Life 2014, 1, 160–169. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable Chitosan as a Dressing for Wound Occlusion and Accelerator in Healing Process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Capel, V.; Vllasaliu, D.; Watts, P.; Clarke, P.A.; Luxton, D.; Grabowska, A.M.; Mantovani, G.; Stolnik, S. Water-Soluble Substituted Chitosan Derivatives as Technology Platform for Inhalation Delivery of SiRNA. Drug Deliv. 2018, 25, 644–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl Methacrylate Modified Chitosan: Synthesis, Characterization and Application in Drug and Gene Delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Ramos, V.; Stanic, V.; Dubini, B.; Mattioli-Belmonte, M.; Tosi, G.; Giardino, R. Osteogenesis Promoted by Calcium Phosphate N,N-Dicarboxymethyl Chitosan. Carbohydr. Polym. 1998, 36, 267–276. [Google Scholar] [CrossRef]
- Kantak, M.N.; Bharate, S.S. Analysis of Clinical Trials on Biomaterial and Therapeutic Applications of Chitosan: A Review. Carbohydr. Polym. 2022, 278, 118999. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485. [Google Scholar]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and Its Derivatives for Tissue Engineering Applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and Its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-Based Gastrointestinal Delivery Systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan Biomaterials Application in Dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.D. Potential Aspects of Chitosan as Pharmaceutical Excipient. Acta Pol. Pharm. 2011, 68, 619–622. [Google Scholar]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [Green Version]
- Kumar, U.S.; Afjei, R.; Ferrara, K.; Massoud, T.F.; Paulmurugan, R. Gold-Nanostar-Chitosan-Mediated Delivery of SARS-CoV-2 DNA Vaccine for Respiratory Mucosal Immunization: Development and Proof-of-Principle. ACS Nano 2021, 15, 17582–17601. [Google Scholar] [CrossRef]
- Beniac, D.R.; Andonov, A.; Grudeski, E.; Booth, T.F. Architecture of the SARS Coronavirus Prefusion Spike. Nat. Struct. Mol. Biol. 2006, 13, 751–752. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Knobler, R.L.; Powell, H.; Buchmeier, M.J. Monoclonal Antibodies to Murine Hepatitis Virus-4 (Strain JHM) Define the Viral Glycoprotein Responsible for Attachment and Cell—Cell Fusion. Virology 1982, 119, 358–371. [Google Scholar] [CrossRef]
- Luytjes, W.; Sturman, L.S.; Bredenbeek, P.J.; Charite, J.; van der Zeijst, B.A.; Horzinek, M.C.; Spaan, W.J. Primary Structure of the Glycoprotein E2 of Coronavirus MHV-A59 and Identification of the Trypsin Cleavage Site. Virology 1987, 161, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, P.A. Clinical Features and Diagnostic Criteria of Atopic Dermatitis in Relation to Age. Pediatr. Allergy Immunol. 2001, 12 (Suppl. 14), 69–74. [Google Scholar] [CrossRef]
- Lopes, C.; Soares, J.; Tavaria, F.; Duarte, A.; Correia, O.; Sokhatska, O.; Severo, M.; Silva, D.; Pintado, M.; Delgado, L.; et al. Chitosan Coated Textiles May Improve Atopic Dermatitis Severity by Modulating Skin Staphylococcal Profile: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0142844. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Škalko-Basnet, N.; Jøraholmen, M.W. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Mar. Drugs 2021, 19, 697. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Nam, J.-P.; Kim, J.-H.; Kim, Y.-M.; Nah, J.-W.; Jang, M.-K. Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria. Int. J. Mol. Sci. 2015, 16, 7995–8007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Ji, Y.; Zhu, Y.; Wu, X.; Mei, L.; Zhang, H.; Deng, J.; Wang, S. Antibacterial Effect of Chitosan and Its Derivative on Enterococcus Faecalis Associated with Endodontic Infection. Exp. Ther. Med. 2020, 19, 3805–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into Chitosan Antibiofilm Activity against Methicillin-Resistant Staphylococcus Aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial Activity of Lysozyme-Chitosan Oligosaccharide Conjugates (LYZOX) against Pseudomonas Aeruginosa, Acinetobacter Baumannii and Methicillin-Resistant Staphylococcus Aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef]
- Abadi, M.S.S.; Mirzaei, E.; Bazargani, A.; Gholipour, A.; Heidari, H.; Hadi, N. Antibacterial Activity and Mechanism of Action of Chitosan Nanofibers against Toxigenic Clostridioides (Clostridium) Difficile Isolates. Ann. Ig. 2020, 32, 72–80. [Google Scholar]
- Zhang, F.; Ramachandran, G.; Mothana, R.A.; Noman, O.M.; Alobaid, W.A.; Rajivgandhi, G.; Manoharan, N. Anti-Bacterial Activity of Chitosan Loaded Plant Essential Oil against Multi Drug Resistant K. Pneumoniae. Saudi J. Biol. Sci. 2020, 27, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of Cefotaxime Impregnated Chitosan as Nano-Antibiotics: De Novo Strategy to Combat Biofilm Forming Multi-Drug Resistant Pathogens. Front. Microbiol. 2016, 7, 330. [Google Scholar] [CrossRef] [Green Version]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal Activity of Low Molecular Weight Chitosan against Clinical Isolates of Candida Spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Ganan, M.; Lorentzen, S.B.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antibiotic Saving Effect of Combination Therapy through Synergistic Interactions between Well-Characterized Chito-Oligosaccharides and Commercial Antifungals against Medically Relevant Yeasts. PLoS ONE 2019, 14, e0227098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida Albicans, Candida Tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, Y.; Zimmerli, S. Common Invasive Fungal Diseases: An Overview of Invasive Candidiasis, Aspergillosis, Cryptococcosis, and Pneumocystis Pneumonia. Swiss Med. Wkly. 2016, 146, w14281. [Google Scholar] [CrossRef] [Green Version]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive Fungal Infections in the ICU: How to Approach, How to Treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [Green Version]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Effects of Chitosan on Candida Albicans: Conditions for Its Antifungal Activity. Biomed Res. Int. 2013, 2013, 527549. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Ma, S.; Tao, H.; Jin, L.; Luo, Y.; Zheng, J.; Xiang, W.; Deng, H. Chitosan-Based Drug Delivery Systems: From Synthesis Strategy to Osteomyelitis Treatment—A Review. Carbohydr. Polym. 2021, 251, 117063. [Google Scholar] [CrossRef]
- Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, G.R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Novel Carboxymethyl Chitin Nanoparticles for Cancer Drug Delivery Applications. Carbohydr. Polym. 2010, 79, 1073–1079. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Dash, M.; Samal, S.K.; Dubruel, P.; Lupusoru, C.E.; Lupusoru, R.-V.; Dragostin, O.; Profire, L. Development, Optimization and Biological Evaluation of Chitosan Scaffold Formulations of New Xanthine Derivatives for Treatment of Type-2 Diabetes Mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the Immense Potential of Chitosan in Fighting a Wide Spectrum of Viruses: A Plausible Molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Hoseini, M.H.M. Chitin and Chitosan as Tools to Combat COVID-19: A Triple Approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, Characterization and in Vitro Antiviral Activity Evaluation of Foscarnet-Chitosan Nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Kubbinga, M.; Nguyen, M.A.; Staubach, P.; Teerenstra, S.; Langguth, P. The Influence of Chitosan on the Oral Bioavailability of Acyclovir—A Comparative Bioavailability Study in Humans. Pharm. Res. 2015, 32, 2241–2249. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, L.; et al. In Vivo Nose-to-Brain Delivery of the Hydrophilic Antiviral Ribavirin by Microparticle Agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Lungare, S.; Bowen, J.; Badhan, R. Development and Evaluation of a Novel Intranasal Spray for the Delivery of Amantadine. J. Pharm. Sci. 2016, 105, 1209–1220. [Google Scholar] [CrossRef]
- WuDunn, D.; Spear, P.G. Initial Interaction of Herpes Simplex Virus with Cells Is Binding to Heparan Sulfate. J. Virol. 1989, 63, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Compton, T.; Nowlin, D.M.; Cooper, N.R. Initiation of Human Cytomegalovirus Infection Requires Initial Interaction with Cell Surface Heparan Sulfate. Virology 1993, 193, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Liao, C.L.; Lee, Y.L.; Lin, Y.L. Highly Sulfated Forms of Heparin Sulfate Are Involved in Japanese Encephalitis Virus Infection. Virology 2001, 286, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis Virus to BHK Cells Selects for Use of Heparan Sulfate as an Attachment Receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrnes, A.P.; Griffin, D.E. Binding of Sindbis Virus to Cell Surface Heparan Sulfate. J. Virol. 1998, 72, 7349–7356. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue Virus Infectivity Depends on Envelope Protein Binding to Target Cell Heparan Sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Kroschewski, H.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Role of Heparan Sulfate for Attachment and Entry of Tick-Borne Encephalitis Virus. Virology 2003, 308, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 Activity of Low Molecular Weight Sulfated Chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Chirkov, S.N. Influence of the Chitosan Oligomer on the Phage Particles and Reproduction of Phage 1-97A in the Culture of Bacillus Thuringiensis. Microbiology 2001, 70, 706–710. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Stepanov, O.A.; Prokof’eva, M.M.; Stocking, K.; Varlamov, V.P.; Levov, A.N.; Vikhoreva, G.A.; Spirin, P.V.; Mikhailov, S.N.; Prassolov, V.S. Replication-Competent Gamma-Retrovirus Mo-MuLV Expressing Green Fluorescent Protein as Efficient Tool for Screening of Inhibitors of Retroviruses That Use Heparan Sulfate as Primary Cell Receptor. Mol. Biol. 2012, 46, 457–466. [Google Scholar] [CrossRef]
- Nishimura, S.I.; Kai, H.; Shinada, K.; Yoshida, T.; Tokura, S.; Kurita, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Regioselective Syntheses of Sulfated Polysaccharides: Specific Anti-HIV-1 Activity of Novel Chitin Sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, K.; Li, D.; Yu, S.; Cai, J.; Chen, L.; Du, Y. Preparation, Characterization and in Vitro Anticoagulant Activity of Highly Sulfated Chitosan. Int. J. Biol. Macromol. 2013, 52, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.A.G.; Fazely, F.; Koch, J.A.; Vercellotti, S.V.; Ruprecht, R.M. N-Carboxymethylchitosan-N, O-Sulfate as an Anti-HIV-1 Agent. Biochem. Biophys. Res. Commun. 1991, 174, 489–496. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated Chitin and Chitosan as Novel Biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and Sulfated Chitosan Derivatives for Biomedical Applications: A Review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Karadeniz, F.; Kim, S.-K. Anti-HIV Activities of Novel Synthetic Peptide Conjugated Chitosan Oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. [Google Scholar] [CrossRef]
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-Stabilized Colloidal Polyelectrolyte Complexes of Chitosan/Hyaluronan: A Tool for the Inhibition of HIV-1 Infection. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 5455–5463. [Google Scholar] [CrossRef]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of Silver Nanoparticles onto Different Surface Structures of Chitin/Chitosan and Correlations with Antimicrobial Activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral Activity of Silver Nanoparticle/Chitosan Composites against H1N1 Influenza A Virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Zhao, H.; Xu, Y.; Yang, Y.; Lv, X.; Wu, P.; Li, X. Inhibition of Influenza Virus Infection with Chitosan–Sialyloligosaccharides Ionic Complex. Carbohydr. Polym. 2014, 107, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Paulson, J.C. Cell Surface Biology Mediated by Low Affinity Multivalent Protein–Glycan Interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, S.-T.; Chen, C.-Y.; Wu, S.-C. Enterovirus 71 Adsorption on Metal Ion-Composite Chitosan Beads. Biotechnol. Prog. 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Pauls, T. Chitosan as an Antiviral. Bachelor’s Thesis, University of Arkansas, Fayetteville, NC, USA, 2016. [Google Scholar]
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The Inhibitory Effects and Mechanisms of 3,6-O-Sulfated Chitosan against Human Papillomavirus Infection. Carbohydr. Polym. 2018, 198, 329–338. [Google Scholar] [CrossRef]
- Ishihara, C.; Yoshimatsu, K.; Tsuji, M.; Arikawa, J.; Saiki, I.; Tokura, S.; Azuma, I. Anti-Viral Activity of Sulfated Chitin Derivatives against Friend Murine Leukaemia and Herpes Simplex Type-1 Viruses. Vaccine 1993, 11, 670–674. [Google Scholar] [CrossRef]
- Hassan, M.; Mohamed, A.; Taher, F.; Kamel, M. Antimicrobial Activities of Chitosan Nanoparticles Prepared from Lucilia Cuprina Maggots (Diptera: Calliphoridae). J. Egypt. Soc. Parasitol. 2016, 46, 563–570. [Google Scholar]
- Bai, B.; Mi, X.; Xiang, X.; Heiden, P.A.; Heldt, C.L. Non-Enveloped Virus Reduction with Quaternized Chitosan Nanofibers Containing Graphene. Carbohydr. Res. 2013, 380, 137–142. [Google Scholar] [CrossRef]
- Mi, X.; Vijayaragavan, K.S.; Heldt, C.L. Virus Adsorption of Water-Stable Quaternized Chitosan Nanofibers. Carbohydr. Res. 2014, 387, 24–29. [Google Scholar] [CrossRef]
- Ciejka, J.; Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K. Biopolymeric Nano/Microspheres for Selective and Reversible Adsorption of Coronaviruses. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 735–742. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel Polymeric Inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milewska, A.; Kaminski, K.; Ciejka, J.; Kosowicz, K.; Zeglen, S.; Wojarski, J.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. HTCC: Broad Range Inhibitor of Coronavirus Entry. PLoS ONE 2016, 11, e0156552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briz, V.; Poveda, E.; Soriano, V. HIV Entry Inhibitors: Mechanisms of Action and Resistance Pathways. J. Antimicrob. Chemother. 2006, 57, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [Green Version]
- Baranova, E.O.; Shastina, N.S.; Shvets, V.I. Polyanionic Inhibitors of HIV Adsorption. Russ. J. Bioorganic Chem. 2011, 37, 527. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Manigandan, V.; Saravanan, R.; Rajesh, R.P.; Chandrika, B. Structural Characterization and in Vitro Biomedical Activities of Sulfated Chitosan from Sepia Pharaonis. Int. J. Biol. Macromol. 2016, 84, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory Effects of Sulfated Chitosans on RAW 264.7 Mouse Macrophages via the Activation of PI3 K/Akt Signaling Pathway. Int. J. Biol. Macromol. 2018, 108, 1310–1321. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.-S.; Hwang, J.-W.; Han, Y.-K.; Lee, J.-S.; Kim, S.-K.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Bahk, Y.Y.; et al. Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-ΚB Inactivation. Molecules 2014, 19, 18232–18247. [Google Scholar] [CrossRef] [Green Version]
- Chirkov, S.N. The Antiviral Activity of Chitosan. Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The Improved Antiviral Activities of Amino-Modified Chitosan Derivatives on Newcastle Virus. Drug Chem. Toxicol. 2021, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, P.; Gao, G.F.; Cheng, S. Carbohydrate-Functionalized Chitosan Fiber for Influenza Virus Capture. Biomacromolecules 2011, 12, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.-E.; Elshaarawy, R.F.M. Polyphosphonium-Oligochitosans Decorated with Nanosilver as New Prospective Inhibitors for Common Human Enteric Viruses. Carbohydr. Polym. 2019, 226, 115261. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.L.; Hassandarvish, P.; Chee, C.F.; Chan, L.W.; Wong, T.W. Chitosan and Its Derivatives as Polymeric Anti-Viral Therapeutics and Potential Anti-SARS-CoV-2 Nanomedicine. Carbohydr. Polym. 2022, 290, 119500. [Google Scholar] [CrossRef]
- Davis, R.; Zivanovic, S.; D’Souza, D.H.; Davidson, P.M. Effectiveness of Chitosan on the Inactivation of Enteric Viral Surrogates. Food Microbiol. 2012, 32, 57–62. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Surgucheva, N.A.; Chirkov, S.N. Coliphages inactivation using chitosan derivatives. Mikrobiologiia 2000, 69, 261–265. [Google Scholar]
- Sabino, E.C.; Buss, L.F.; Carvalho, M.P.S.; Prete, C.A., Jr.; Crispim, M.A.E.; Fraiji, N.A.; Pereira, R.H.M.; Parag, K.V.; da Silva Peixoto, P.; Kraemer, M.U.G.; et al. Resurgence of COVID-19 in Manaus, Brazil, despite High Seroprevalence. Lancet 2021, 397, 452–455. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Callaway, E. Delta Coronavirus Variant: Scientists Brace for Impact. Nature 2021, 595, 17–18. [Google Scholar] [CrossRef]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of Mortality in Patients Infected with SARS-CoV-2 Variant of Concern 202012/1: Matched Cohort Study. BMJ 2021, 372, n579. [Google Scholar] [CrossRef]
- Chen, Y.; Zuiani, A.; Fischinger, S.; Mullur, J.; Atyeo, C.; Travers, M.; Lelis, F.J.N.; Pullen, K.M.; Martin, H.; Tong, P.; et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell 2020, 183, 1496–1507.e16. [Google Scholar] [CrossRef]
- Burioni, R.; Topol, E.J. Assessing the Human Immune Response to SARS-CoV-2 Variants. Nat. Med. 2021, 27, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. MRNA Vaccine-Elicited Antibodies to SARS-CoV-2 and Circulating Variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nie, J.; Wu, J.; Zhang, L.; Ding, R.; Wang, H.; Zhang, Y.; Li, T.; Liu, S.; Zhang, M.; et al. SARS-CoV-2 501Y.V2 Variants Lack Higher Infectivity but Do Have Immune Escape. Cell 2021, 184, 2362–2371.e9. [Google Scholar] [CrossRef]
- London School of Hygiene & Tropical Medicine COVID-19 Vaccine Tracker. Available online: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ (accessed on 5 August 2022).
- Zhang, Y.-N.; Paynter, J.; Sou, C.; Fourfouris, T.; Wang, Y.; Abraham, C.; Ngo, T.; Zhang, Y.; He, L.; Zhu, J. Mechanism of a COVID-19 Nanoparticle Vaccine Candidate That Elicits a Broadly Neutralizing Antibody Response to SARS-CoV-2 Variants. Sci. Adv. 2021, 7, eabj3107. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Burgers, W.A. SARS-CoV-2 Evolution and Vaccines: Cause for Concern? Lancet Respir. Med. 2021, 9, 333–335. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and MRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Lupala, C.S.; Kumar, V.; Su, X.-D.; Wu, C.; Liu, H. Computational Insights into Differential Interaction of Mammalian Angiotensin-Converting Enzyme 2 with the SARS-CoV-2 Spike Receptor Binding Domain. Comput. Biol. Med. 2022, 141, 105017. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2523. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Andreano, E.; Piccini, G.; Licastro, D.; Casalino, L.; Johnson, N.V.; Paciello, I.; Dal Monego, S.; Pantano, E.; Manganaro, N.; Manenti, A.; et al. SARS-CoV-2 Escape from a Highly Neutralizing COVID-19 Convalescent Plasma. Proc. Natl. Acad. Sci. USA 2021, 118, e2103154118. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; de Oliveira, T.; Vermeulen, M.; van der Berg, K.; et al. SARS-CoV-2 501Y.V2 Escapes Neutralization by South African COVID-19 Donor Plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar] [CrossRef]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 Variant of Concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Ortega-Rivera, O.A.; Shin, M.D.; Moreno-Gonzalez, M.A.; Steinmetz, N.F. Injectable Slow-Release Hydrogel Formulation of a Plant Virus-Based COVID-19 Vaccine Candidate. Biomacromolecules 2022, 23, 1812–1825. [Google Scholar] [CrossRef]
- Modak, C.; Jha, A.; Sharma, N.; Kumar, A. Chitosan Derivatives: A Suggestive Evaluation for Novel Inhibitor Discovery against Wild Type and Variants of SARS-CoV-2 Virus. Int. J. Biol. Macromol. 2021, 187, 492–512. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Liu, K.; Liu, D.; Guo, X.; Ge, Y.; Li, J.; Cui, L.; et al. HTCC as a Highly Effective Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. bioRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, D.; Mishra, V.; Das, D.; Kaur, K.; Suresh, M.R. Dendritic Cell Targeted Chitosan Nanoparticles for Nasal DNA Immunization against SARS CoV Nucleocapsid Protein. Mol. Pharm. 2012, 9, 946–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, O.; Tavares, J.; de Sousa, A.; Borchard, G.; Junginger, H.E.; Cordeiro-da-Silva, A. Evaluation of the Immune Response Following a Short Oral Vaccination Schedule with Hepatitis B Antigen Encapsulated into Alginate-Coated Chitosan Nanoparticles. Eur. J. Pharm. Sci. 2007, 32, 278–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, P.D.; Okino, C.H.; Fernando, F.S.; Pavani, C.; Casagrande, V.M.; Lopez, R.F.V.; Montassier, M.d.F.S.; Montassier, H.J. Inactivated Infectious Bronchitis Virus Vaccine Encapsulated in Chitosan Nanoparticles Induces Mucosal Immune Responses and Effective Protection against Challenge. Vaccine 2018, 36, 2630–2636. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/Curcumin Loaded Albumin Nanoparticles Coated by Chitosan as Muco-Inhalable Delivery System Observing Anti-Inflammatory and Anti COVID-19 Characterizations in Oleic Acid Triggered Lung Injury and in Vitro COVID-19 Experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Dwivedi, P.; Tiwary, D.; Narvi, S.S.; Tewari, R.P.; Shukla, K.P. Curcuma Longa Aided Ag/CS Nanocomposite Coating of Surfaces as SARS-CoV-2 Contamination Minimizing Measure towards Containment of COVID-19: A Perspective. Lett. Appl. NanoBioSci 2020, 9, 1485–1493. [Google Scholar]
- Vörös-Horváth, B.; Živković, P.; Bánfai, K.; Bóvári-Biri, J.; Pongrácz, J.; Bálint, G.; Pál, S.; Széchenyi, A. Preparation and Characterization of ACE2 Receptor Inhibitor-Loaded Chitosan Hydrogels for Nasal Formulation to Reduce the Risk of COVID-19 Viral Infection. ACS Omega 2022, 7, 3240–3253. [Google Scholar] [CrossRef]
- Orkhan, F.; Melike, U.; Cihan, G.; Faruk, D.O.; Samet, B.; Ilknur, U.; Alemdar, J. Others RBD and ACE2 Embedded Chitosan Nanoparticles as a Prevention Approach for SARS-CoV 2. Biomed. J. Sci. Tech. Res. 2021, 37, 29193–29197. [Google Scholar]
- Rezaei, F.S.; Khorshidian, A.; Beram, F.M.; Derakhshani, A.; Esmaeili, J.; Barati, A. 3D Printed Chitosan/Polycaprolactone Scaffold for Lung Tissue Engineering: Hope to Be Useful for COVID-19 Studies. RSC Adv. 2021, 11, 19508–19520. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Abdel-Salam, A.I.; Moatasim, Y.; Gomaa, M.R.; Abdel Fattah, N.F.; Emam, M.H.; Ali, F.; ElShehaby, H.A.; Ragab, E.A.; Alam El-Din, H.M.; et al. Antiviral Activity of Chitosan Nanoparticles Encapsulating Silymarin (Sil-CNPs) against SARS-CoV-2 (in Silico and in Vitro Study). RSC Adv. 2022, 12, 15775–15786. [Google Scholar] [CrossRef]
- Hathout, R.M.; Kassem, D.H. Positively Charged Electroceutical Spun Chitosan Nanofibers Can Protect Health Care Providers From COVID-19 Infection: An Opinion. Front. Bioeng. Biotechnol. 2020, 8, 885. [Google Scholar] [CrossRef]
- Wang, I.-J.; Chen, Y.-C.; Su, C.; Tsai, M.-H.; Shen, W.-T.; Bai, C.-H.; Yu, K.-P. Effectiveness of the Nanosilver/TiO2-Chitosan Antiviral Filter on the Removal of Viral Aerosols. J. Aerosol Med. Pulm. Drug Deliv. 2021, 34, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Maharani, D.K.; Sanjaya, I.G.M.; Amaria, A.; Anggraeni, M.A.; Jannah, L.R. Molecular Docking Analysis Chitosan-Zeolite-ZnO Nanocomposite and Its Potency Against SARS-CoV-2. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1125, 012006. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Che, X.; Chang, J.; Yang, Y.; Yu, L. Evaluation on the Immune Efficiency of Bovine Coronavirus N Protein-Loaded Chitosan Microspheres. Zhongguo Yufang Shouyi Xuebao/Chin. J. Prev. Vet. Med. 2009, 31, 882–886. [Google Scholar]
- Mohammadi, Z.; Eini, M.; Rastegari, A.; Tehrani, M.R. Chitosan as a Machine for Biomolecule Delivery: A Review. Carbohydr. Polym. 2021, 256, 117414. [Google Scholar] [CrossRef] [PubMed]
- Ejeromedoghene, O.; Oderïnde, O.; Egejuru, G.; Adewuyï, S. Chitosan-Drug Encapsulation as a Potential Candidate for COVID-19 Drug Delivery Systems: A Review. J. Turk. Chem. Soc. Sect. Chem. 2020, 7, 851–864. [Google Scholar] [CrossRef]
- Tatlow, D.; Tatlow, C.; Tatlow, S.; Tatlow, S. A Novel Concept for Treatment and Vaccination against COVID-19 with an Inhaled Chitosan-Coated DNA Vaccine Encoding a Secreted Spike Protein Portion. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1874–1878. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A Review of the Antiviral Activity of Chitosan, Including Patented Applications and Its Potential Use against COVID-19. J. Appl. Microbiol. 2022, 132, 41–58. [Google Scholar] [CrossRef]
- Kalathiya, U.; Padariya, M.; Mayordomo, M.; Lisowska, M.; Nicholson, J.; Singh, A.; Baginski, M.; Fahraeus, R.; Carragher, N.; Ball, K.; et al. Highly Conserved Homotrimer Cavity Formed by the SARS-CoV-2 Spike Glycoprotein: A Novel Binding Site. J. Clin. Med. Res. 2020, 9, 1473. [Google Scholar] [CrossRef]
- Alitongbieke, G.; Li, X.-M.; Wu, Q.-C.; Lin, Z.-C.; Huang, J.-F.; Xue, Y.; Liu, J.-N.; Lin, J.-M.; Pan, T.; Chen, Y.-X.; et al. Effect of β-Chitosan on the Binding Interaction between SARS-CoV-2 S-RBD and ACE2. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing Use of Theranostic Nanoparticles as a Life-Saving Strategy for Treating COVID-19 Patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef]
- Pyrć, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; Mellor, R.; Kalber, T.L.; Fernandes-Reyes, D.; et al. SARS-CoV-2 Inhibition in Human Airway Epithelial Cells Using a Mucoadhesive, Amphiphilic Chitosan That May Serve as an Anti-Viral Nasal Spray. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Cho, N.J.; Glenn, J.S. Materials Science Approaches in the Development of Broad-Spectrum Antiviral Therapies. Nat. Mater. 2020, 19, 813–816. [Google Scholar] [CrossRef] [PubMed]


| Chitosan Composite/Derivative | Anti-COVID Application | Antiviral Mechanism | References |
|---|---|---|---|
| Chitosan nanoparticle in aerosol formulations for anti-COVID-19 drugs (Novochizol™) | Chitosan NPs in Novochizol™ adhere to mucosal membranes and deliver drugs, siRNA, and peptides | Slow release of anti-COVID-19 drug in the infected area by slow degradation of chitosan | [76] |
| Chitosan and genipin nano/micro spheres | Antiviral activity against HCoV-NL63 and HCoV-OC43 | Sphere complex exerts electrostatic interaction with Viral nucleic acid | [112] |
| A cationically modified chitosan derivative, N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC), and hydrophobically-modified HTCC | Chitosan derivatives as inhibitors for coronavirus NL63 | Act as inhibitors of HCoV-NL63 replication | [113] |
| Cationic modifications of chitosan, N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC), and hydrophobically-modified derivative (HM-HTCC) | Inhibition of the coronavirus HCoV-NL63 | HTCC and HM-HTCC inhibit interaction of a virus with its receptor | [114] |
| Chitosan hydrogels with dicarboxylic acids (malic and glutaric acid) | Viral spike protein to the ACE2 receptors | Controlling the risk of SARS-CoV-2 infection. | [159] |
| Chitosan nanoparticles (CNPs) | CNPs encapsulated with silymarin (Sil–CNPs) | Mechanism was studied | [161] |
| Chitosan nanofibers made from N,N,N-trimethyl chitosan (TMC), the single N-quaternized (QCS) and the double N-diquaternized (DQCS) | Making Personal Protective Equipment (PPE) with chitosan fibers | SARS-CoV-2 viral repulsion by Cationic amino group | [163] |
| Zero-valent nanosilver/titania-chitosan (nano-Ag0/TiO2-CS) filter bed | Removal of viral aerosols to minimize the risk of airborne transmission | Reducing infection caused by COVID-19 tested using MS2 bacteriophages | [164] |
| Chitosan-Zeolite-ZnO nanocomposite | Identification of nanocomposite and SARS-CoV-2 glycoprotein receptors RCSB and PDB interaction by molecular docking | Antiviral activity chitosan–zeolite and ZnO against SARS-CoV-2 The SARS-CoV-2 ligand and receptor | [165] |
| A cationically modified chitosan derivative, HTCC | Chitosan derivative HTCC as inhibitors for coronavirus MERS-CoV and SARS-CoV-2 infection | Inhibiting receptor protein | [153] |
| Chitosan nanoparticles | Chitosan nanoparticles act as carrier molecules for DNA vaccine expressing SARS-CoV nucleocapsid protein | Activating T cells to produce IgG and nasal IgA antibodies against SARS-CoV | [154] |
| Chitosan and its derivatives (In silico studies) | Inhibition of SARS-CoV-2 viral binding with host cells | Inhibition of viral spike protein binding to the ACE2 receptors | [171] |
| β-chitosan | Inhibition of SARS-CoV-2 viral binding with host cells | Binding interaction between SARS-CoV-2 S-RBD and ACE2 receptors | [172] |
| Nanochitosan | Application as vaccine adjuvant or vehicles for delivery of | Vaccine delivery via the intranasal route to fight against SARS-CoV-2 | [173] |
| Amphiphilic chitosan Npalmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan (GCPQ), | As a COVID-19 prophylactic agent | Reduce the infectivity of SARS-CoV-2 in A549ACE2+ and Vero E6 cells and in human airway epithelial cells | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopal, J.; Muthu, M.; Pushparaj, S.S.C.; Sivanesan, I. Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope? Antibiotics 2023, 12, 665. https://doi.org/10.3390/antibiotics12040665
Gopal J, Muthu M, Pushparaj SSC, Sivanesan I. Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope? Antibiotics. 2023; 12(4):665. https://doi.org/10.3390/antibiotics12040665
Chicago/Turabian StyleGopal, Judy, Manikandan Muthu, Suraj Shiv Charan Pushparaj, and Iyyakkannu Sivanesan. 2023. "Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope?" Antibiotics 12, no. 4: 665. https://doi.org/10.3390/antibiotics12040665
APA StyleGopal, J., Muthu, M., Pushparaj, S. S. C., & Sivanesan, I. (2023). Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope? Antibiotics, 12(4), 665. https://doi.org/10.3390/antibiotics12040665







