Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis
Abstract
:1. Introduction
2. Materials and Methods
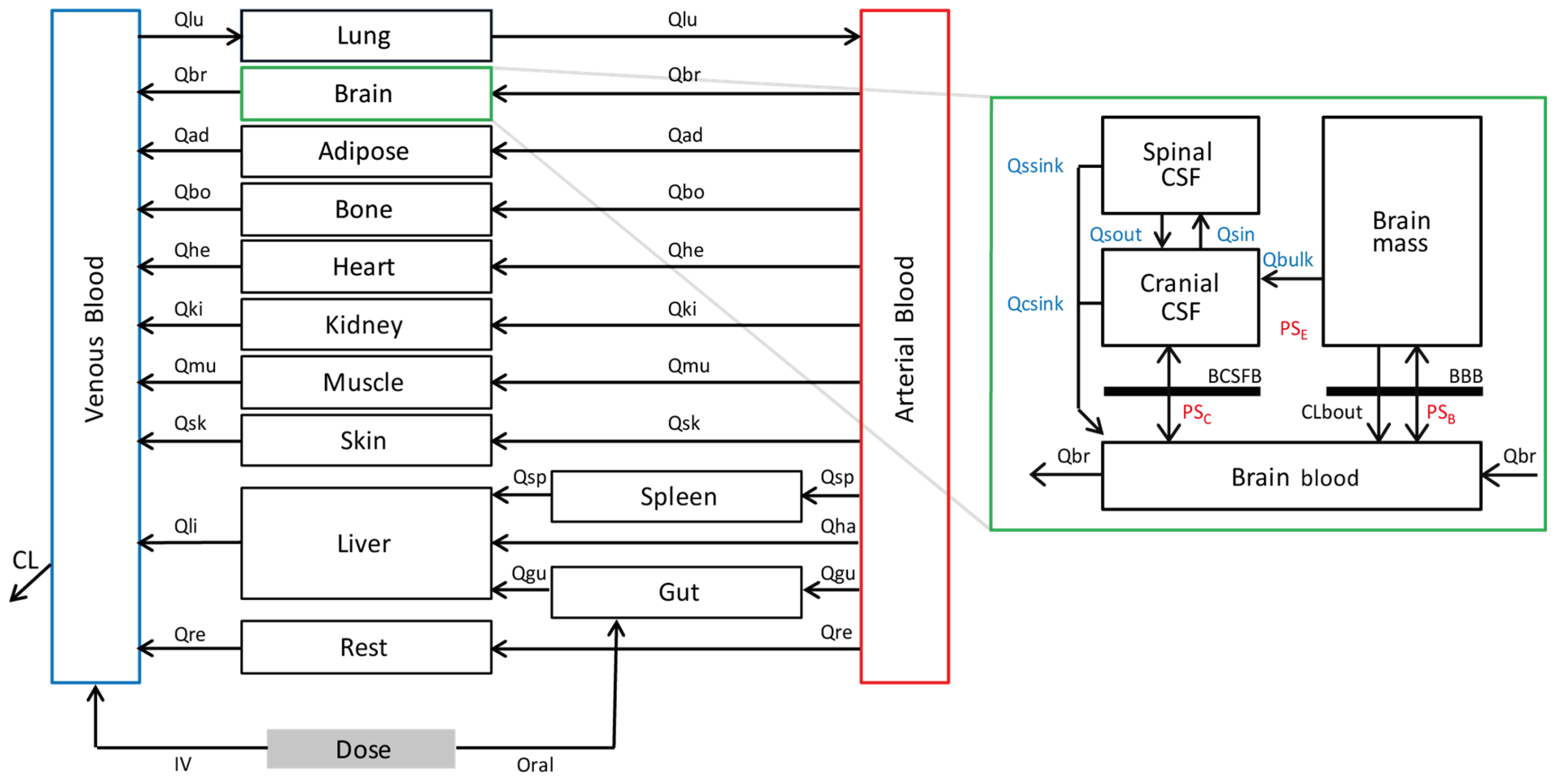
2.1. Development of a Linezolid PBPK Model
2.2. Transporter-Mediated Clearance of Linezolid
2.3. Evaluation of the CNS Model-Plasma and CSF Exposures
2.4. Extrapolation of the CNS Model-Target Attainment in TB Patients
3. Results
3.1. Transporter-Mediated Clearance of Linezolid
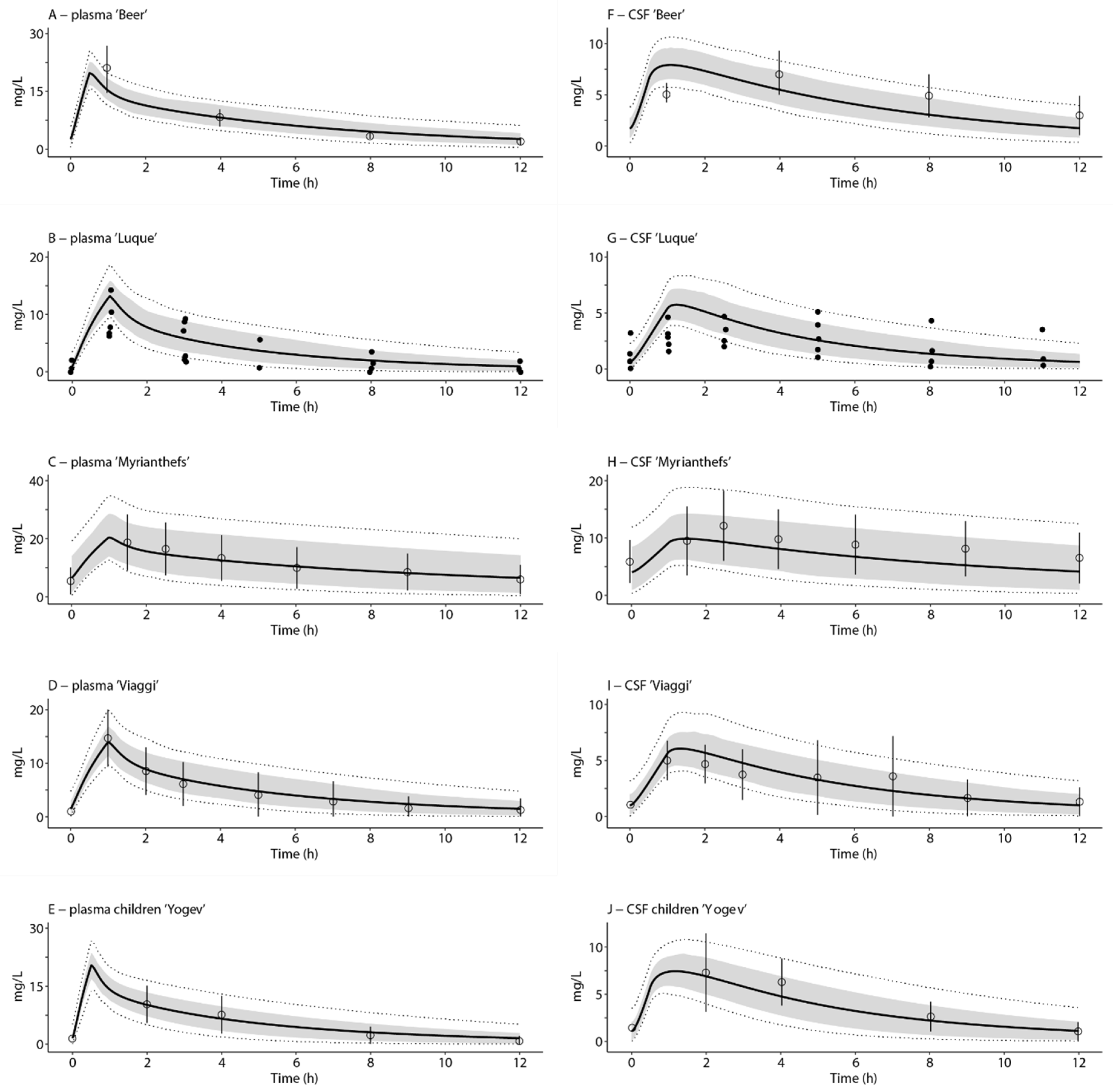
3.2. Evaluation of the CNS Model–Plasma and CSF Exposures
3.3. Extrapolation of the CNS Model-Target Attainment in TB Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfizer Inc. (per FDA). Product Information: ZYVOX(R) Intravenous Injection, Oral Tablets Suspension, Linezolid Intravenous Injection, Oral Tablets Suspension; New York, NY, USA. 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021130s032,021131s026,021132s031lbl.pdf (accessed on 29 March 2023).
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. 2019. Available online: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ (accessed on 29 March 2023).
- Singh, B.; Cocker, D.; Ryan, H.; Sloan, D.J. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst. Rev. 2019, 3, CD012836. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Te Brake, L.; Atherton, R.; Ruslami, R.; Dooley, K.E.; Aarnoutse, R.; Van Crevel, R. Intensified antibiotic treatment of tuberculous meningitis. Expert Rev. Clin. Pharmacol. 2019, 12, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.; Engelhardt, K.W.; Pfausler, B.; Broessner, G.; Helbok, R.; Lackner, P.; Brenneis, C.; Kaehler, S.T.; Georgopoulos, A.; Schmutzhard, E. Pharmacokinetics of Intravenous Linezolid in Cerebrospinal Fluid and Plasma in Neurointensive Care Patients with Staphylococcal Ventriculitis Associated with External Ventricular Drains. Antimicrob. Agents Chemother. 2007, 51, 379–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque, S.; Grau, S.; Alvarez-Lerma, F.; Ferrández, O.; Campillo, N.; Horcajada, J.P.; Basas, M.; Lipman, J.; Roberts, J. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int. J. Antimicrob. Agents 2014, 44, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Myrianthefs, P.; Markantonis, S.L.; Vlachos, K.; Anagnostaki, M.; Boutzouka, E.; Panidis, D.; Baltopoulos, G. Serum and Cerebrospinal Fluid Concentrations of Linezolid in Neurosurgical Patients. Antimicrob. Agents Chemother. 2006, 50, 3971–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaggi, B.; Di Paolo, A.; Danesi, R.; Polillo, M.; Ciofi, L.; Del Tacca, M.; Malacarne, P. Linezolid in the central nervous system: Comparison between cerebrospinal fluid and plasma pharmacokinetics. Scand. J. Infect. Dis. 2011, 43, 721–727. [Google Scholar] [CrossRef]
- Davis, A.G.; Wasserman, S.; Stek, C.; Maxebengula, M.; Liang, C.J.; Stegmann, S.; Koekemoer, S.; Jackson, A.; Kadernani, Y.; Bremer, M.; et al. A phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for HIV-associated tuberculous meningitis (The LASER-TBM Trial). Clin. Infect. Dis. 2022, ciac932. [Google Scholar] [CrossRef]
- Sun, F.; Ruan, Q.; Wang, J.; Chen, S.; Jin, J.; Shao, L.; Zhang, Y.; Zhang, W. Linezolid Manifests a Rapid and Dramatic Therapeutic Effect for Patients with Life-Threatening Tuberculous Meningitis. Antimicrob. Agents Chemother. 2014, 58, 6297–6301. [Google Scholar] [CrossRef] [Green Version]
- Kempker, R.R.; Smith, A.G.C.; Avaliani, T.; Gujabidze, M.; Bakuradze, T.; Sabanadze, S.; Avaliani, Z.; Collins, J.M.; Blumberg, H.M.; Alshaer, M.H.; et al. Cycloserine and Linezolid for Tuberculosis Meningitis: Pharmacokinetic Evidence of Potential Usefulness. Clin. Infect. Dis. 2022, 75, 682–689. [Google Scholar] [CrossRef]
- Bolhuis, M.S.; Akkerman, O.W.; Sturkenboom, M.G.G.; Ghimire, S.; Srivastava, S.; Gumbo, T.; Alffenaar, J.-W.C. Linezolid-based Regimens for Multidrug-resistant Tuberculosis (TB): A Systematic Review to Establish or Revise the Current Recommended Dose for TB Treatment. Clin. Infect. Dis. 2018, 67 (Suppl. S3), S327–S335. [Google Scholar] [CrossRef]
- Millard, J.; Pertinez, H.; Bonnett, L.; Hodel, E.M.; Dartois, V.; Johnson, J.L.; Caws, M.; Tiberi, S.; Bolhuis, M.; Alffenaar, J.-W.C.; et al. Linezolid pharmacokinetics in MDR-TB: A systematic review, meta-analysis and Monte Carlo simulation. J. Antimicrob. Chemother. 2018, 73, 1755–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Thuong, N.T.T.; Van Pham, T.; Heemskerk, D.; Pouplin, T.; Tran, C.T.H.; Nguyen, M.T.H.; Nguyen, P.H.; Phan, L.P.; Nguyen, C.V.V.; et al. Pharmacokinetics and Pharmacodynamics of Intensive Antituberculosis Treatment of Tuberculous Meningitis. Clin. Pharmacol. Ther. 2020, 107, 1023–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, E.M.; Dian, S.; Brake, L.T.; Ganiem, A.R.; Yunivita, V.; Van Laarhoven, A.; Van Crevel, R.; Ruslami, R.; Aarnoutse, R.E. Model-Based Meta-analysis of Rifampicin Exposure and Mortality in Indonesian Tuberculous Meningitis Trials. Clin. Infect. Dis. 2020, 71, 1817–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, E.W.; Guglieri-Lopez, B.; Ordonez, A.A.; Ritchie, B.; Klunk, M.H.; Sharma, R.; Chang, Y.S.; Sanchez-Bautista, J.; Frey, S.; Lodge, M.A.; et al. Noninvasive 11C-rifampin positron emission tomography reveals drug biodistribution in tuberculous meningitis. Sci. Transl. Med. 2018, 10, eaau0965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamei, M. Recent Advances in Development and Application of Physiologically-Based Pharmacokinetic (PBPK) Models: A Transition from Academic Curiosity to Regulatory Acceptance. Curr. Pharmacol. Rep. 2016, 2, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaohua, L.; Neuhoff, S.; Johnson, T.N.; Rostami-Hodjegan, A.; Jamei, M. Development of a permeability-limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: Estimating time varying CSF drug concentrations and their variability using in vitro data. Drug Metab. Pharmacokinet. 2016, 31, 224–233. [Google Scholar] [CrossRef]
- Simcyp. 19.1 ed. Available online: https://www.certara.com/ (accessed on 27 February 2021).
- Verscheijden, L.F.M.; Koenderink, J.B.; De Wildt, S.N.; Russel, F.G.M. Development of a physiologically-based pharmacokinetic pediatric brain model for prediction of cerebrospinal fluid drug concentrations and the influence of meningitis. PLoS Comput. Biol. 2019, 15, e1007117. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef]
- Te Brake, L.H.M.; Van Den Heuvel, J.J.M.W.; Buaben, A.O.; Van Crevel, R.; Bilos, A.; Russel, F.G.; Aarnoutse, R.E.; Koenderink, J.B. Moxifloxacin Is a Potent In Vitro Inhibitor of OCT- and MATE-Mediated Transport of Metformin and Ethambutol. Antimicrob. Agents Chemother. 2016, 60, 7105–7114. [Google Scholar] [CrossRef] [Green Version]
- Gandelman, K.; Zhu, T.; Fahmi, O.A.; Glue, P.; Lian, K.; Obach, R.S.; Damle, B. Unexpected Effect of Rifampin on the Pharmacokinetics of Linezolid: In Silico and In Vitro Approaches to Explain Its Mechanism. J. Clin. Pharmacol. 2011, 51, 229–236. [Google Scholar] [CrossRef]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, B.C.; Barker, B.C.; Markewitz, B.A. Decreased Serum Linezolid Levels in a Critically Ill Patient Receiving Concomitant Linezolid and Rifampin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.E.; Yu, J.; Ragueneau-Majlessi, I.; Isoherranen, N. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification. Drug Metab. Dispos. 2015, 43, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.-W.; Marriott, D.J.E.; Neely, M. Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics. Ther. Drug Monit. 2020, 42, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Harausz, E.P.; Garcia-Prats, A.J.; Seddon, J.; Schaaf, H.S.; Hesseling, A.C.; Achar, J.; Bernheimer, J.; Cruz, A.T.; D’Ambrosio, L.; Detjen, A.; et al. New and Repurposed Drugs for Pediatric Multidrug-Resistant Tuberculosis. Practice-based Recommendations. Am. J. Respir. Crit. Care Med. 2017, 195, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prats, A.J.; Schaaf, H.S.; Draper, H.R.; Garcia-Cremades, M.; Winckler, J.; Wiesner, L.; Hesseling, A.C.; Savic, R.M. Pharmacokinetics, optimal dosing, and safety of linezolid in children with multidrug-resistant tuberculosis: Combined data from two prospective observational studies. PLoS Med. 2019, 16, e1002789. [Google Scholar] [CrossRef] [Green Version]
- Alffenaar, J.-W.C.; Kosterink, J.G.W.; Van Altena, R.; van der Werf, T.; Uges, D.R.A.; Proost, J.H. Limited Sampling Strategies for Therapeutic Drug Monitoring of Linezolid in Patients With Multidrug-Resistant Tuberculosis. Ther. Drug Monit. 2010, 32, 97–101. [Google Scholar] [CrossRef]
- Alffenaar, J.-W.C.; Van Altena, R.; Harmelink, I.M.; Filguera, P.; Molenaar, E.; Wessels, A.M.A.; Van Soolingen, D.; Kosterink, J.G.W.; Uges, N.R.A.; Van Der Werf, T.S. Comparison of the Pharmacokinetics of Two Dosage Regimens of Linezolid in Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Patients. Clin. Pharmacokinet. 2010, 49, 559–565. [Google Scholar] [CrossRef]
- Diacon, A.H.; De Jager, V.R.; Dawson, R.; Narunsky, K.; Vanker, N.; Burger, D.A.; Everitt, D.; Pappas, F.; Nedelman, J.; Mendel, C.M. Fourteen-Day Bactericidal Activity, Safety, and Pharmacokinetics of Linezolid in Adults with Drug-Sensitive Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2020, 64, e02012-19. [Google Scholar] [CrossRef] [Green Version]
- Yogev, R.; Damle, B.; Levy, G.; Nachman, S. Pharmacokinetics and Distribution of Linezolid in Cerebrospinal Fluid in Children and Adolescents. Pediatr. Infect. Dis. J. 2010, 29, 827–830. [Google Scholar] [CrossRef]
- Török, M.E.; Yen, N.T.B.; Chau, T.T.H.; Mai, N.T.H.; Phu, N.H.; Mai, P.P.; Dung, N.T.; Chau, N.V.V.; Bang, N.D.; Tien, N.A.; et al. Timing of Initiation of Antiretroviral Therapy in Human Immunodeficiency Virus (HIV)-Associated Tuberculous Meningitis. Clin. Infect. Dis. 2011, 52, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- van Laarhoven, A.; Dian, S.; Aguirre-Gamboa, R.; Avila-Pacheco, J.; Ricaño-Ponce, I.; Ruesen, C.; Annisa, J.; Koeken, V.A.C.M.; Chaidir, L.; Li, Y.; et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: An observational cohort study. Lancet Infect. Dis. 2018, 18, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, G.E.; Bang, N.D.; Dung, N.H.; Quy, H.T.; Oanh, D.T.T.; Thoa, N.T.C.; Hien, N.Q.; Thuc, N.T.; Hai, N.N.; Lan, N.T.N.; et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 2004, 351, 1741–1751. [Google Scholar] [CrossRef] [Green Version]
- Heinrichs, M.T.; Drusano, G.L.; Brown, D.L.; Maynard, M.S.; Sy, S.K.; Rand, K.H.; Peloquin, C.A.; Louie, A.; Derendorf, H. Dose optimization of moxifloxacin and linezolid against tuberculosis using mathematical modeling and simulation. Int. J. Antimicrob. Agents 2019, 53, 275–283. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Usón, E.; Sainz, J.; Martín, A.; Sánchez-Izquierdo, J.A.; Maynar, J.; Rodríguez-Gascón, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. Off. Publ. Int. Soc. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Stalker, D.J.; Jungbluth, G.L.; Hopkins, N.K.; Batts, D.H. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 2003, 51, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Maitre, T.; Bonnet, M.; Calmy, A.; Raberahona, M.; Rakotoarivelo, R.A.; Rakotosamimanana, N.; Ambrosioni, J.; Miró, J.M.; Debeaudrap, P.; Muzoora, C.; et al. Intensified tuberculosis treatment to reduce the mortality of HIV-infected and uninfected patients with tuberculosis meningitis (INTENSE-TBM): Study protocol for a phase III randomized controlled trial. Trials 2022, 23, 928. [Google Scholar] [CrossRef]
- Rangaka, M.X.; Cavalcante, S.C.; Marais, B.J.; Thim, S.; Martinson, N.A.; Swaminathan, S.; Chaisson, R.E. Controlling the seedbeds of tuberculosis: Diagnosis and treatment of tuberculosis infection. Lancet 2015, 386, 2344–2353. [Google Scholar] [CrossRef] [Green Version]
- Wynalda, M.A.; Hauer, M.J.; Wienkers, L.C. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab. Dispos. 2000, 28, 1014–1017. [Google Scholar]
- Gallagher, E.; Minn, I.; Chambers, J.E.; Searson, P.C. In vitro characterization of pralidoxime transport and acetylcholinesterase reactivation across MDCK cells and stem cell-derived human brain microvascular endothelial cells (BC1-hBMECs). Fluids Barriers CNS 2016, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Mahnke, H.; Ballent, M.; Baumann, S.; Imperiale, F.; von Bergen, M.; Lanusse, C.; Lifschitz, A.L.; Honscha, W.; Halwachs, S. The ABCG2 Efflux Transporter in the Mammary Gland Mediates Veterinary Drug Secretion across the Blood-Milk Barrier into Milk of Dairy Cows. Drug Metab. Dispos. 2016, 44, 700–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wu, J.; Bao, X.; Honea, N.; Xie, Y.; Kim, S.; Sparreboom, A.; Sanai, N. Quantitative and Mechanistic Understanding of AZD1775 Penetration across Human Blood–Brain Barrier in Glioblastoma Patients Using an IVIVE–PBPK Modeling Approach. Clin. Cancer Res. 2017, 23, 7454–7466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Shawahna, R.; Uchida, Y.; Declèves, X.; Ohtsuki, S.; Yousif, S.; Dauchy, S.; Jacob, A.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.-O.; et al. Transcriptomic and Quantitative Proteomic Analysis of Transporters and Drug Metabolizing Enzymes in Freshly Isolated Human Brain Microvessels. Mol. Pharm. 2011, 8, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Al-Majdoub, Z.M.; Al Feteisi, H.; Achour, B.; Warwood, S.; Neuhoff, S.; Rostami-Hodjegan, A.; Barber, J. Proteomic Quantification of Human Blood–Brain Barrier SLC and ABC Transporters in Healthy Individuals and Dementia Patients. Mol. Pharm. 2019, 16, 1220–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, P.; Fisher, J.W.; Yoshida, K.; Zhang, L.; Burckart, G.J.; Wang, J. Physiologically Based Pharmacokinetic Prediction of Linezolid and Emtricitabine in Neonates and Infants. Clin. Pharmacokinet. 2016, 56, 383–394. [Google Scholar] [CrossRef]
- Tsuji, Y.; Hiraki, Y.; Matsumoto, K.; Mizoguchi, A.; Sadoh, S.; Kobayashi, T.; Takemura, Y.; Sakamoto, S.; Morita, K.; Kamimura, H.; et al. Pharmacokinetics and protein binding of linezolid in cerebrospinal fluid and serum in a case of post-neurosurgical bacterial meningitis. Scand. J. Infect. Dis. 2011, 43, 982–985. [Google Scholar] [CrossRef]
- Tsona, A.; Metallidis, S.; Foroglou, N.; Selviaridis, P.; Chrysanthidis, T.; Lazaraki, G.; Papaioannou, M.; Nikolaidis, J. Linezolid Penetration Into Cerebrospinal Fluid and Brain Tissue. J. Chemother. 2010, 22, 17–19. [Google Scholar] [CrossRef]

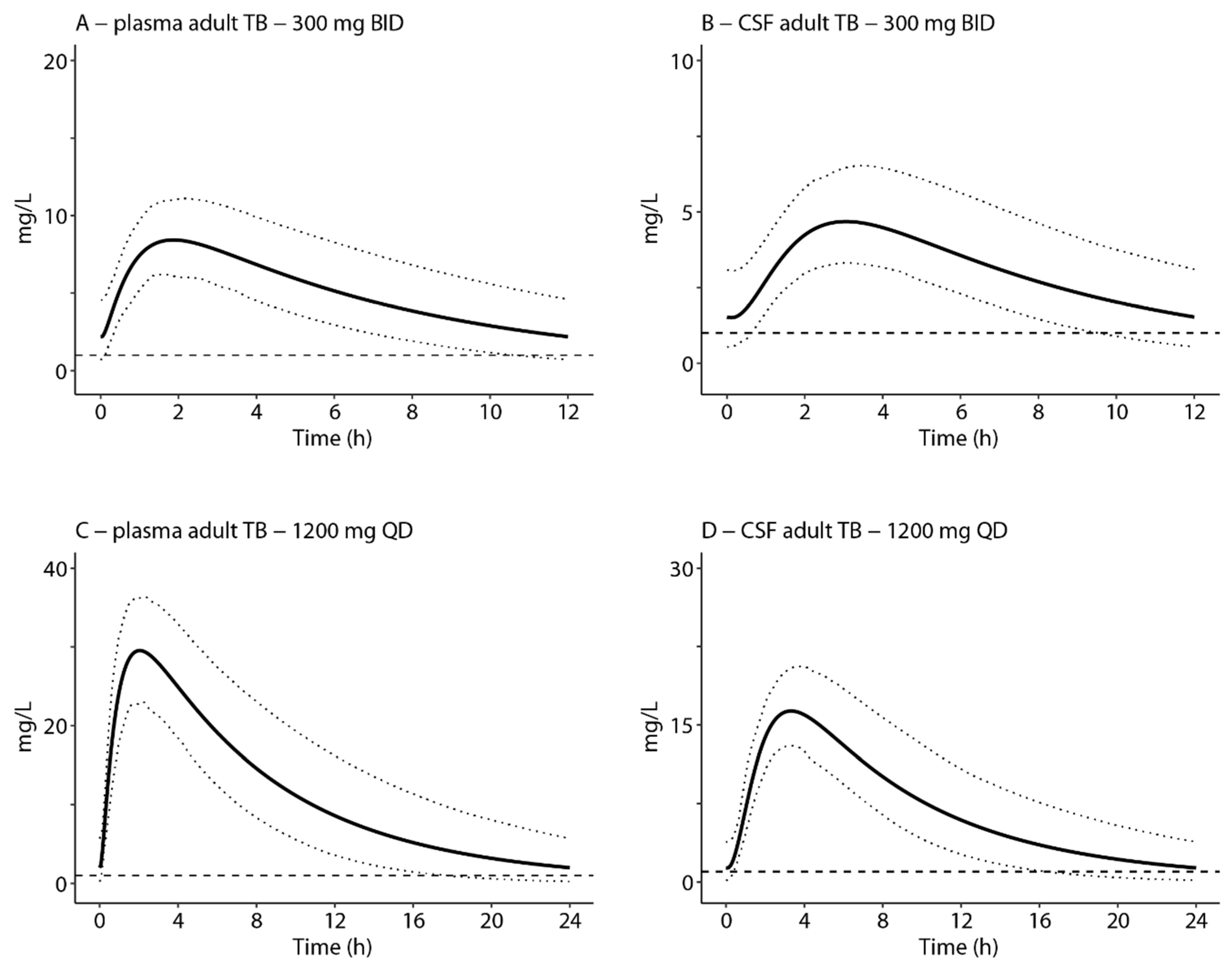
| Dose | AUC0–24/MIC Plasma a | Reaching Threshold Plasma (%) b | AUC0–24/MIC Cranial CSF c | Reaching Threshold Cranial CSF (%) b | T > MIC (%) d |
|---|---|---|---|---|---|
| Adults | |||||
| 300 mg BID oral | 118 (75–189) | 45 | 74 (45–117) | 4 | 100 |
| 600 mg BID oral | 281 (168–491) | 99 | 181 (99–300) | 87 | 100 |
| 1200 mg QD oral | 262 (166–423) | 99 | 166 (100–266) | 87 | 100 |
| Children | |||||
| ~10 mg/kg BID oral | 202 (117–360) | 93 | 135 (74–225) | 56 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litjens, C.H.C.; Verscheijden, L.F.M.; Svensson, E.M.; van den Broek, P.H.H.; van Hove, H.; Koenderink, J.B.; Russel, F.G.M.; Aarnoutse, R.E.; te Brake, L.H.M. Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis. Antibiotics 2023, 12, 702. https://doi.org/10.3390/antibiotics12040702
Litjens CHC, Verscheijden LFM, Svensson EM, van den Broek PHH, van Hove H, Koenderink JB, Russel FGM, Aarnoutse RE, te Brake LHM. Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis. Antibiotics. 2023; 12(4):702. https://doi.org/10.3390/antibiotics12040702
Chicago/Turabian StyleLitjens, Carlijn H. C., Laurens F. M. Verscheijden, Elin M. Svensson, Petra H. H. van den Broek, Hedwig van Hove, Jan B. Koenderink, Frans G. M. Russel, Rob E. Aarnoutse, and Lindsey H. M. te Brake. 2023. "Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis" Antibiotics 12, no. 4: 702. https://doi.org/10.3390/antibiotics12040702





