Extracellular Vesicles of Pseudomonas: Friends and Foes
Abstract
:1. Introduction
2. What Are Bacterial Extracellular Vesicles?
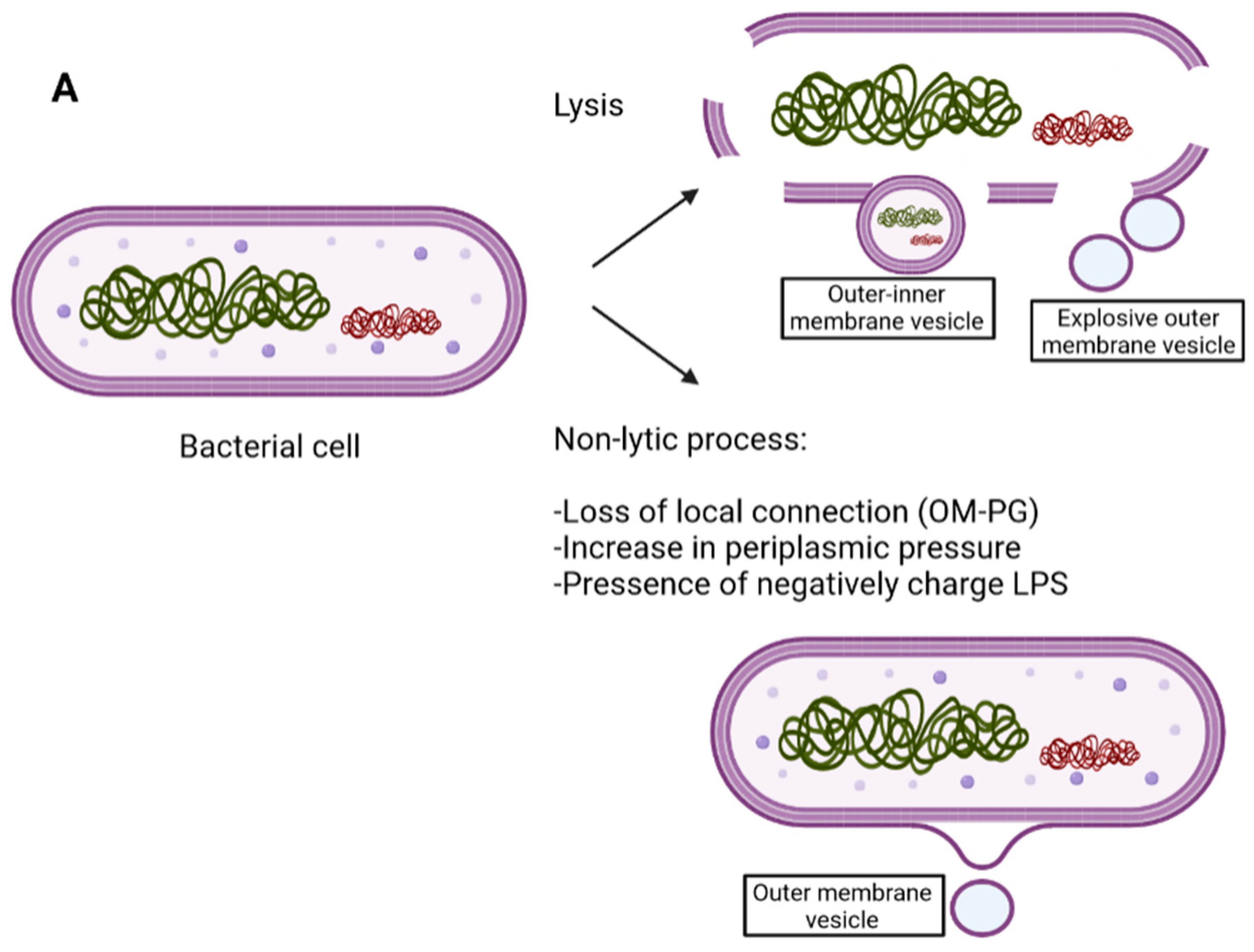
3. Gram-Negative Structure and EV Biogenesis
4. Components of EVs of Pseudomonas aeruginosa and Other Species
| Type of Molecule | Molecule Name | Reference |
|---|---|---|
| Bacterial protein | OprD, OprE, OprF, OprG, OprH, OprI, PagL, PcoB | [33,34,35] |
| Β-lactamases | [36,37] | |
| FlgK, FlgE, Peptidyl-prolyl cis-trans isomerase, LptD, PilQ, EstA, Gbt, OprC, OpdT, FecA, OpdC, OprB, PslD, LolB, PonA, OprM, LasA, Lipoprotein nlpD/lppB homolog, PilA, OmlA, WspA, among many others. | [34] | |
| PaAP | [33] | |
| Cif | [38] | |
| Cytoplasmic proteins such as: 50S ribosomal proteins, ATP synthase subunit and cytochrome c oxidase * | [34] | |
| Nucleic acid | DNA | [17,39,40] |
| RNA, sRNA | [41,42] | |
| Lipid | Glycerophospholipid | [43,44] |
| LPS (high levels of B-bands) | [17,25] | |
| Others | Gentamicin (after treatment) *1 | [17,45] |
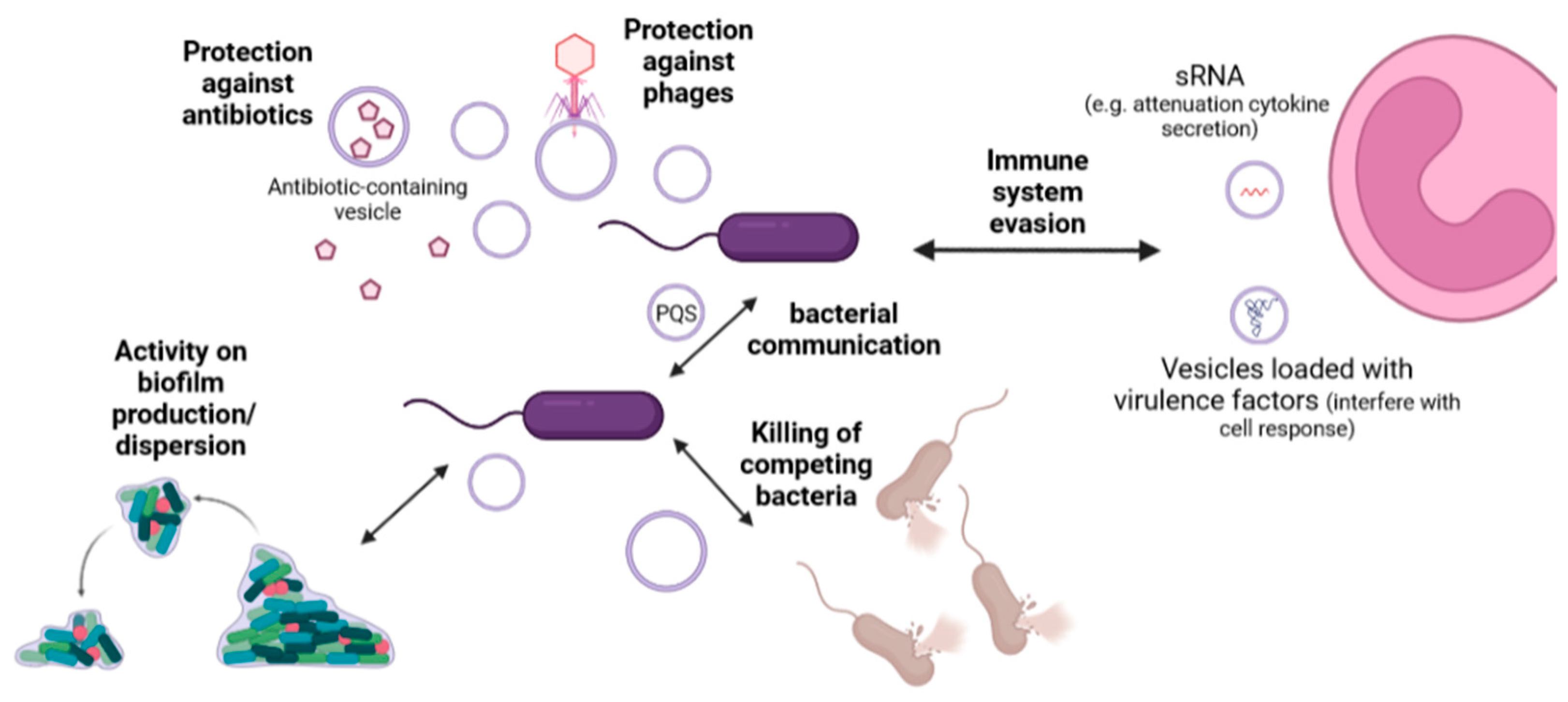
5. Physiological Roles of Extracellular Vesicles
6. Extracellular Vesicles as Biotechnological Tools
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Iglewski, B.H. Pseudomonas. In Medical Microbiology; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Curran, C.S.; Bolig, T.; Torabi-Parizi, P. Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection. Am. J. Respir. Crit. Care Med. 2017, 197, 708–727. [Google Scholar] [CrossRef]
- Kung Vanderlene, L.; Ozer Egon, A.; Hauser Alan, R. The Accessory Genome of Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2010, 74, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2018, 43, 273–303. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Bishop, D.; Work, E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem. J. 1965, 96, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M.; Brown, A.C. Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles. Front. Immunol. 2021, 12, 733064. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Juodeikis, R.; Carding, S.R. Outer Membrane Vesicles: Biogenesis, Functions, and Issues. Microbiol. Mol. Biol. Rev. 2022, 86, e00032-00022. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Combo, S.; Mendes, S.; Nielsen, K.M.; da Silva, G.J.; Domingues, S. The Discovery of the Role of Outer Membrane Vesicles against Bacteria. Biomedicines 2022, 10, 2399. [Google Scholar] [CrossRef]
- Pérez-Cruz, C.; Carrión, O.; Delgado, L.; Martinez, G.; López-Iglesias, C.; Mercade, E. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: Implications for DNA content. Appl. Environ. Microbiol. 2013, 79, 1874–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. Outer-Inner Membrane Vesicles Naturally Secreted by Gram-Negative Pathogenic Bacteria. PLoS ONE 2015, 10, e0116896. [Google Scholar] [CrossRef] [Green Version]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Lee, W.C.; Yeo, K.J.; Ryu, K.-S.; Kumarasiri, M.; Hesek, D.; Lee, M.; Mobashery, S.; Song, J.H.; Kim, S.I.; et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012, 26, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmar, A.T.; Collet, J.-F. Lpp, the Braun lipoprotein, turns 50—Major achievements and remaining issues. FEMS Microbiol. Lett. 2018, 365, fny199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wensink, J.; Witholt, B. Outer-Membrane Vesicles Released by Normally Growing Escherichia coli Contain Very Little Lipoprotein. Eur. J. Biochem. 1981, 116, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Rocchetta, H.L.; Burrows, L.L.; Pacan, J.C.; Lam, J.S. Three rhamnosyltransferases responsible for assembly of the A-band D-rhamnan polysaccharide in Pseudomonas aeruginosa: A fourth transferase, WbpL, is required for the initiation of both A-band and B-band lipopolysaccharide synthesis. Mol. Microbiol. 1998, 28, 1103–1119. [Google Scholar] [CrossRef]
- Rocchetta, H.L.; Burrows, L.L.; Lam, J.S. Genetics of O-Antigen Biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 1999, 63, 523–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, J.; Taylor, V.; Islam, S.; Hao, Y.; Kocíncová, D. Genetic and Functional Diversity of Pseudomonas aeruginosa Lipopolysaccharide. Front. Microbiol. 2011, 2, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Saxena, A.; Beveridge, T.J. Effect of surface lipopolysaccharide on the nature of membrane vesicles liberated from the Gram-negative bacterium Pseudomonas aeruginosa. J. Electron Microsc. 2003, 52, 465–469. [Google Scholar] [CrossRef]
- Hayashi, J.-i.; Hamada, N.; Kuramitsu, H.K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol. Lett. 2002, 216, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Hunt, J.R.; Kremer, N.; Krasity, B.C.; Apicella, M.A.; McFall-Ngai, M.J.; Ruby, E.G. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. eLife 2014, 3, e01579. [Google Scholar] [CrossRef]
- Aschtgen, M.-S.; Lynch Jonathan, B.; Koch, E.; Schwartzman, J.; McFall-Ngai, M.; Ruby, E. Rotation of Vibrio fischeri Flagella Produces Outer Membrane Vesicles That Induce Host Development. J. Bacteriol. 2016, 198, 2156–2165. [Google Scholar] [CrossRef] [Green Version]
- Aschtgen, M.-S.; Wetzel, K.; Goldman, W.; McFall-Ngai, M.; Ruby, E. Vibrio fischeri-derived outer membrane vesicles trigger host development. Cell. Microbiol. 2016, 18, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, Y.; Uchiyama, H.; Nomura, N. Multifunctional membrane vesicles in Pseudomonas aeruginosa. Environ. Microbiol. 2012, 14, 1349–1362. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauman, S.J.; Kuehn, M.J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006, 8, 2400–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.-S.; Kim, D.-K.; Choi, S.J.; Lee, J.; Choi, J.-P.; Rho, S.; Park, S.-H.; Kim, Y.-K.; Hwang, D.; Gho, Y.S. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. PROTEOMICS 2011, 11, 3424–3429. [Google Scholar] [CrossRef]
- Tashiro, Y.; Ichikawa, S.; Shimizu, M.; Toyofuku, M.; Takaya, N.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Variation of Physiochemical Properties and Cell Association Activity of Membrane Vesicles with Growth Phase in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2010, 76, 3732–3739. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Beveridge, T.J.; Kadurugamuwa, J.; Walther-Rasmussen, J.; Høiby, N. Chromosomal β-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 9–13. [Google Scholar] [CrossRef]
- González, L.J.; Bahr, G.; Nakashige, T.G.; Nolan, E.M.; Bonomo, R.A.; Vila, A.J. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat. Chem. Biol. 2016, 12, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacEachran Daniel, P.; Ye, S.; Bomberger Jennifer, M.; Hogan Deborah, A.; Swiatecka-Urban, A.; Stanton Bruce, A.; O’Toole George, A. The Pseudomonas aeruginosa Secreted Protein PA2934 Decreases Apical Membrane Expression of the Cystic Fibrosis Transmembrane Conductance Regulator. Infect. Immun. 2007, 75, 3902–3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renelli, M.; Matias, V.; Lo, R.Y.; Beveridge, T.J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [Google Scholar] [CrossRef]
- Schooling Sarah, R.; Hubley, A.; Beveridge Terry, J. Interactions of DNA with Biofilm-Derived Membrane Vesicles. J. Bacteriol. 2009, 191, 4097–4102. [Google Scholar] [CrossRef] [Green Version]
- Koeppen, K.; Hampton, T.H.; Jarek, M.; Scharfe, M.; Gerber, S.A.; Mielcarz, D.W.; Demers, E.G.; Dolben, E.L.; Hammond, J.H.; Hogan, D.A.; et al. A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016, 12, e1005672. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cruz, C.; Briansó, F.; Sonnleitner, E.; Bläsi, U.; Mercadé, E. RNA release via membrane vesicles in Pseudomonas aeruginosa PAO1 is associated with the growth phase. Environ. Microbiol. 2021, 23, 5030–5041. [Google Scholar] [CrossRef]
- Tashiro, Y.; Inagaki, A.; Shimizu, M.; Ichikawa, S.; Takaya, N.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Characterization of Phospholipids in Membrane Vesicles Derived from Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 2011, 75, 605–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysse, C.; Cullinane, M.; Dénervaud, V.; Burrowes, E.; Dow, J.M.; Morrissey, J.P.; Tam, L.; Trevors, J.T.; O’Gara1, F. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 2005, 151, 2529–2542. [Google Scholar] [CrossRef] [Green Version]
- Kadurugamuwa, J.L.; Beveridge, T.J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: Conceptually new antibiotics. J. Bacteriol. 1996, 178, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Mashburn, L.M.; Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005, 437, 422–425. [Google Scholar] [CrossRef]
- Toyofuku, M. Bacterial communication through membrane vesicles. Biosci. Biotechnol. Biochem. 2019, 83, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not Just for Quorum Sensing Anymore. Front. Cell. Infect. Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.-Y.; Luo, Z.-Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef] [Green Version]
- Kadurugamuwa, J.L.; Beveridge, T.J. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other Gram-negative bacteria. Microbiology 1999, 145, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Kesty, N.C.; Mason, K.M.; Reedy, M.; Miller, S.E.; Kuehn, M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004, 23, 4538–4549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Uematsu, K.; Hirayama, H.; Horikoshi, K. Novel Toluene Elimination System in a Toluene-Tolerant Microorganism. J. Bacteriol. 2000, 182, 6451–6455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.L.; Duque, E.; Gallegos, M.-T.; Godoy, P.; Ramos-González, M.I.; Rojas, A.; Terán, W.; Segura, A. Mechanisms of Solvent Tolerance in Gram-Negative Bacteria. Annu. Rev. Microbiol. 2002, 56, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J. The “hole” story of predatory outer-membrane vesicles. Can. J. Microbiol. 2018, 64, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosgodage, U.S.; Matewele, P.; Mastroianni, G.; Kraev, I.; Brotherton, D.; Awamaria, B.; Nicholas, A.P.; Lange, S.; Inal, J.M. Peptidylarginine Deiminase Inhibitors Reduce Bacterial Membrane Vesicle Release and Sensitize Bacteria to Antibiotic Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyniak, D.; Olszak, T.; Drulis-Kawa, Z. Outer Membrane Vesicles (OMVs) of Pseudomonas aeruginosa Provide Passive Resistance but Not Sensitization to LPS-Specific Phages. Viruses 2022, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Robles, T.; Dillard Rebecca, S.; Cairns Lynne, S.; Silva-Valenzuela Cecilia, A.; Housman, M.; Ali, A.; Wright Elizabeth, R.; Camilli, A. Vibrio cholerae Outer Membrane Vesicles Inhibit Bacteriophage Infection. J. Bacteriol. 2018, 200, e00792-00717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, Y.; Ichikawa, S.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Pseudomonas Quinolone Signal Affects Membrane Vesicle Production in not only Gram-Negative but also Gram-Positive Bacteria. Microbes Environ. 2010, 25, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Clarke Anthony, J.; Beveridge Terry, J. Gram-Negative Bacteria Produce Membrane Vesicles Which Are Capable of Killing Other Bacteria. J. Bacteriol. 1998, 180, 5478–5483. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Clarke, A.J.; Beveridge, T.J. A major autolysin of Pseudomonas aeruginosa: Subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles. J. Bacteriol. 1996, 178, 2479–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan Nick, D.; Beveridge Terry, J. Gentamicin Delivery to Burkholderia cepacia Group IIIa Strains via Membrane Vesicles from Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 2003, 47, 2962–2965. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, T.J.; Makin, S.A.; Kadurugamuwa, J.L.; Li, Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997, 20, 291–303. [Google Scholar] [CrossRef]
- Beveridge Terry, J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Kamaguchi, A.; Ohyama, T.; Sakai, E.; Nakamura, R.; Watanabe, T.; Baba, H.; Nakayama, K. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia. Microbiology 2003, 149, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kamaguchi, A.; Nakayama, K.; Ichiyama, S.; Nakamura, R.; Watanabe, T.; Ohta, M.; Baba, H.; Ohyama, T. Effect of Porphyromonas gingivalis Vesicles on Coaggregation of Staphylococcus aureus to Oral Microorganisms. Curr. Microbiol. 2003, 47, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Onishi, S.; Kuramitsu Howard, K.; Sharma, A. Porphyromonas gingivalis Vesicles Enhance Attachment, and the Leucine-Rich Repeat BspA Protein Is Required for Invasion of Epithelial Cells by “Tannerella forsythia”. Infect. Immun. 2006, 74, 5023–5028. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, H.; Osaki, T.; Kurata, S.; Fukuda, M.; Kawakami, H.; Ochiai, K.; Hanawa, T.; Kamiya, S. Outer Membrane Vesicles of Helicobacter pylori TK1402 are Involved in Biofilm Formation. BMC Microbiol. 2009, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Sato, K.; Yukitake, H.; Noiri, Y.; Ebisu, S.; Nakayama, K. A Porphyromonas gingivalis Mutant Defective in a Putative Glycosyltransferase Exhibits Defective Biosynthesis of the Polysaccharide Portions of Lipopolysaccharide, Decreased Gingipain Activities, Strong Autoaggregation, and Increased Biofilm Formation. Infect. Immun. 2010, 78, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- Schooling Sarah, R.; Beveridge Terry, J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef] [Green Version]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Cooke Adam, C.; Florez, C.; Dunshee Elise, B.; Lieber Avery, D.; Terry Michelle, L.; Light Caitlin, J.; Schertzer Jeffrey, W. Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa. mSphere 2020, 5, e01109-01120. [Google Scholar] [CrossRef] [PubMed]
- Kyung Lee, M.; Armstrong, D.A.; Hazlett, H.F.; Dessaint, J.A.; Mellinger, D.L.; Aridgides, D.S.; Christensen, B.C.; Ashare, A. Exposure to extracellular vesicles from Pseudomonas aeruginosa result in loss of DNA methylation at enhancer and DNase hypersensitive site regions in lung macrophages. Epigenetics 2021, 16, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G.; et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [Google Scholar] [CrossRef] [Green Version]
- Yum, H.-K.; Park, I.N.; Shin, B.-M.; Choi, S.-J. Recurrent Pseudomonas aeruginosa Infection in Chronic Lung Diseases: Relapse or Reinfection? Tuberc. Respir. Dis. 2014, 77, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.; Oster, P.; Arnold, R.; Tatley, M.; Næss, L.; Aaberge, I.; Galloway, Y.; McNicholas, A.; O’Hallahan, J.; Rosenqvist, E.; et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum. Vaccines Immunother. 2013, 9, 1241–1253. [Google Scholar] [CrossRef] [Green Version]
- Zare Banadkoki, E.; Rasooli, I.; Ghazanfari, T.; Siadat, S.D.; Shafiee Ardestani, M.; Owlia, P. Pseudomonas aeruginosa PAO1 outer membrane vesicles-diphtheria toxoid conjugate as a vaccine candidate in a murine burn model. Sci. Rep. 2022, 12, 22324. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Zou, J.; Wu, W.; Jing, H.; Gou, Q.; Li, H.; Gu, J.; Zou, Q.; Zhang, J. Immunization with Pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 2018, 36, 1047–1054. [Google Scholar] [CrossRef]
- Ito, S.; Nakamura, J.; Fukuta, M.; Ura, T.; Teshigawara, T.; Fukushima, J.; Mizuki, N.; Okuda, K.; Shimada, M. Prophylactic and therapeutic vaccine against Pseudomonas aeruginosa keratitis using bacterial membrane vesicles. Vaccine 2021, 39, 3152–3160. [Google Scholar] [CrossRef]
- Killough, M.; Rodgers, A.M.; Ingram, R.J. Pseudomonas aeruginosa: Recent Advances in Vaccine Development. Vaccines 2022, 10, 1100. [Google Scholar] [CrossRef]
- Toyofuku, M.; Morinaga, K.; Hashimoto, Y.; Uhl, J.; Shimamura, H.; Inaba, H.; Schmitt-Kopplin, P.; Eberl, L.; Nomura, N. Membrane vesicle-mediated bacterial communication. ISME J. 2017, 11, 1504–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, P. Pressures in the pipeline. Nat. Rev. Drug Discov. 2003, 2, 337. [Google Scholar] [CrossRef]
- van der Ley, P.; Steeghs, L.; Hamstra Hendrik, J.; ten Hove, J.; Zomer, B.; van Alphen, L. Modification of Lipid A Biosynthesis in Neisseria meningitidis lpxL Mutants: Influence on Lipopolysaccharide Structure, Toxicity, and Adjuvant Activity. Infect. Immun. 2001, 69, 5981–5990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Kim, K.-S.; Lee, S.-R.; Kim, E.; Kim, M.-S.; Lee, E.-Y.; Gho, Y.S.; Kim, J.-W.; Bishop, R.E.; Chang, K.-T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim. Et Biophys. Acta (BBA) -Biomembr. 2009, 1788, 2150–2159. [Google Scholar] [CrossRef] [Green Version]
- Scorza, F.B.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High Yield Production Process for Shigella Outer Membrane Particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [Green Version]
- Bernadac, A.; Gavioli, M.; Lazzaroni, J.-C.; Raina, S.; Lloube, R. Escherichia coli tol-pal Mutants Form Outer Membrane Vesicles. J. Bacteriol. 1998, 180, 4872–4878. [Google Scholar] [CrossRef] [Green Version]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef] [Green Version]
- Berleman, J.E.; Allen, S.; Danielewicz, M.A.; Remis, J.P.; Gorur, A.; Cunha, J.; Hadi, M.Z.; Zusman, D.R.; Northen, T.R.; Witkowska, H.E.; et al. The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]

| Application | Advantages | Reference |
|---|---|---|
| Vaccines | EVs can mimic a bacterium without causing the disease. | [11] |
| Have natural adjuvant properties and induce adaptive immune responses. | [77,78] | |
| EVs can be easily phagocytized and processed by antigen-presenting cells | [11,77] | |
| Vehicles for drug delivery | EVs are strategic vehicles for the simultaneous delivery of hydrophobic and hydrophilic molecules | [9] |
| Direct killing of pathogens | EVs can kill competing Gram-positive and Gram-negative bacteria, using molecules such as murein hydrolase | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriquez, T.; Falciani, C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics 2023, 12, 703. https://doi.org/10.3390/antibiotics12040703
Henriquez T, Falciani C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics. 2023; 12(4):703. https://doi.org/10.3390/antibiotics12040703
Chicago/Turabian StyleHenriquez, Tania, and Chiara Falciani. 2023. "Extracellular Vesicles of Pseudomonas: Friends and Foes" Antibiotics 12, no. 4: 703. https://doi.org/10.3390/antibiotics12040703






