Abstract
(1) Background: To explore the impact of the degree of inflammation on voriconazole exposure in critically ill patients affected by COVID-associated pulmonary aspergillosis (CAPA); (2) Methods: Critically ill patients receiving TDM-guided voriconazole for the management of proven or probable CAPA between January 2021 and December 2022 were included. The concentration/dose ratio (C/D) was used as a surrogate marker of voriconazole total clearance. A receiving operating characteristic (ROC) curve analysis was performed by using C-reactive protein (CRP) or procalcitonin (PCT) values as the test variable and voriconazole C/D ratio > 0.375 (equivalent to a trough concentration [Cmin] value of 3 mg/L normalized to the maintenance dose of 8 mg/kg/day) as the state variable. Area under the curve (AUC) and 95% confidence interval (CI) were calculated; (3) Results: Overall, 50 patients were included. The median average voriconazole Cmin was 2.47 (1.75–3.33) mg/L. The median (IQR) voriconazole concentration/dose ratio (C/D) was 0.29 (0.14–0.46). A CRP value > 11.46 mg/dL was associated with the achievement of voriconazole Cmin > 3 mg/L, with an AUC of 0.667 (95% CI 0.593–0.735; p < 0.001). A PCT value > 0.3 ng/mL was associated with the attainment of voriconazole Cmin > 3 mg/L (AUC 0.651; 95% CI 0.572–0.725; p = 0.0015). (4) Conclusions: Our findings suggest that in critically ill patients with CAPA, CRP and PCT values above the identified thresholds may cause the downregulation of voriconazole metabolism and favor voriconazole overexposure, leading to potentially toxic concentrations.
1. Introduction
The SARS-CoV-2 pandemic has been responsible for the most intensive care unit (ICU) admission in the last three years, accounting for remarkable morbidity and mortality [1]. Bacterial and fungal superinfections have been widely reported in critically ill COVID-19 patients, with prevalence ranging from 16% to 40% [2,3,4].
COVID-19-associated pulmonary aspergillosis (CAPA) emerged as a severe fungal superinfection in ICU-admitted patients, affecting up to 40% of patients undergoing mechanical ventilation [5,6]. COVID-19 itself, coupled with the use of immunomodulatory agents (i.e., corticosteroids and/or tocilizumab), may strongly compromise the immune system, leading to the emergence of opportunistic superinfections [6]. This clinical picture was similar to those of invasive pulmonary aspergillosis previously observed in critically ill patients affected by severe influenza pneumonia requiring ICU management [7]. Notably, the occurrence of CAPA seems to increase the mortality rate (i.e., greater than 50%) among COVID-19-patients [5,6,8]. In previous studies, the treatment of CAPA with voriconazole was associated with a trend toward a decrease in mortality rate [5,9].
Implementing a therapeutic drug monitoring (TDM) strategy for guiding voriconazole dosing should be considered mandatory among ICU patients due to the well-recognized huge inter- and intra-individual pharmacokinetic variability that may make unpredictable drug exposure at fixed dosing regimens [10,11]. Specifically, a recent international position paper strongly recommended the routine implementation of a TDM-guided strategy when voriconazole is administered in critically ill patients [10]. Indeed, the application of a TDM-guided strategy showed to improve clinical efficacy and safety of voriconazole, by minimizing the risk of treatment withdrawal because of adverse events [12]. Although real-world evidence investigating the role of a TDM-guided strategy for voriconazole in critically ill patients is limited, the occurrence of underexposure associated with negative clinical outcome was reported [13].
Among the different factors possibly impacting on voriconazole exposure, recent evidence found that the degree of inflammation may significantly affect voriconazole concentrations [14,15,16,17]. Voriconazole metabolism is mediated by CYP2C9, CYP2C19, and CYP3A4 [18]. Indeed, an ever-growing number of studies showed that several pro-inflammatory cytokines, especially IL-6, may moderately downregulate the activity of CYP3A4 and weakly-to-moderately downregulate those of CYP2C9 and CYP2C19 [19,20]. Considering that inflammation represents a common feature that shows remarkable proportion among ICU patients, a relevant impact on the occurrence of voriconazole overexposure and toxicity could not be ruled out [14]. However, no studies investigated this issue in the challenging scenario of CAPA.
The aim of this study was to explore the impact of the degree of inflammation by using C-reactive protein (CRP) and procalcitonin (PCT) serum levels as inflammatory biomarkers on voriconazole exposure in critically ill patients affected by CAPA.
2. Results
Overall, a total of 50 critically ill patients received TDM-guided voriconazole for treating probable CAPA during the study period (Table 1).

Table 1.
Demographic and clinical variables of critically ill patients undergoing at least one TDM assessment of voriconazole during treatment for COVID-associated pulmonary aspergillosis.
Median (interquartile [IQR]) age was 65.5 (60.0–73.75) years, with a slight male preponderance (54.0%). Thirty-three out of 50 patients (66.0%) had comorbidities, with obesity (30.0%), type 2 diabetes mellitus (20.0%), and cancer (16.0%) being the most frequent ones. Most patients (90.0%) underwent mechanical ventilation, whereas vasopressor support was needed in 44.0% of cases. Extracorporeal membrane oxygenation (ECMO) was applied in three patients. COVID-19 treatment was implemented in all of the included critically ill patients, and the most frequent administered drugs were dexamethasone (88.0%), low molecular weight heparin (52.0%), and tocilizumab (44.0%).
The median (IQR) baseline galactomannan (GM) index on bronchoalveolar lavage (BAL) before starting voriconazole treatment was 3.41 (1.84–4.63). Aspergillus spp. was isolated on BAL cultures in nine cases (18.0%), with Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus fumigatus and flavus reported in five, two, and one case each, respectively. Bacterial superinfections were present in 42 out of 50 patients (84.0%), with Pseudomonas aeruginosa (24.0%), Klebsiella pneumoniae (24.0%), and Enterococcus faecalis (16.0%) being the most prevalent clinical isolates. Gram-negative pathogens were carbapenem-resistant in 11 cases (22.0%).
The median (IQR) number of TDM assessments of voriconazole trough concentrations (Cmin) per patient was 3 (2–6). The median (IQR) average voriconazole Cmin was 2.47 (1.75–3.33) mg/L, whereas the median (IQR) average voriconazole daily dose was 7.69 (6.99–8.13) mg/kg. The median (IQR) voriconazole concentration/dose ratio (C/D) was 0.29 (0.14–0.46). Potentially interacting medications were co-administered in 68.0% of cases (34/50), but none of these was a strong inhibitor or inducer of CYP3A4 or 2C9/2C19 isoenzymes.
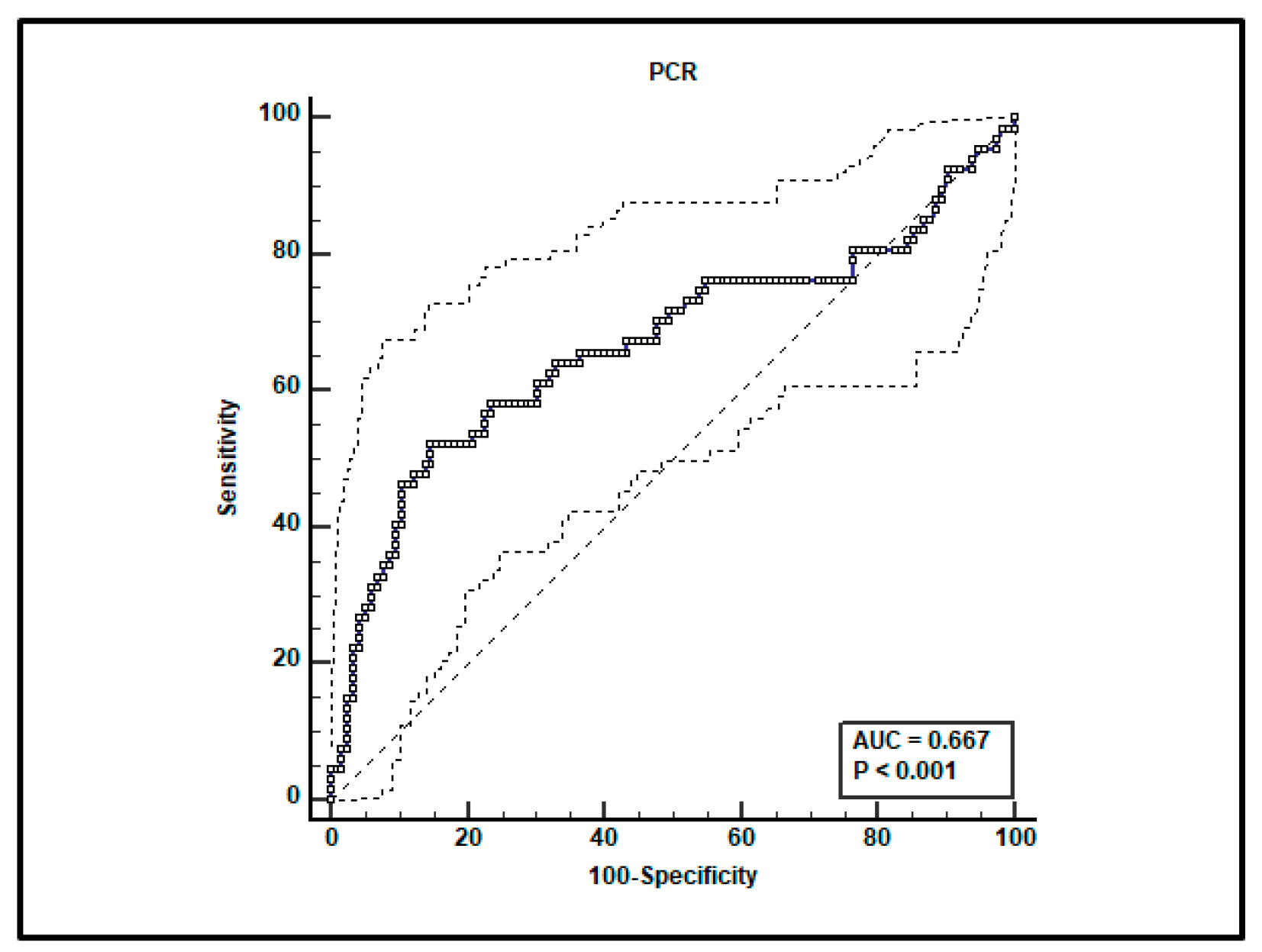
A total of 182 paired voriconazole Cmin-serum C-reactive protein (CRP) determinations were performed in the included patients. Receiving operating characteristics (ROC) curve analysis showed that the CRP value > 11.46 mg/dL was associated with voriconazole Cmin > 3 mg/L, with a sensitivity of 52.2% (95% confidence interval [CI] 39.7–64.6%) and specificity of 85.2% (95% CI 77.4–91.1%). The area under curve (AUC) was 0.667 (95% CI 0.593–0.735; p < 0.001; Figure 1).
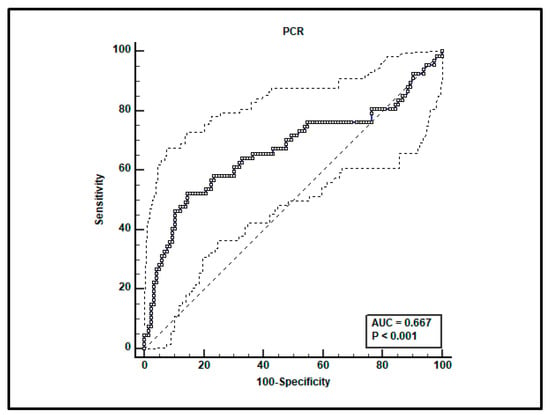
Figure 1.
ROC curve analysis for voriconazole Cmin > 3 mg/L. An optimal cut-off of CRP value > 11.46 mg/dL was found with a sensitivity of 52.2% and specificity of 85.2%.
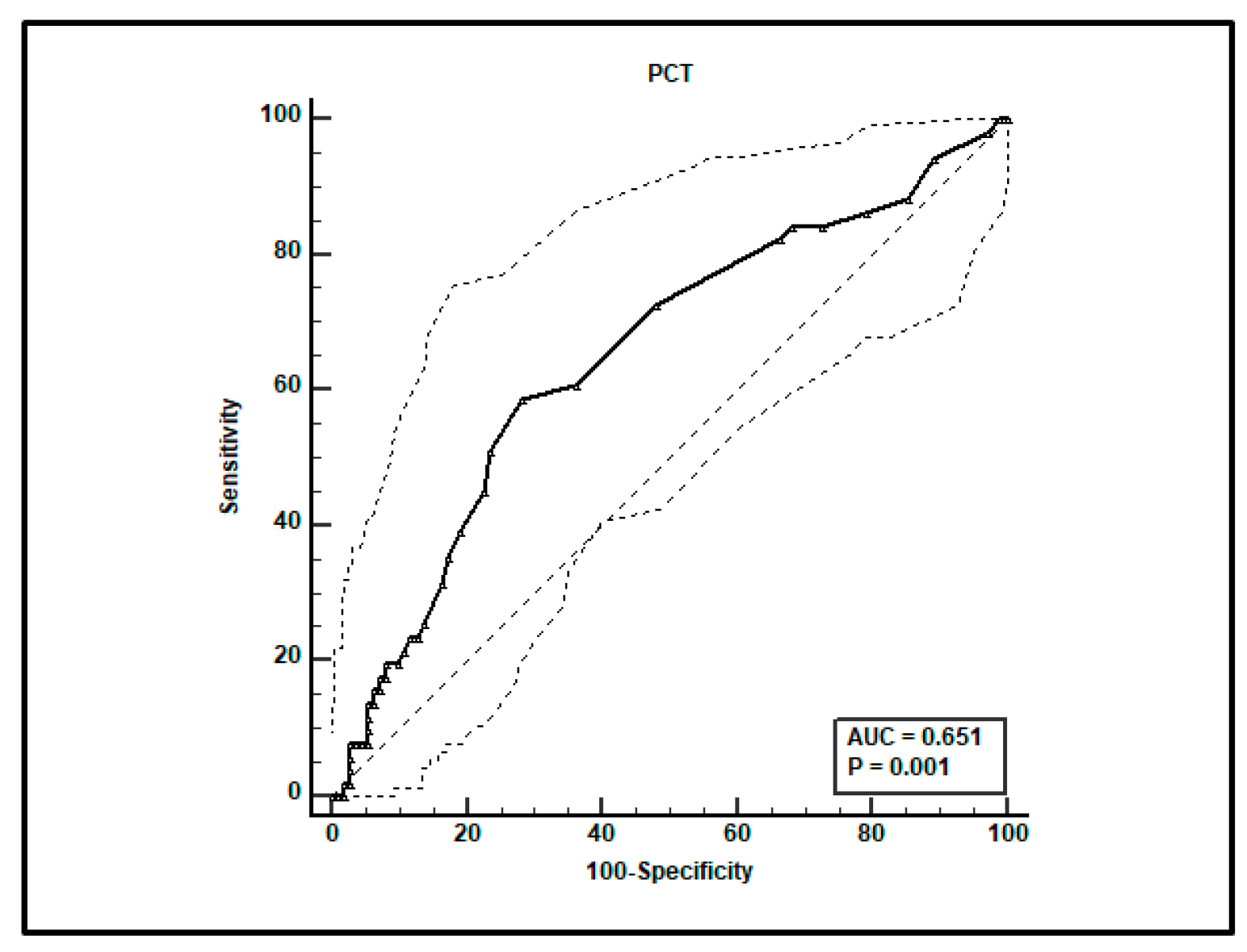
A total of 161 paired voriconazole Cmin-serum procalcitonin (PCT) determinations were performed in the included patients. ROC curve analysis showed that a PCT value > 0.3 ng/mL was associated with the achievement of toxic voriconazole Cmin > 3 mg/L, with sensitivity of 58.8% (95% CI 44.2–72.4%) and specificity of 71.8% (95% CI 62.4–80.0%). The AUC was 0.651 (95% CI 0.572–0.725; p = 0.0015; Figure 2).

Figure 2.
ROC curve analysis for voriconazole Cmin > 3 mg/L. An optimal cut-off of PCT value > 0.3 ng/mL was found with a sensitivity of 58.8% and specificity of 71.8%.
In two cases, antifungal treatment was escalated to liposomal amphotericin B because of isolation of Aspergillus niger resistant to voriconazole in one case, and because of lack in attainment of effective voriconazole concentrations in the other case. The ICU mortality rate was 58.0%.
3. Discussion
To the best of our knowledge, this is the first study that described the impact of inflammatory status on voriconazole exposure in critically ill patients affected by CAPA. Interestingly, we found that both CRP and PCT thresholds may be significantly associated with an increased risk of attaining toxic voriconazole levels. Indeed, previous real-world evidence found that voriconazole Cmin higher than 3 mg/L and 4 mg/L was associated with increased risk of hepatotoxicity and neurotoxicity, respectively [21,22].
Several factors may be responsible for the large inter- and intra-individual variability in voriconazole Cmin commonly retrieved during treatment with standard fixed dosing regimens [18]. Specifically, voriconazole pharmacokinetics may be strongly affected by genetic polymorphisms of CYP2C19 isoenzyme, by drug–drug interactions with CYP2C9, 2C19, or 3A4 modulators, and/or by the inflammatory status [14,18,23,24]. In regard to the latter, several real-world studies carried out, mainly in patients with hematological malignancies undergoing hematopoietic stem cell/bone marrow transplantation and/or in solid organ transplant recipients, showed in the last decade that thresholds of inflammatory biomarkers like CRP or PCT may be significantly associated with toxic voriconazole levels [14]. Dote et al. [25] found in a cohort of 63 mixed patients (mainly hematological) that CRP values > 4.7 mg/dL were associated with an increased risk of voriconazole Cmin exceeding 4 mg/L. In a case-control study including 266 hematological patients, Gautier-Veyret et al. [26] found at multivariate analysis that patients with CRP levels > 9.6 mg/dL showed a 27-fold higher risk of having voriconazole Cmin ≥ 4 mg/L. Cheng et al. [27] found among 73 elderly patients that at ROC curve analysis serum PCT levels ≥ 1.31 ng/mL were associated with voriconazole Cmin > 5 mg/L.
To the best of our knowledge, in the ICU scenario the impact of inflammation on voriconazole exposure was investigated previously only among 33 critically ill patients with sepsis or respiratory failure. The findings showed that a serum CRP level > 10 mg/dL was significantly associated with voriconazole Cmin > 5.5 mg/L [28].
Overall, the findings are consistent with the CRP and PCT thresholds identified at ROC analysis in our homogeneous cohort of CAPA critically ill patients and may support the contention that voriconazole exposure is significantly affected by the degree of inflammation even in the novel scenario of CAPA among ICU admitted patients. Notably, two previous real-world experiences in COVID-19 patients affected by CAPA have raised the potential issue of the impact of inflammatory status on voriconazole exposure [29,30]. Le Daré et al. [29] described a case of critically ill COVID-19 patient affected by acute respiratory distress syndrome due to CAPA superinfection in which unexpected variations in voriconazole exposure were reported. Specifically, a remarkable impact of inflammatory degree on voriconazole Cmin was observed, considering that the metabolite ratios (expressed as voriconazole N-oxide/voriconazole ratio) ranged from <0.3 during the inflammatory period to >1 after resolution of inflammation [29]. These findings were consistent with a significant decrease in CRP levels. Furthermore, a case series including 13 patients affected by CAPA (of which 12 required ICU admission) found higher voriconazole levels (in terms of Cmin) compared to those retrieved in 13 non-COVID-19 patients treated with voriconazole, leading to significantly higher transaminase levels [30]. Notably, a mild and positive correlation was found between voriconazole Cmin and CRP levels [30]. However, in none of these real-world experience concerning CAPA scenario was a CRP or PCT threshold value predictive of a significant higher risk of voriconazole overexposure identified.
On this basis, implementing a TDM-based expert clinical pharmacological advice program for optimizing voriconazole exposure should become mandatory even in CAPA critically ill patients [20,31], as previously recommended for other settings [10,32]. In this scenario, the careful assessment of the degree of inflammation should be taken into account in the personalization of voriconazole therapy. The identification of specific thresholds based on serum levels of CRP, PCT, and IL-6 predictive for voriconazole overexposure could be associated with information on genetic polymorphisms of CYP2C19 isoenzyme and of clinically relevant drug–drug interactions in order to provide the best accurate voriconazole dosing adjustment when TDM results are interpreted.
Limitations of our study have to be recognized. The retrospective monocentric study design and the limited sample size should be acknowledged. The role of CYP2C19 genetic polymorphisms and of drug–drug interactions in affecting voriconazole exposure could not be ruled out. However, the absence of co-treatment with strong CYP450 inhibitors or inducers may suggest a minor role of drug–drug interactions. Finally, we recognize that assessing a more specific pro-inflammatory biomarker like IL-6 rather than CRP or PCT would have been more appropriate for addressing this issue. Unfortunately, available data on serum IL-6 values were limited in our study patients and this precluded us from performing ROC curve analysis, which was eventually useful at identifying a specific IL-6 threshold. Conversely, the fact that ours is the first real-life experience exploring the impact of inflammation on voriconazole exposure in a cohort of critically ill patients affected by CAPA is a point of strength.
4. Materials and Methods
All the critically ill COVID-19 patients who were treated with voriconazole intravenously because of probable or proven CAPA at the COVID ICU of the IRCCS Azienda Ospedaliero–Universitaria of Bologna between 1 January 2021 and 31 December 2022 were retrospectively assessed. Patients were included if probable or proven CAPA was identified according to the 2020 European Confederation of Medical Mycology (ECMM)/International Society for Human and Animal Mycology (ISHAM) consensus criteria [33], and if underwent at least one therapeutic drug monitoring (TDM) of voriconazole trough level (Cmin) with concomitant assessment of the inflammatory biomarkers CRP and PCT.
Demographic (age, sex, height, weight, body mass index (BMI), underlying diseases (i.e., obesity, cardiovascular disease, diabetes mellitus, chronic kidney disease, hepatic cirrhosis, cancer, immunosuppression)) and clinical/laboratory data (need for mechanical ventilation and vasopressors, implementation of ECMO, COVID-19 antiviral therapies, serum CRP and PCT) were retrieved for each patient. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Voriconazole dosage, concomitant medications, number of TDM assessment per patient, baseline GM index on BAL specimen, positive BAL cultures for Aspergillus spp., bacterial superinfections, and ICU mortality rate were also retrieved. Clinically relevant drug interactions of voriconazole with concomitant medication were defined according to the EMA guidelines [34]. Co-administered drugs potentially increasing voriconazole exposure were defined as strong inhibitors whether causing a > 5-fold increase in voriconazole plasma AUC values or ≥an 80% decrease in clearance; moderate inhibitors whether causing a >2-fold increase in voriconazole plasma AUC values or a 50–80% decrease in clearance; mild inhibitors whether causing a 1.25- to 2-fold increase in voriconazole plasma AUC values or a <50% reduction in clearance. Conversely, co-administered drugs potentially reducing voriconazole exposure were defined as strong, moderate, or mild inducers whether causing a >80%, a 50–80%, or a <50% decrease in voriconazole plasma AUC values.
Voriconazole was prescribed at the discretion of the treating physician or infectious disease consultant according to current clinical practice implemented at the IRCCS Azienda Ospedaliero-Universitaria of Bologna. Voriconazole was started at the recommended dosage of 6 mg/kg every 12 h for the first 24 h (loading dose), followed by 4 mg/kg every 12 h as a maintenance dose (MD), and was subsequently optimized by means of a real-time TDM-based clinical pharmacological advice strategy.
Blood samples for assessing plasma voriconazole Cmin were collected 5–15 min before one drug administration after at least 48 h from starting treatment. Voriconazole blood concentrations were measured by means of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) commercially available method (Chromsystems Instruments and Chemicals GmbH, Munich, Germany) [35]. TDM results were made available via the intranet to the MD clinical pharmacologist who provided an expert clinical pharmacological advice for prompt dosing adaptation by ICU physicians within the same day of TDM sampling. The desired voriconazole Cmin range was set at 1.0–3.0 mg/L in agreement with international guidelines [10], and recent findings [21,22]. Two recent meta-analyses showed that voriconazole Cmin > 3.0–4.0 mg/L may be associated with higher risk of hepatotoxicity [21,22].
Continuous data were presented as median and interquartile range (IQR), whereas categorial variables were expressed as count and percentage. The C/D was used as a surrogate marker of total clearance by dividing voriconazole Cmin by daily dose per kg of body weight. ROC curve analysis was carried out by using on the one hand CRP or PCT values as the test variable, and on the other hand voriconazole C/D ratio > 0.375 (equivalent to a Cmin value of 3 mg/L normalized to the MD of 8 mg/kg/day) as the state variable and defined as toxic level [22,36]. AUC along with 95% CI were calculated. The optimal cut-off point was computed by means of the Youden Index method. The Youden Index was calculated according to the following equation: sensitivity (%) + specificity (%) − 100. A p value < 0.05 was considered significant. Statistical analysis was performed by using the MedCalc for Windows (MedCalc statistical software, version 19.6.1, MedCalc Software Ltd., Ostend, Belgium). The study was approved by the Ethics Committee of IRCCS Azienda Ospedaliero-Universitaria of Bologna (n. 442/2021/Oss/AOUBo approved on 28 June 2021).
5. Conclusions
Our findings suggest that in critically ill patients with CAPA, CRP and PCT values above the identified thresholds may cause downregulation of voriconazole metabolism and favor voriconazole overexposure. This may lead to potentially toxic concentrations, which might cause hepato- and/or neuro-toxicity. In this scenario, a real-time TDM-based clinical pharmacological advice strategy should be implemented for granting optimal management of voriconazole treatment of CAPA. In clinical settings in which a real-time TDM strategy is unavailable, the identified CRP and PCT thresholds could be useful for providing clinicians a guide for promptly adopting voriconazole dosing adjustments in order to minimize the attainment of toxic concentrations. Large prospective clinical studies are warranted for confirming our findings and for assessing the potential impact on voriconazole efficacy and/or safety.
Author Contributions
Conceptualization, M.G. and F.P.; methodology, M.G.; formal analysis, M.G.; data curation, M.G., G.F. and Z.P.; writing—original draft preparation, M.G.; writing—review and editing, A.Z., M.B., P.V. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of IRCCS Azienda Ospedaliero-Universitaria of Bologna (n. 442/2021/Oss/AOUBo approved on 28 June 2021).
Informed Consent Statement
Signed informed consent was waived due to the retrospective and observational nature of the investigation according to hospital agreements.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
M.G. received personal fees from Angelini; P.V. has served as a consultant for Biomerieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureau for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma and Pfizer; F.P participated in speaker bureau for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis, and in advisory board for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfizer, and Thermo-Fisher. The authors report no other conflicts of interest in this work.
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of Hospital-Acquired Bacterial and Fungal Superinfections in COVID-19: A Prospective Observational Study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary Infections in Patients Hospitalized with COVID-19: Incidence and Predictive Factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clin. Infect. Dis. 2021, 73, e3606–e3614. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensive Med. 2021, 1, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Reizine, F.; Pinceaux, K.; Lederlin, M.; Autier, B.; Guegan, H.; Gacouin, A.; Luque-Paz, D.; Boglione-Kerrien, C.; Bacle, A.; Le Daré, B.; et al. Influenza- and COVID-19-Associated Pulmonary Aspergillosis: Are the Pictures Different? J. Fungi 2021, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.-P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal Infections in Mechanically Ventilated Patients with COVID-19 during the First Wave: The French Multicentre MYCOVID Study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Dupont, D.; Menotti, J.; Turc, J.; Miossec, C.; Wallet, F.; Richard, J.-C.; Argaud, L.; Paulus, S.; Wallon, M.; Ader, F.; et al. Pulmonary Aspergillosis in Critically Ill Patients with Coronavirus Disease 2019 (COVID-19). Med. Mycol. 2021, 59, 110–114. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Therapeutic Drug Monitoring of Anti-Infective Agents in Critically Ill Patients. Expert Rev. Clin. Pharmacol. 2016, 9, 961–979. [Google Scholar] [CrossRef]
- Park, W.B.; Kim, N.-H.; Kim, K.-H.; Lee, S.H.; Nam, W.-S.; Yoon, S.H.; Song, K.-H.; Choe, P.G.; Kim, N.J.; Jang, I.-J.; et al. The Effect of Therapeutic Drug Monitoring on Safety and Efficacy of Voriconazole in Invasive Fungal Infections: A Randomized Controlled Trial. Clin. Infect. Dis. 2012, 55, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Duettmann, W.; Raggam, R.B.; Seeber, K.; Troppan, K.; Fruhwald, S.; Prueller, F.; Wagner, J.; Valentin, T.; Zollner-Schwetz, I.; et al. Potential Factors for Inadequate Voriconazole Plasma Concentrations in Intensive Care Unit Patients and Patients with Hematological Malignancies. Antimicrob. Agents Chemother. 2013, 57, 3262–3267. [Google Scholar] [CrossRef]
- Li, X.; Lai, F.; Jiang, Z.; Li, M.; Chen, Z.; Cheng, J.; Cui, H.; Wen, F. Effects of Inflammation on Voriconazole Levels: A Systematic Review. Br. J. Clin. Pharmacol. 2022, 88, 5166–5182. [Google Scholar] [CrossRef] [PubMed]
- Encalada Ventura, M.A.; Span, L.F.R.; van den Heuvel, E.R.; Groothuis, G.M.M.; Alffenaar, J.-W.C. Influence of Inflammation on Voriconazole Metabolism. Antimicrob. Agents Chemother. 2015, 59, 2942–2943. [Google Scholar] [CrossRef]
- Encalada Ventura, M.A.; van Wanrooy, M.J.P.; Span, L.F.R.; Rodgers, M.G.G.; van den Heuvel, E.R.; Uges, D.R.A.; van der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.W.C. Longitudinal Analysis of the Effect of Inflammation on Voriconazole Trough Concentrations. Antimicrob. Agents Chemother. 2016, 60, 2727–2731. [Google Scholar] [CrossRef]
- Veringa, A.; Ter Avest, M.; Span, L.F.R.; van den Heuvel, E.R.; Touw, D.J.; Zijlstra, J.G.; Kosterink, J.G.W.; van der Werf, T.S.; Alffenaar, J.-W.C. Voriconazole Metabolism Is Influenced by Severe Inflammation: A Prospective Study. J. Antimicrob. Chemother. 2017, 72, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Profile of Voriconazole. Clin. Pharm. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- White, C.M. Inflammation Suppresses Patients’ Ability to Metabolize Cytochrome P450 Substrate Drugs. Ann. Pharm. 2021, 56, 10600280211047864. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pea, F. The Cytokine Release Syndrome and/or the Proinflammatory Cytokines as Underlying Mechanisms of Downregulation of Drug Metabolism and Drug Transport: A Systematic Review of the Clinical Pharmacokinetics of Victim Drugs of This Drug–Disease Interaction Under Different Clinical Conditions. Clin. Pharm. 2022, 61, 1519–1544. [Google Scholar] [CrossRef]
- Jin, H.; Wang, T.; Falcione, B.A.; Olsen, K.M.; Chen, K.; Tang, H.; Hui, J.; Zhai, S. Trough Concentration of Voriconazole and Its Relationship with Efficacy and Safety: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1772–1785. [Google Scholar] [CrossRef]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Takesue, Y. Optimal Trough Concentration of Voriconazole with Therapeutic Drug Monitoring in Children: A Systematic Review and Meta-Analysis. J. Infect. Chemother. 2021, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, H.; Sun, J.; Cheng, X.; Xie, J.; Dong, H.; Chen, L.; Wang, X.; Xing, J.; Dong, Y. Efficacy and Safety of Voriconazole and CYP2C19 Polymorphism for Optimised Dosage Regimens in Patients with Invasive Fungal Infections. Int. J. Antimicrob. Agents 2014, 44, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, K.; Liu, F.; Luo, X.; He, S.; Hu, L.; Yang, C.; Huang, L.; Feng, Y. The Influence of CYP2C19 Polymorphisms on Voriconazole Trough Concentrations: Systematic Review and Meta-Analysis. Mycoses 2021, 64, 860–873. [Google Scholar] [CrossRef]
- Dote, S.; Sawai, M.; Nozaki, A.; Naruhashi, K.; Kobayashi, Y.; Nakanishi, H. A Retrospective Analysis of Patient-Specific Factors on Voriconazole Clearance. J. Pharm. Health Care Sci. 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Veyret, E.; Truffot, A.; Bailly, S.; Fonrose, X.; Thiebaut-Bertrand, A.; Tonini, J.; Cahn, J.-Y.; Stanke-Labesque, F. Inflammation Is a Potential Risk Factor of Voriconazole Overdose in Hematological Patients. Fundam. Clin. Pharmacol. 2019, 33, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, R.; Liu, F.; Li, Y.; Chen, H.; Yao, P.; Sun, F.; Xia, P. Therapeutic Drug Monitoring and Safety of Voriconazole in Elderly Patients. Int. Immunopharmacol. 2020, 78, 106078. [Google Scholar] [CrossRef]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of Voriconazole Plasma Concentrations on Treatment Response in Critically Ill Patients. J. Clin. Pharm. Ther. 2019, 44, 572–578. [Google Scholar] [CrossRef]
- Le Daré, B.; Boglione-Kerrien, C.; Reizine, F.; Gangneux, J.-P.; Bacle, A. Toward the Personalized and Integrative Management of Voriconazole Dosing during COVID-19-Associated Pulmonary Aspergillosis. Crit. Care 2021, 25, 152. [Google Scholar] [CrossRef]
- Bakir Ekinci, P.; Kara, E.; Er, A.G.; Inkaya, A.C.; Demirkan, K.; Uzun, O. Challenge in Treating COVID-19 Associate Pulmonary Aspergillosis: Supratherapeutic Voriconazole Levels. Br. J. Clin. Pharmacol. 2022, 88, 1387–1391. [Google Scholar] [CrossRef]
- Gatti, M.; De Ponti, F.; Pea, F. Clinically Significant Drug Interactions Between Psychotropic Agents and Repurposed COVID-19 Therapies. CNS Drugs 2021, 35, 345–384. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency—EMA Guideline on the Investigation of Drug Interactions 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf (accessed on 20 March 2023).
- Chromsystems Instruments & Chemicals GmbH. MassTox TDM Series A. 2022. Available online: https://chromsystems.com/masstox-tdm-series-a (accessed on 20 March 2023).
- Luong, M.-L.; Al-Dabbagh, M.; Groll, A.H.; Racil, Z.; Nannya, Y.; Mitsani, D.; Husain, S. Utility of Voriconazole Therapeutic Drug Monitoring: A Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1786–1799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).