Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Human Cells
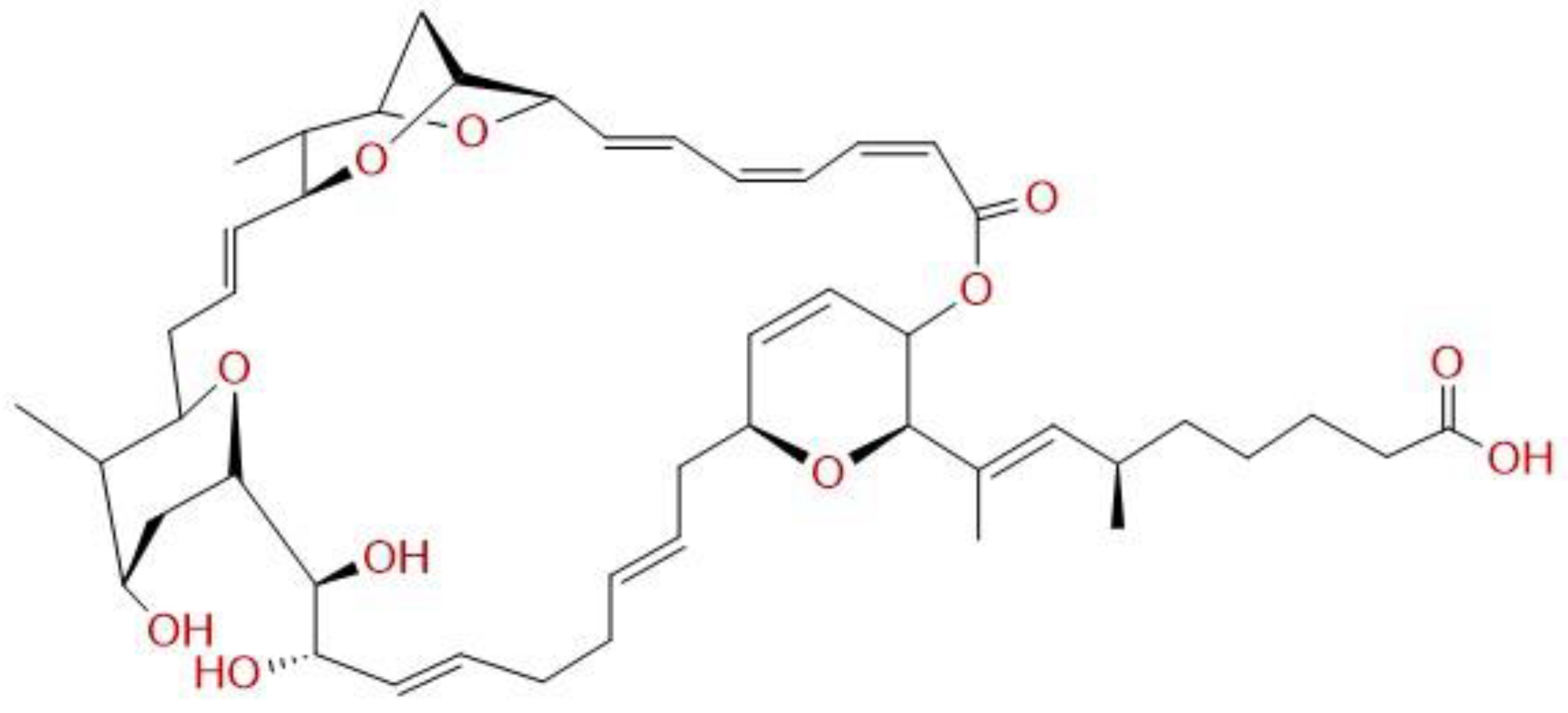
2.2. Chemicals
2.3. Determination of the Minimal Inhibitory Concentration (MIC) for Chlamydiae
2.4. Testing Recoverable Chlamydiae
2.5. Assessing Growth Pattern Changes of Species from the Genus Lactobacillus under Sorangicin A Application
2.6. Efficacy of Sorangicin A against C. trachomatis Serovar D in the Human Fallopian Tube Ex Vivo Model
2.7. Application of Sorangicin A in an In Vivo Mouse Model of Chlamydia Muridarum Infection
2.8. Microbiome Analysis
2.9. Measurements of SorA Levels
2.10. Statistics
3. Results
3.1. Sorangicin A Is Active against C. trachomatis in Cell Culture and the Fallopian Tube Model
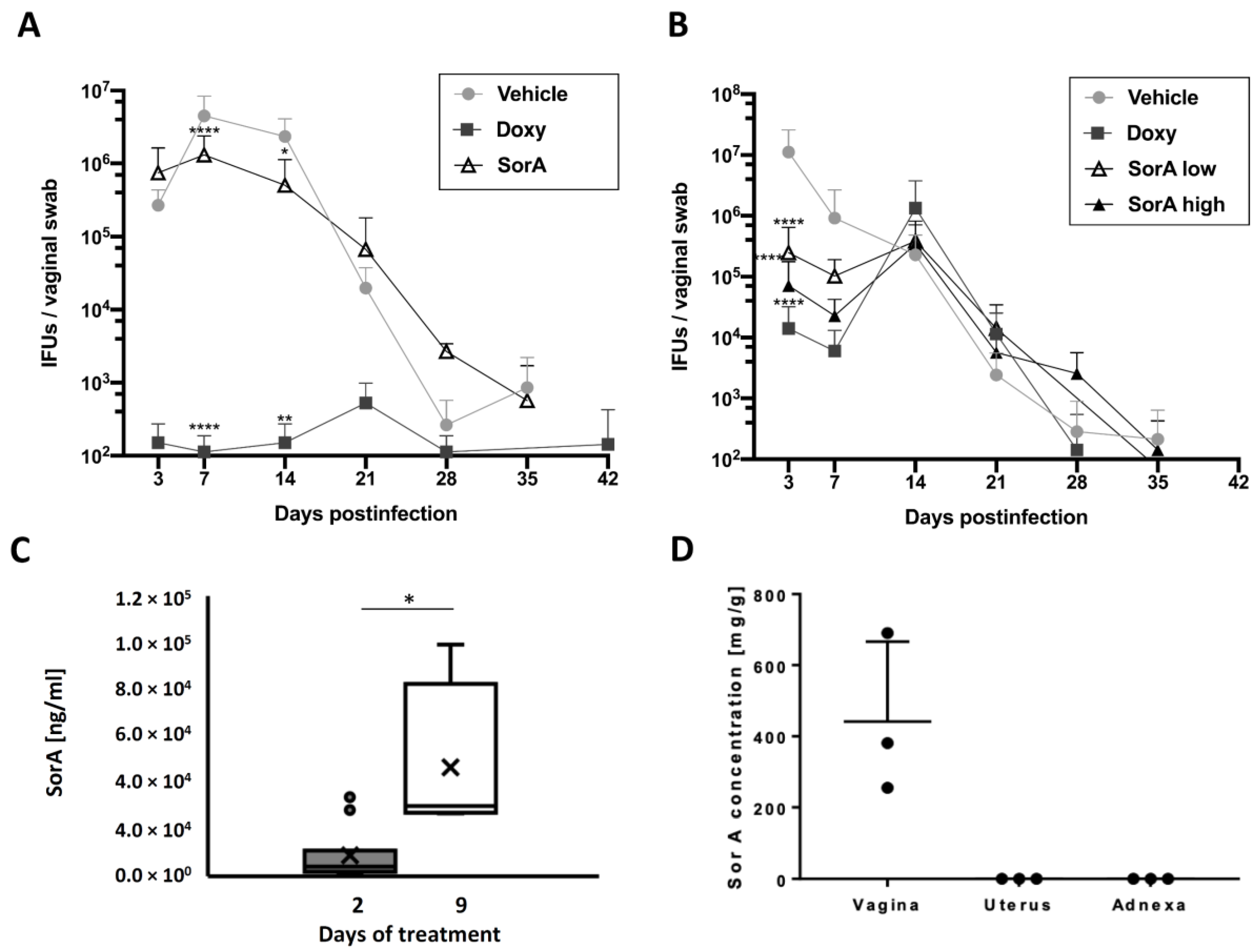
3.2. Efficacy of Topical and Systemic Sorangicin A Application on Chlamydial Shedding Differs in a Mouse Infection Model
3.3. Accumulation of Sorangicin A Is Detected at the Site of Topical Treatment Only
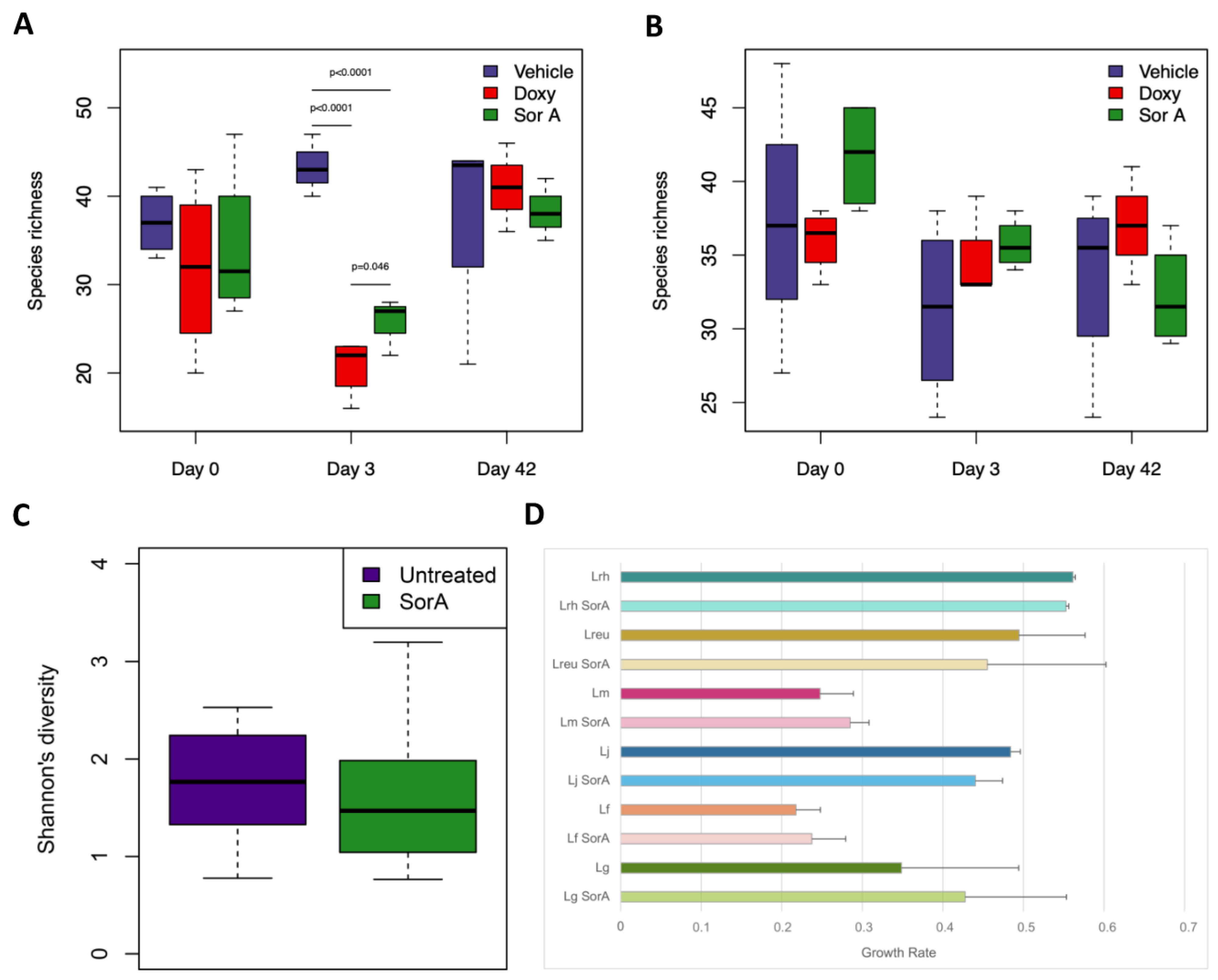
3.4. Impact of Sorangicin A on the Gut and Vaginal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, Gonorrhoea, Trichomoniasis and Syphilis: Global Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Starnbach, M.N. Action Needed on Chlamydia Vaccines. Trends Microbiol. 2018, 26, 639–640. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bolan, G.A. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Golden, M.R.; Handsfield, H.H.; Hogben, M.; Helmers, J.R.L.; Holmes, K.K. Effect of Expedited Treatment of Sex Partners on Recurrent or Persistent Gonorrhea or Chlamydial Infection. N. Engl. J. Med. 2005, 352, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, F.; Dolatian, M.; Jorjani, M.; Afrakhteh, M.; Majd, H.A.; Abdi, F.; Pakzad, R. Urogenital Chlamydia Trachomatis Treatment Failure with Azithromycin: A Meta-Analysis. Int. J. Reprod. Biomed. 2019, 17, 603–620. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia Trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef]
- Smith, A.; Pennefather, P.M.; Kaye, S.B.; Hart, C.A. Fluoroquinolones. Drugs 2001, 61, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Thaçi, D.; Schöfer, H. Topische Antibiotika zur Therapie von Hautinfektionen. Hautarzt 2005, 56, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Shima, K.; Ledig, S.; Loeper, N.; Schiefer, A.; Pfarr, K.; Hoerauf, A.; Graspeuntner, S.; Rupp, J. Effective Inhibition of Rifampicin-Resistant Chlamydia Trachomatis by the Novel DNA-Dependent RNA Polymerase Inhibitor Corallopyronin A. Int. J. Antimicrob. Agents 2018, 52, 523–524. [Google Scholar] [CrossRef]
- Loeper, N.; Graspeuntner, S.; Ledig, S.; Kaufhold, I.; Hoellen, F.; Schiefer, A.; Henrichfreise, B.; Pfarr, K.; Hoerauf, A.; Shima, K.; et al. Elaborations on Corallopyronin A as a Novel Treatment Strategy Against Genital Chlamydial Infections. Front. Microbiol. 2019, 10, 943. [Google Scholar] [CrossRef]
- Weissman, K.J.; Müller, R. Myxobacterial Secondary Metabolites: Bioactivities and Modes-of-Action. Nat. Prod. Rep. 2010, 27, 1276–1295. [Google Scholar] [CrossRef]
- Irschik, H.; Jansen, R.; Gerth, K.; Höfle, G.; Reichenbach, H. The Sorangicins, Novel and Powerful Inhibitors of Eubacterial RNA Polymerase Isolated from Myxobacteria. J. Antibiot. 1987, 40, 7–13. [Google Scholar] [CrossRef]
- Wenholz, D.S.; Miller, M.; Dawson, C.; Bhadbhade, M.; Black, D.S.; Griffith, R.; Dinh, H.; Cain, A.; Lewis, P.; Kumar, N. Inhibitors of Bacterial RNA Polymerase Transcription Complex. Bioorg. Chem. 2022, 118, 105481. [Google Scholar] [CrossRef]
- Lilic, M.; Chen, J.; Boyaci, H.; Braffman, N.; Hubin, E.A.; Herrmann, J.; Müller, R.; Mooney, R.; Landick, R.; Darst, S.A.; et al. The Antibiotic Sorangicin A Inhibits Promoter DNA Unwinding in a Mycobacterium Tuberculosis Rifampicin-Resistant RNA Polymerase. Proc. Natl. Acad. Sci. USA 2020, 117, 30423–30432. [Google Scholar] [CrossRef] [PubMed]
- Shima, K.; Szaszák, M.; Solbach, W.; Gieffers, J.; Rupp, J. Impact of a Low-Oxygen Environment on the Efficacy of Antimicrobials against Intracellular Chlamydia Trachomatis. Antimicrob. Agents Chemother. 2011, 55, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.A.; Rettig, H.C.; Lupatsii, M.; Schlüter, B.; Schäfer, K.; Friedrich, D.; Graspeuntner, S.; Rupp, J. Culturomics Approaches Expand the Diagnostic Accuracy for Sexually Transmitted Infections. Int. J. Mol. Sci. 2021, 22, 10815. [Google Scholar] [CrossRef]
- Jerchel, S.; Knebel, G.; König, P.; Bohlmann, M.K.; Rupp, J. A Human Fallopian Tube Model for Investigation of C. Trachomatis Infections. J. Vis. Exp. JoVE 2012, 66, e4036. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An Improved Dual-Indexing Approach for Multiplexed 16S RRNA Gene Sequencing on the Illumina MiSeq Platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Graspeuntner, S.; Bohlmann, M.K.; Gillmann, K.; Speer, R.; Kuenzel, S.; Mark, H.; Hoellen, F.; Lettau, R.; Griesinger, G.; König, I.R.; et al. Microbiota-Based Analysis Reveals Specific Bacterial Traits and a Novel Strategy for the Diagnosis of Infectious Infertility. PLoS ONE 2018, 13, e0191047. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.-A.; Hugenholtz, P. A Standardized Bacterial Taxonomy Based on Genome Phylogeny Substantially Revises the Tree of Life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Vegan: Community Ecology Package, R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 February 2023).
- Srivastava, A.; Talaue, M.; Liu, S.; Degen, D.; Ebright, R.Y.; Sineva, E.; Chakraborty, A.; Druzhinin, S.Y.; Chatterjee, S.; Mukhopadhyay, J.; et al. New Target for Inhibition of Bacterial RNA Polymerase: ‘Switch Region’. Curr. Opin. Microbiol. 2011, 14, 532–543. [Google Scholar] [CrossRef]
- Juul, N.; Jensen, H.; Hvid, M.; Christiansen, G.; Birkelund, S. Characterization of In Vitro Chlamydial Cultures in Low-Oxygen Atmospheres. J. Bacteriol. 2007, 189, 6723–6726. [Google Scholar] [CrossRef]
- Dietz, I.; Jerchel, S.; Szaszák, M.; Shima, K.; Rupp, J. When Oxygen Runs Short: The Microenvironment Drives Host–Pathogen Interactions. Microbes Infect. 2012, 14, 311–316. [Google Scholar] [CrossRef]
- Schaffer, K.; Taylor, C.T. The Impact of Hypoxia on Bacterial Infection. FEBS J. 2015, 282, 2260–2266. [Google Scholar] [CrossRef]
- Christensen, J.J.; Holten-Andersen, W.; Nielsen, P.B. Chlamydia Trachomatis: In Vitro Susceptibility to Antibiotics Singly and in Combination. Acta Pathol. Microbiol. Immunol. Scand. 1986, 94, 329–332. [Google Scholar] [CrossRef]
- Notomi, T.; Ikeda, Y.; Nagayama, A. Minimum Inhibitory and Minimal Lethal Concentration against Chlamydia Trachomatis Dependent on the Time of Addition and the Duration of the Presence of Antibiotics. Chemotherapy 1999, 45, 242–248. [Google Scholar] [CrossRef]
- Shima, K.; Klinger, M.; Solbach, W.; Rupp, J. Activities of First-Choice Antimicrobials against Gamma Interferon-Treated Chlamydia Trachomatis Differ in Hypoxia. Antimicrob. Agents Chemother. 2013, 57, 2828–2830. [Google Scholar] [CrossRef]
- Molina-Quiroz, R.C.; Silva, C.A.; Molina, C.F.; Leiva, L.E.; Reyes-Cerpa, S.; Contreras, I.; Santiviago, C.A. Exposure to Sub-Inhibitory Concentrations of Cefotaxime Enhances the Systemic Colonization of Salmonella Typhimurium in BALB/c Mice. Open Biol. 2015, 5, 150070. [Google Scholar] [CrossRef]
- Roth, A.; König, P.; van Zandbergen, G.; Klinger, M.; Hellwig-Bürgel, T.; Däubener, W.; Bohlmann, M.K.; Rupp, J. Hypoxia Abrogates Antichlamydial Properties of IFN-γ in Human Fallopian Tube Cells in Vitro and Ex Vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 19502–19507. [Google Scholar] [CrossRef]
- Kessler, M.; Zielecki, J.; Thieck, O.; Mollenkopf, H.-J.; Fotopoulou, C.; Meyer, T.F. Chlamydia Trachomatis Disturbs Epithelial Tissue Homeostasis in Fallopian Tubes via Paracrine Wnt Signaling. Am. J. Pathol. 2012, 180, 186–198. [Google Scholar] [CrossRef]
- Käding, N.; Schmidt, N.; Scholz, C.; Graspeuntner, S.; Rupp, J.; Shima, K. Impact of First-Line Antimicrobials on Chlamydia Trachomatis-Induced Changes in Host Metabolism and Cytokine Production. Front. Microbiol. 2021, 12, 676747. [Google Scholar] [CrossRef]
- Barron, A.L.; White, H.J.; Rank, R.G.; Soloff, B.L.; Moses, E.B. A New Animal Model for the Study of Chlamydia Trachomatis Genital Infections: Infection of Mice with the Agent of Mouse Pneumonitis. J. Infect. Dis. 1981, 143, 63–66. [Google Scholar] [CrossRef]
- Miyairi, I.; Ramsey, K.H.; Patton, D.L. Duration of Untreated Chlamydial Genital Infection and Factors Associated with Clearance: Review of Animal Studies. J. Infect. Dis. 2010, 201, S96–S103. [Google Scholar] [CrossRef]
- O’Meara, C.P.; Andrew, D.W.; Beagley, K.W. The Mouse Model of Chlamydia Genital Tract Infection: A Review of Infection, Disease, Immunity and Vaccine Development. Curr. Mol. Med. 2014, 14, 396–421. [Google Scholar] [CrossRef]
- Connolly, K.L.; Eakin, A.E.; Gomez, C.; Osborn, B.L.; Unemo, M.; Jerse, A.E. Pharmacokinetic Data Are Predictive of In Vivo Efficacy for Cefixime and Ceftriaxone against Susceptible and Resistant Neisseria Gonorrhoeae Strains in the Gonorrhea Mouse Model. Antimicrob. Agents Chemother. 2019, 63, e01644-18. [Google Scholar] [CrossRef]
- Cester, C.C.; Laurentie, M.P.; García-Villar, R.; Toutain, P.L. Spiramycin Concentrations in Plasma and Genital-Tract Secretions after Intravenous Administration in the Ewe. J. Vet. Pharmacol. Ther. 1990, 13, 7–14. [Google Scholar] [CrossRef]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Müller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the Collateral Damage of Antibiotics on Gut Bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef]
- Menard, J.-P. Antibacterial Treatment of Bacterial Vaginosis: Current and Emerging Therapies. Int. J. Womens Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Méndez-García, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic Use and Microbiome Function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical Modifications to Increase the Therapeutic Potential of Antimicrobial Peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.; Ee, P. Macromolecular Conjugate and Biological Carrier Approaches for the Targeted Delivery of Antibiotics. Antibiotics 2017, 6, 14. [Google Scholar] [CrossRef]
- Jammal, J.; Zaknoon, F.; Mor, A. Eliciting Improved Antibacterial Efficacy of Host Proteins in the Presence of Antibiotics. FASEB J. 2018, 32, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ohmer, M.; Tzivelekidis, T.; Niedenführ, N.; Volceanov-Hahn, L.; Barth, S.; Vier, J.; Börries, M.; Busch, H.; Kook, L.; Biniossek, M.L.; et al. Infection of HeLa Cells with CHLAMYDIA TRACHOMATIS nhibits Protein Synthesis and Causes Multiple Changes to Host Cell Pathways. Cell. Microbiol. 2019, 21, e12993. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graspeuntner, S.; Koethke, K.; Scholz, C.; Semmler, L.; Lupatsii, M.; Kirchhoff, L.; Herrmann, J.; Rox, K.; Wittstein, K.; Käding, N.; et al. Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment. Antibiotics 2023, 12, 795. https://doi.org/10.3390/antibiotics12050795
Graspeuntner S, Koethke K, Scholz C, Semmler L, Lupatsii M, Kirchhoff L, Herrmann J, Rox K, Wittstein K, Käding N, et al. Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment. Antibiotics. 2023; 12(5):795. https://doi.org/10.3390/antibiotics12050795
Chicago/Turabian StyleGraspeuntner, Simon, Katharina Koethke, Celeste Scholz, Lea Semmler, Mariia Lupatsii, Laura Kirchhoff, Jennifer Herrmann, Katharina Rox, Kathrin Wittstein, Nadja Käding, and et al. 2023. "Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment" Antibiotics 12, no. 5: 795. https://doi.org/10.3390/antibiotics12050795
APA StyleGraspeuntner, S., Koethke, K., Scholz, C., Semmler, L., Lupatsii, M., Kirchhoff, L., Herrmann, J., Rox, K., Wittstein, K., Käding, N., Hanker, L. C., Stadler, M., Brönstrup, M., Müller, R., Shima, K., & Rupp, J. (2023). Sorangicin A Is Active against Chlamydia in Cell Culture, Explanted Fallopian Tubes, and Topical In Vivo Treatment. Antibiotics, 12(5), 795. https://doi.org/10.3390/antibiotics12050795







