New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities
Abstract
:1. Introduction
2. Results
2.1. Chemistry
Optimization Study for the Synthesis of 1b
2.2. Spectral Data
2.2.1. 2-((4-Methoxyphenoxy)methyl)-N-(thiazol-2-ylcarbamothioyl)benzamide (1a) (C19H17N3O3S2; MW = 399.48 g/mol; m.p. 128–131 °C; yield 65%)
2.2.2. N-(benzo[d]thiazol-2-ylcarbamothioyl)-2-((4-methoxyphenoxy)methyl)benzamide (1b) (C23H19N3O3S2; MW = 449.53 g/mol; m.p. 169–172 °C; yield 41%, after optimization 76%)
2.2.3. 2-((4-Methoxyphenoxy)methyl)-N-(pyridin-2-ylcarbamothioyl)benzamide (1c) (C21H19N3O3S; MW = 393.446 g/mol; m.p. 110–114 °C; yield 56%)
2.2.4. 2-((4-Methoxyphenoxy)methyl)-N-((6-methylpyridin-2-yl)carbamothioyl)benzamide (1d) (C22H21N3O3S; MW = 407.476 g/mol; m.p. 95–98 °C; yield 52%)
2.2.5. N-((5-chloropyridin-2-yl)carbamothioyl)-2-((4-methoxyphenoxy)methyl)benzamide (1e) (C21H18ClN3O3S; MW = 427.893 g/mol; m.p. 131–135 °C; yield 73%)
2.2.6. N-((3,5-dibromopyridin-2-yl)carbamothioyl)-2-((4-methoxyphenoxy)methyl)benzamide (1f) (C21H17Br2N3O3S; MW = 551.25 g/mol; m.p. 156–159 °C; yield 63%)
2.2.7. 2-((4-Methoxyphenoxy)methyl)-N-(pyrimidin-2-ylcarbamothioyl)benzamide (1g) (C20H18N4O3S; MW = 394.436 g/mol; m.p. 157–160 °C; yield 54%)
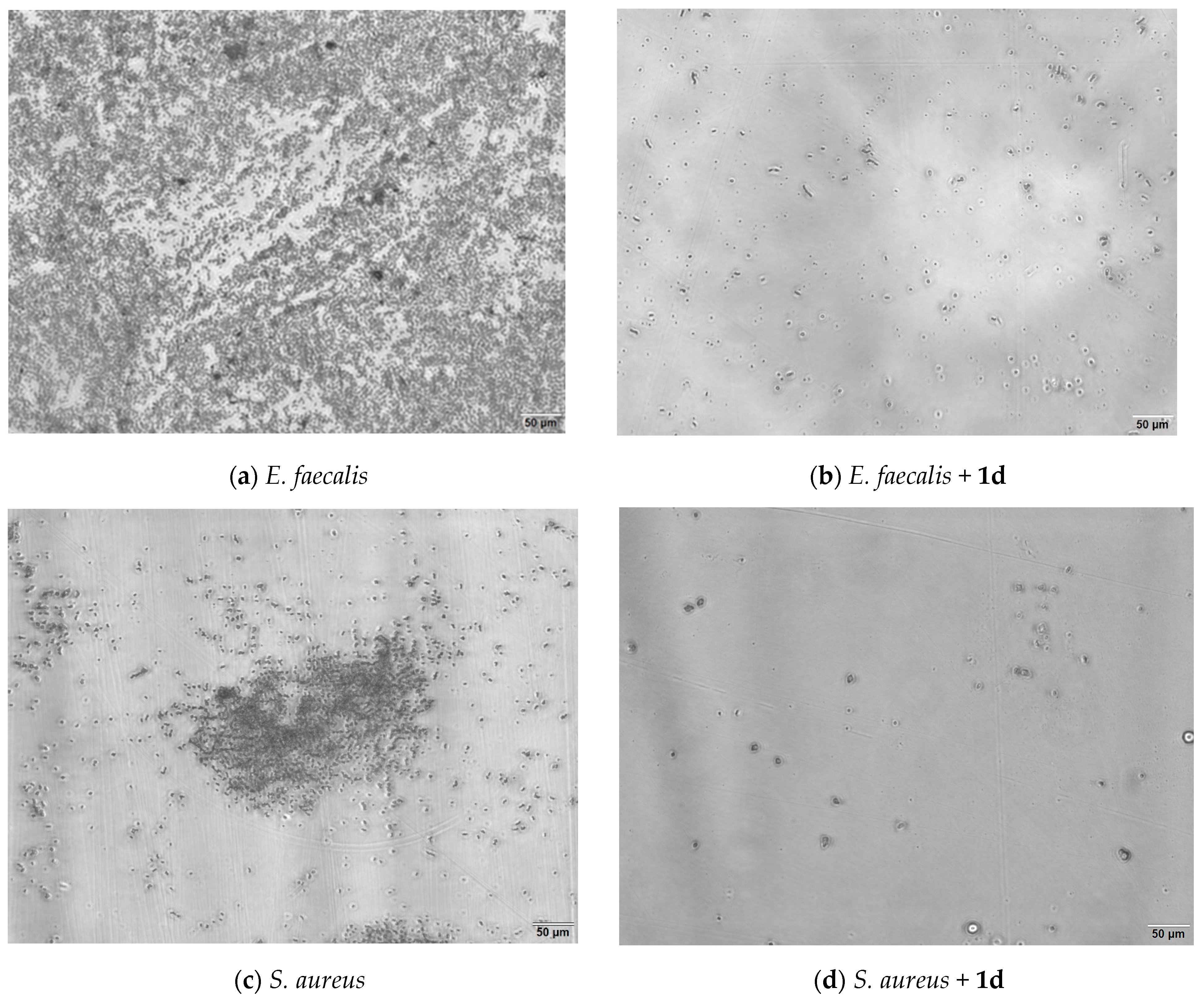
2.3. Antimicrobial Activity Results
2.4. Total Antioxidant Activity
2.5. Cytotoxicity Assay

2.6. Validation of Quantitative Analysis Method by HPLC
2.6.1. Specificity


2.6.2. LOD and LOQ
2.6.3. Precision Results
2.6.4. Accuracy Results
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for the Synthesis of the New Compounds (1a–1g)
4.1.2. Optimization Study for the Synthesis of 1b
4.2. Measurements
4.2.1. TLC Analysis
4.2.2. Melting Points
4.2.3. IR Spectra
4.2.4. NMR Spectra
4.2.5. FT-ICR Mass Spectrometry
4.3. Biological Evaluation of the Antimicrobial Activity
4.4. Total Antioxidant Activity
4.5. Cytotoxicity and Cell Cycle Assay
4.6. RP-HPLC Analysis
4.6.1. Chromatographic Conditions
4.6.2. Materials and Equipment
4.6.3. Mobile Phase Preparation
4.6.4. Samples Preparation
Specificity
LOD/LOQ
Linearity
Precision and Accuracy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Keche, A.P.; Kamble, V.M. Synthesis and anti-inflammatory and antimicrobial activities of some novel 2-methylquinazolin-4(3H)-one derivatives bearing urea, thiourea and sulphonamide functionalities. Arab. J. Chem. 2014, 12, 1522–1531. [Google Scholar] [CrossRef]
- Abd Halim, A.N.; Ngaini, Z. Synthesis and Bacteriostatic Activities of Bis(thiourea) Derivatives with Variable Chain Length. J. Chem. 2016, 2016, 2739832. [Google Scholar] [CrossRef]
- Arslan, H.; Duran, N.; Borekci, G.; Koray Ozer, C.; Akbay, C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules 2009, 14, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Elaasser, M.M.; Nissan, Y.M. Antimicrobial and anticancer activity of some novel fluorinated thiourea derivatives carrying sulfonamide moieties: Synthesis, biological evaluation and molecular docking. Chem. Cent. J. 2017, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Antypenko, L.; Meyer, F.; Kholodniak, O.; Sadykova, Z.; Jirásková, T.; Troianova, A.; Buhaiova, V.; Cao, S.; Kovalenko, S.; Garbe, L.A.; et al. Novel acyl thiourea derivatives: Synthesis, antifungal activity, gene toxicity, drug-like and molecular docking screening. Arch. Pharm. Chem. Life Sci. 2018, 352, e1800275. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, I.V.; Nurkenov, O.A.; Akhmetova, S.B.; Seidakhmetova, R.B.; Zhambekov, Z.M. Synthesis and antibacterial and antifungal activities of thiourea derivatives of the alkaloid anabasine. Pharm. Chem. J. 2011, 45, 15–18. [Google Scholar] [CrossRef]
- Calixto, S.D.; Simão, T.L.B.V.; Palmeira-Mello, M.V.; Viana, G.M.; Assumpção, P.W.M.C.; Rezende, M.G.; do Espirito Santo, C.C.; de Oliveira Mussi, V.; Rodrigues, C.R.; Lasunskaia, E.; et al. Antimycobacterial and anti-inflammatory activities of thiourea derivatives focusing on treatment approaches for severe pulmonary tuberculosis. Bioorg. Med. Chem. 2022, 53, 116506. [Google Scholar] [CrossRef]
- Doğan, Ş.D.; Gündüz, M.G.; Doğan, H.; Vagolu, S.K.; Lherbet, C.; Sriram, D. Design and synthesis of thiourea-based derivatives as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur. J. Med. Chem. 2020, 199, 112402. [Google Scholar] [CrossRef]
- Tatar, E.; Karakuş, S.; Küçükgüzel, Ş.G.; Öktem Okullu, S.; Ünübol, N.; Kocagöz, T.; De Clercq, E.; Andrei, G.; Snoeck, R.; Pannecouque, C.; et al. Design, Synthesis, and Molecular Docking Studies of a Conjugated Thiadiazole-Thiourea Scaffold as Antituberculosis Agents. Biol. Pharm. Bull. 2016, 39, 502–515. [Google Scholar] [CrossRef]
- Duan, L.-P.; Xue, J.; Xu, L.-L.; Zhang, H.-B. Synthesis 1-Acyl-3-(2’-aminophenyl) thioureas as Anti-Intestinal Nematode Prodrugs. Molecules 2010, 15, 6941–6947. [Google Scholar] [CrossRef]
- Krishna Reddy, R.C.; Rasheed, S.; Subba Rao, D.; Adam, S.; Venkata Rami Reddy, Y.; Raju, C.N. New Urea and Thiourea Derivatives of Piperazine Doped with Febuxostat: Synthesis and Evaluation of Anti-TMV and Antimicrobial Activities. Sci. World J. 2013, 2013, 682603. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Venkatachalam, T.K.; Uckun, F.M. Novel Thiourea Compounds as Dual-Function Microbicides. Biol. Reprod. 2000, 63, 196–205. [Google Scholar] [CrossRef]
- Abbas, S.Y.; Al-Harbi, R.A.K.; Sh El-Sharief, M.A.M. Synthesis and anticancer activity of thiourea derivatives bearing a benzodioxole moiety with EGFR inhibitory activity, apoptosis assay and molecular docking study. Eur. J. Med. Chem. 2020, 198, 112363. [Google Scholar] [CrossRef]
- Arafa, W.A.A.; Ghoneim, A.A.; Mourad, A.K. N-Naphthoyl Thiourea Derivatives: An Efficient Ultrasonic-Assisted Synthesis, Reaction, and In Vitro Anticancer Evaluations. ACS Omega 2022, 7, 6210–6222. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Hussain, H.; Shah, S.W.A.; Umar, M.N.; Ullah, A. Synthesis, acute toxicity, analgesic activity and cytotoxicity of Some bisthiourea derivatives. Pak. J. Pharm. Sci. 2017, 30, 1351–1356. [Google Scholar]
- Hardjono, S.; Siswandono, S.; Andayani, R. Evaluation of N-benzoylthiourea derivatives as possible analgesic agents by predicting their physicochemical and pharmacokinetic properties, toxicity, and analgesic activity. Indones. J. Biotechnol. 2017, 22, 76–85. [Google Scholar] [CrossRef]
- Shalas, A.F.; Rudyanto, M. Synthesis and structure-activity relationship of 1-allyl-3-(2-chlorobenzoyl)thiourea as analgesic. Int. J. Pharm. Sci. 2016, 8, 297–298. [Google Scholar]
- Alagarsamy, V.; Murugananthan, G.; Venkateshperumal, R. Synthesis, Analgesic, Anti-inflammatory and Antibacterial Activities of Some Novel 2-Methyl-3-substituted Quinazolin-4-(3H)-ones. Biol. Pharm. Bull. 2003, 26, 1711–1714. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Al-Mohammadi, M.M.; Alamry, K.A.; Khan, K.A. Synthesis and biological evaluation of fluoropyrazolesulfonylurea and thiourea derivatives as possible antidiabetic agents. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. S1), 157–163. [Google Scholar] [CrossRef]
- Naz, S.; Zahoor, M.; Umar, M.N.; AlQahtany, F.S.; Elnahas, Y.M.; Ullah, R. In vivo glucose-6-phosphatase inhibitory, toxicity and antidiabetic potentials of 2-picolylamine thioureas in Swiss albino mice. Saudi J. Biol. Sci. 2020, 27, 3267–3273. [Google Scholar] [CrossRef]
- Thakur, A.S.; Deshmukh, R.; Jha, A.K.; Kumar, P.S. Molecular docking study and anticonvulsant activity of synthesized 4-((4,6-dimethyl-6H-1,3-thiazin-2-yl)phenylsulfonyl)urea/thiourea derivatives. J. King. Saud. Univ. Sci. 2018, 30, 330–336. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea Derivatives in Drug Design and Medicinal Chemistry: A Short Review. JMCDD 2016, 2, 10–20. [Google Scholar] [CrossRef]
- Manna, D.; Roy, G.; Mugesh, G. Antithyroid drugs and their analogues: Synthesis, structure, and mechanism of action. Acc. Chem. Res. 2013, 46, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Zahoor, M.; Umar, M.N.; Alghamdi, S.; Sahibzada, M.U.K.; UlBari, W. Synthesis, characterization, and pharmacological evaluation of thiourea derivatives. Open Chem. J. 2020, 18, 764–777. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Wu, W.-Y.; Chen, F.-L.; Guan, X.-L.; Fu, X.-H.; Jiang, P.; Wan, R. Design, synthesis, crystal structure and insecticidal evaluation of novel arylpyrazole derivatives containing cyhalothroyl thiourea moiety. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 911–918. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Cao, X.; Chen, C. Synthesis and Bio-Evaluation of Novel Salicylic Acid-Oriented Thiourea Derivatives with Potential Applications in Agriculture. Lett. Drug Des. Discov. 2013, 11, 98–103. [Google Scholar] [CrossRef]
- Mody, V.; Ray, S.D. α-Naphthylthiourea, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; pp. 445–447. [Google Scholar]
- Kalhor, M.; Salehifar, M. Synthesis, characterization, and antibacterial activities of some novel N,N’-disubstituted thiourea, 2-amino thiazole, and imidazole-2-thione derivatives. Med. Chem. Res. 2014, 23, 2947–2954. [Google Scholar] [CrossRef]
- Barzaga, R.; Lestón-Sánchez, L.; Aguilar-Galindo, F.; Estévez-Hernández, O.; Díaz-Tendero, S. Synergy Effects in Heavy Metal Ion Chelation with Aryl- and Aroyl-Substituted Thiourea Derivatives. Inorg. Chem. 2021, 60, 11984–12000. [Google Scholar] [CrossRef]
- Nordin, N.A.; Chai, T.W.; Tan, B.L.; Choi, C.L.; Abd Halim, A.N.; Hussain, H.; Ngaini, Z. Novel Synthetic Monothiourea Aspirin Derivatives Bearing Alkylated Amines as Potential Antimicrobial Agents. J. Chem. 2017, 2017, 2378186. [Google Scholar] [CrossRef]
- Cui, P.; Li, X.; Zhu, M.; Wang, B.; Liu, J.; Chen, H. Design, synthesis and antibacterial activities of thiouracil derivatives containing acyl thiourea as SecA inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2234–2237. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Q.; Chen, Z.; He, M.; Jin, L.; Hu, D. Synthesis and Bioactivity of Pyrazole Acyl Thiourea Derivatives. Molecules 2012, 17, 5139–5150. [Google Scholar] [CrossRef] [PubMed]
- Nabo, S.; Zhonghua, S.; Zhiwen, Z.; Liang, H.; Jianquan, W.; Chengxia, T.; Xinghai, L. Design, Synthesis, Fungicidal Activity and Docking Study of Acyl Thiourea Derivatives Containing Pyrazole Moiety. Chin. J. Org. Chem. 2017, 37, 2705–2710. [Google Scholar]
- Roman, R.; Pintilie, L.; Nuță, D.; Limban, C. A QSAR Study on Thiourea Derivatives—New Approches in Drug Development. Farmacia 2022, 70, 228–240. [Google Scholar] [CrossRef]
- Limban, C.; Missir, A.V.; Chiriţă, I.C.; Bădiceanu, C.D.; Drăghici, C.; Balotescu, M.C.; Stamatoiu, O. New thioureides of 2-(4-methyl-phenoxymethyl)-benzoic and 2-(4-methoxy-phenoxymethyl)-benzoic acids with biological activity. Rev. Roum. Chim. 2008, 53, 595–602. [Google Scholar]
- Saeed, S.; Rashid, N.; Wong, W.-T.; Hussain, R. 1-(4,6-Dimethylpyrimidin-2-yl)-3-(3,5-dinitrobenzoyl)thiourea monohydrate. Acta Crystallogr. E 2011, 67, o1094. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Bhatti, M.H.; Jones, P.G. Synthesis, spectroscopic characterization, mass spectrometry, and crystal structure of N-{[(4-bromophenyl)amino]carbonothioyl}benzamide. Turk. J. Chem. 2010, 34, 761–770. [Google Scholar] [CrossRef]
- Patel, A.G.; Raval, K.N.; Patel, S.P.; Patel, K.S.; Patel, S.V. Review on benzothiazole including synthetic and pharmacological activity. J. Pharm. Res. 2012, 1, 1–4. [Google Scholar]
- Saeed, S.; Rashid, N.; Jones, P.G.; Hussain, R.; Bhatti, M.H. Synthesis, spectroscopic characteriyation, crystal structure and antifungal activity of thiourea derivatives containing a thiazole moiety. Cent. Eur. J. Chem. 2010, 8, 550–558. [Google Scholar]
- Saeed, S.; Rashid, N.; Ali, M.; Hussain, R.; Jones, P.G. Synthesis, spectroscopic characterization, crystal structure and pharmacological properties of some novel thiophene-thiourea core derivatives. Eur. J. Chem. 2010, 1, 221–227. [Google Scholar] [CrossRef]
- Kea, S.-Y.; Xue, S.-J. Synthesis and herbicidal activity of N-(o-fluorophenoxyacetyl)thioureas derivatives and related fused heterocyclic compounds. Arkivoc 2006, 2006, 63–68. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Jones, G.P.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common themes in antimicrobial and anticancer drug resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Limban, C.; Chifiriuc, M.C.; Caproiu, M.T.; Dumitrascu, F.; Ferbinteanu, M.; Pintilie, L.; Stefaniu, A.; Vlad, I.M.; Bleotu, C.; Marutescu, L.G.; et al. New Substituted Benzoylthiourea Derivatives: From Design to Antimicrobial Applications. Molecules 2020, 25, 1478. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Carbone, D.; Schillaci, D.; Giovannetti, E.; Cirrincione, G.; Diana, P. Thiazoles, Their Benzofused Systems, and Thiazolidinone Derivatives: Versatile and Promising Tools to Combat Antibiotic Resistance. J. Med. Chem. 2020, 63, 7923–7956. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring—A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- García-Báez, E.V.; Padilla-Martínez, I.I.; Tamay-Cach, F.; Cruz, A. Benzothiazoles from Condensation of o-Aminothiophenoles with Carboxylic Acids and Their Derivatives: A Review. Molecules 2021, 26, 6518. [Google Scholar] [CrossRef]
- Murthi, Y.; Pathak, D. Synthesis and Antimicrobial screening of Substituted 2-Mercaptobenzothiazoles. J. Pharm. Res. 2008, 7, 153–155. [Google Scholar]
- Mahran, M.A.; William, S.; Ramzy, F.; Sembel, A.M. Synthesis and in vitro Evaluation of New Benzothiazole Derivatives as Schistosomicidal Agents. Molecules 2007, 12, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.; Trigg, R.; Warrington, J. Benzothiazolines as antituberculous agents. J. Med. Chem. 1971, 14, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; De Crescenzo, G.A.; Getman, D.P.; Lu, H.-F.; Sikorski, J.A.; Walker, J.L.; McDonald, J.J.; Houseman, K.A.; Kocan, G.P.; Kishore, N. Discovery of novel benzothiazolesulfonamides as potent inhibitors of HIV-1 protease. Bioorg. Med. Chem. 2003, 11, 4769–4777. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef]
- Zhuang, J.; Ma, S. Recent Development of Pyrimidine-Containing Antimicrobial Agents. Chem. Med. Chem. 2020, 15, 1875–1886. [Google Scholar] [CrossRef]
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Struga, M. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef]
- Stefanska, J.; Nowicka, G.; Struga, M.; Szulczyk, D.; Koziol, A.E.; Augustynowicz-Kopec, E.; Napiorkowska, A.; Bielenica, A.; Filipowski, W.; Filipowska, A.; et al. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015, 63, 225–236. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.C.; Alencar, S.M.D.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fatiha, M.; Abdelkader, T. Study of antioxidant activity of pyrimidinium betaines by DPPH radical scavenging method. J. Anal. Pharm. Res. 2019, 8, 33–36. [Google Scholar] [CrossRef]
- Bem, M.; Baratoiu, R.; Radutiu, C.; Lete, C.; Mocanu, S.; Ionita, G.; Lupu, S.; Caproiu, M.T.; Madalan, A.M.; Patrascu, B.; et al. Synthesis and structural characterization of some novel methoxyamino derivatives with acid-basa and redox behavior. J. Mol. Struct. 2018, 1173, 291–299. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2010, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Remes, C.; Paun, A.; Zarafu, I.; Tudose, M.; Caproiu, M.T.; Ionita, G.; Ionita, P. Chemical and biological evaluation of some new antipyrine derivatives with particular properties. Bioorg. Chem. 2012, 41–42, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Paun, A.; Zarafu, I.; Caproiu, M.T.; Draghici, C.; Maganu, M.; Cotar, A.I.; Ionita, P. Synthesis and microbiological evaluation of several benzocaine derivatives. C. R. Chim. 2013, 16, 665–671. [Google Scholar] [CrossRef]
- Zarafu, I.; Olar, R.; Chifiriuc, M.C.; Bleotu, C.; Ionita, P.; Multescu, M.; Ionita, G.; Gradisteanu, G.; Tatibouet, A.; Badea, M. Synthesis, thermal, spectral, antimicrobial and cytotoxicity profile of the Schiff bases bearing pyrazolone moiety and their Cu(II) complexes. J. Therm. Anal. Calorim. 2018, 134, 1851–1861. [Google Scholar] [CrossRef]
- Sudhamani, H.; Syam Prasad, G.; Venkataramaiah, C.; Raju, C.N.; Rajendra, W. In silico and in vitro antioxidant activity profiles of urea and thiourea derivatives of 5-hydroxytryptophan. J. Recept. Signal. Transduct. Res. 2019, 39, 373–381. [Google Scholar] [CrossRef]
- Huong, D.Q.; Bay, M.V.; Nam, P.C. Antioxidant activity of thiourea derivatives: An experimental and theoretical study. J. Mol. Liq. 2021, 340, 117149. [Google Scholar] [CrossRef]
- Armour, A.D.; Powell, H.M.; Boyce, S.T. Fluorescein Diacetate for Determination of Cell Viability in Tissue-Engineered Skin. Tissue Eng. Part C Methods 2008, 14, 89–96. [Google Scholar] [CrossRef]
- Nakanishi, M.; Shimada, M.; Niida, H. Genetic instability in cancer cells by impaired cell cycle checkpoints. Cancer Sci. 2006, 97, 984–989. [Google Scholar] [CrossRef]
- Limban, C.; Missir, A.V.; Chiriţă, I.C.; Guţă, R.; Nănău-Andreescu, D.; Niţulescu, G.M.; Drăghici, C.; Căproiu, M.T.; Delcaru, C.; Chifiriuc, M.C. Synthesis, structural characterization and microbiological assays of some new 2-methoxy-O-acyl-oximino-dibenz[b,e]oxepins. Rev. Roum. Chim. 2010, 55, 313–319. [Google Scholar] [CrossRef]
- Bele, A.A.; Khale, A. An overview on the Thin Layer Chromatography. IJPSR 2011, 2, 256–267. [Google Scholar]
- Sadapha, P.; Dhamak, K. Review Article on High-Performance Liquid Chromatography (HPLC) Method Development and Validation. Int. J. Pharm. Sci. Rev. Res. 2022, 74, 23–29. [Google Scholar] [CrossRef]
- International Conference on Harmonization. ICH Q2 (R1): Validation of Analytical Procedures: Text and Methodology, ICH Secretariat, Geneva, 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 22 February 2023).
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 11.1, 2.2.46. Chromatographic Separation Techniques. Available online: https://pheur.edqm.eu/app/11-1/content (accessed on 22 February 2023).
- Kowalska, M.; Woźniak, M.; Kijek, M.; Mitrosz, P.; Szakiel, J.; Turek, P. Management of validation of HPLC method for determination of acetylsalicylic acid impurities in a new pharmaceutical product. Sci. Rep. 2022, 12, 1. [Google Scholar] [CrossRef]
- ICH Guideline Q2(R2) on Validation of Analytical Procedures, EMA/CHMP/ICH/82072/2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 22 February 2023).





| Strain/ Compound | Staphylococcus aureus ATCC 25923 | Enterococcus faecalis ATCC 29212 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 |
|---|---|---|---|---|
| 1a | 1250 | 1250 | 1250 | 2500 |
| 1b | 1250 | 1250 | 1250 | 2500 |
| 1c | 1250 | 1250 | 1250 | 1250 |
| 1d | 2500 | 1250 | 1250 | 2500 |
| 1e | 2500 | 1250 | 1250 | 1250 |
| 1f | 2500 | 1250 | 1250 | 2500 |
| 1g | 2500 | 1250 | 1250 | >5000 |
| Ciprofloxacin | 0.15 | 0.62 | 0.012 | 0.15 |
| DMSO | 12.5% | 12.5% | 12.5% | 12.5% |
| Strain/ Compound | Staphylococcus aureus ATCC 25923 | Enterococcus faecalis ATCC 29212 | Escherichia coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 |
|---|---|---|---|---|
| 1a | 2500 | 2500 | 2500 | 2500 |
| 1b | 2500 | 1250 | 625 | 1250 |
| 1c | 1250 | 1250 | 1250 | 1250 |
| 1d | 1250 | 1250 | 625 | 1250 |
| 1e | >5000 | >5000 | 5000 | 1250 |
| 1f | >5000 | 2500 | 1250 | 2500 |
| 1g | 2500 | 1250 | 1250 | 1250 |
| DMSO | 25% | 12.5% | 6.25% | 12.5% |
| Compound | 1a | 1b | 1c | 1d | 1e | 1f | 1g |
|---|---|---|---|---|---|---|---|
| TAC (%) | 14.39 | 12.73 | 10.50 | 43.17 | 10.17 | 24.81 | 15.13 |
| STDEV | SLOPE | LOD | LOQ | ||
|---|---|---|---|---|---|
| 490.324 | 930,686.3 | µg/mL | % | µg/mL | % |
| 0.0017 | 0.0057 | 0.053 | 0.0177 | ||
| Solution No. | Compound 1d Peak Area | ||
|---|---|---|---|
| Concentration (µg/mL) | Sample I | Sample II | |
| 1. | 0.05 | 4072 | 3998 |
| 2. | 0.06 | 4604 | 4710 |
| 3. | 0.08 | 6406 | 6552 |
| 4. | 0.10 | 7545 | 7346 |
| 5. | 0.20 | 17,849 | 17,837 |
| 6. | 20.00 | 117,640 | 116,577 |
| 7. | 30.00 | 173,649 | 173,564 |
| 8. | 40.00 | 3,274,981 | 3,276,413 |
| Sample No. | Compound 1d Results—20 µg/mL Solution | |||||
|---|---|---|---|---|---|---|
| Precision 1 Results (1) | Precision 2 Results (2) | Inter-Day Precision (3) | ||||
| Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Area of the Peak | |
| 1. | 5.668 | 1,683,470 | 5.587 | 1,691,833 | ||
| 2. | 5.668 | 1,671,584 | 5.606 | 1,690,608 | ||
| 3. | 5.668 | 1,692,685 | 5.602 | 1,736,221 | ||
| 4. | 5.668 | 1,676,085 | 5.598 | 1,697,748 | ||
| 5. | 5.668 | 1,674,530 | 5.582 | 1,704,577 | ||
| 6. | 5.668 | 1,675,112 | 5.601 | 1,697,552 | ||
| Average | 5.668 | 167,8911 | 5.596 | 1,703,090 | 5.632 | 1,691,000 |
| σ (4) | 0.00 | 7820.54 | 0.01 | 16,980.00 | 0.04 | 17,840.85 |
| RSD (5), % | 0.00 | 0.47 | 0.17 | 1.00 | 0.68 | 1.06 |
| Sample No. | Compound 1d Results—30 µg/mL Solution | |||||
|---|---|---|---|---|---|---|
| Precision 1 Results (1) | Precision 2 Results (2) | Inter-Day Precision (3) | ||||
| Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Area of the Peak | |
| 1. | 5.670 | 2,518,986 | 5.596 | 2,614,065 | ||
| 2. | 5.668 | 2,510,959 | 5.600 | 2,594,510 | ||
| 3. | 5.664 | 2,537,030 | 5.611 | 2,607,145 | ||
| 4. | 5.665 | 2,510,454 | 5.610 | 2,564,753 | ||
| 5. | 5.665 | 2,518,396 | 5.605 | 2,603,224 | ||
| 6. | 5.664 | 2,514,887 | 5.717 | 2,617,823 | ||
| Average | 5.666 | 2,518,452 | 5.623 | 2,600,253 | 5.645 | 2,559,353 |
| σ (4) | 0.00 | 9780.11 | 0.05 | 19,230.37 | 0.04 | 45,127.76 |
| RSD (5), % | 0.04 | 0.39 | 0.82 | 0.74 | 0.68 | 1.76 |
| Sample No. | Compound 1d Results—40 µg/mL Solution | |||||
|---|---|---|---|---|---|---|
| Precision 1 Results (1) | Precision 2 Results (2) | Inter-Day Precision (3) | ||||
| Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Compound 1d, Area of the Peak | Retention Time [min] | Area of the Peak | |
| 1. | 5.639 | 3,274,981 | 5.602 | 3,259,885 | ||
| 2. | 5.629 | 3,276,413 | 5.594 | 3,293,681 | ||
| 3. | 5.636 | 3,288,268 | 5.590 | 3,297,946 | ||
| 4. | 5.628 | 3,281,385 | 5.603 | 3,291,424 | ||
| 5. | 5.620 | 3,287,538 | 5.591 | 3,297,122 | ||
| 6. | 5.622 | 3,288,522 | 5.598 | 3,328,312 | ||
| Average | 5.629 | 3,282,851 | 5.596 | 3,294,728 | 5.613 | 3,288,790 |
| σ (4) | 0.01 | 6148.27 | 0.01 | 21,771.63 | 0.02 | 16,465.46 |
| RSD (5), % | 0.13 | 0.19 | 0.10 | 0.66 | 0.32 | 0.50 |
| Sample No. | Compound 1d Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 µg/mL Solution | 30 µg/mL Solution | 40 µg/mL Solution | |||||||
| Real (µg/mL) | Determined (µg/mL) | Recovery (%) | Real (µg/mL) | Determined (µg/mL) | Recovery % | Real (µg/mL) | Determined (µg/mL) | Recovery (%) | |
| 1. | 20.0800 | 20.2859 | 101.03 | 30.2000 | 30.3540 | 100.51 | 39.8000 | 39.4638 | 99.16 |
| 2. | 20.0800 | 20.1427 | 100.31 | 30.2000 | 30.2572 | 100.19 | 39.8000 | 39.4810 | 99.20 |
| 3. | 20.0800 | 20.3970 | 101.58 | 30.2000 | 30.5714 | 101.23 | 39.8000 | 39.6239 | 99.56 |
| 4. | 20.0800 | 20.1970 | 100.58 | 30.2000 | 30.2512 | 100.17 | 39.8000 | 39.5409 | 99.35 |
| 5. | 20.0800 | 20.1782 | 100.49 | 30.2000 | 30.3469 | 100.49 | 39.8000 | 39.6151 | 99.54 |
| 6. | 20.0800 | 20.1852 | 100.52 | 30.2000 | 30.3046 | 100.35 | 39.8000 | 39.6269 | 99.57 |
| Average: | 100.21 | ||||||||
| Standard Deviation: | 0.70 | ||||||||
| Relative standard deviation (RSD%): | 0.70 | ||||||||
| Confidence interval (Probability 95%): | 99.89–100.53 | ||||||||
| Sample No. | Compound 1d Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 µg/mL Solution | 30 µg/mL Solution | 40 µg/mL Solution | |||||||
| Real (µg/mL) | Determined (µg/mL) | Recovery (%) | Real (µg/mL) | Determined (µg/mL) | Recovery % | Real (µg/mL) | Determined (µg/mL) | Recovery (%) | |
| 1. | 20.5000 | 20.3394 | 99.22 | 31.0000 | 31.4265 | 101.3758 | 39.9000 | 39.1906 | 98.2220 |
| 2. | 20.5000 | 20.3246 | 99.14 | 31.0000 | 31.1914 | 100.6174 | 39.9000 | 39.5969 | 99.2403 |
| 3. | 20.5000 | 20.8730 | 101.82 | 31.0000 | 31.3433 | 101.1074 | 39.9000 | 39.6482 | 99.3688 |
| 4. | 20.5000 | 20.4105 | 99.56 | 31.0000 | 30.8337 | 99.4634 | 39.9000 | 39.5698 | 99.1723 |
| 5. | 20.5000 | 20.4926 | 99.96 | 31.0000 | 31.2962 | 100.9554 | 39.9000 | 39.6383 | 99.3440 |
| 6. | 20.5000 | 20.4081 | 99.55 | 31.0000 | 31.4717 | 101.5215 | 39.9000 | 40.0132 | 100.2838 |
| Average: | 100.00 | ||||||||
| Standard Deviation: | 1.01 | ||||||||
| Relative standard deviation (RSD%): | 1.01 | ||||||||
| Confidence interval (Probability 95%): | 99.53–100.47 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, R.; Pintilie, L.; Căproiu, M.T.; Dumitrașcu, F.; Nuță, D.C.; Zarafu, I.; Ioniță, P.; Chifiriuc, M.C.; Chiriță, C.; Moroșan, A.; et al. New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities. Antibiotics 2023, 12, 807. https://doi.org/10.3390/antibiotics12050807
Roman R, Pintilie L, Căproiu MT, Dumitrașcu F, Nuță DC, Zarafu I, Ioniță P, Chifiriuc MC, Chiriță C, Moroșan A, et al. New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities. Antibiotics. 2023; 12(5):807. https://doi.org/10.3390/antibiotics12050807
Chicago/Turabian StyleRoman, Roxana, Lucia Pintilie, Miron Teodor Căproiu, Florea Dumitrașcu, Diana Camelia Nuță, Irina Zarafu, Petre Ioniță, Mariana Carmen Chifiriuc, Cornel Chiriță, Alina Moroșan, and et al. 2023. "New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities" Antibiotics 12, no. 5: 807. https://doi.org/10.3390/antibiotics12050807
APA StyleRoman, R., Pintilie, L., Căproiu, M. T., Dumitrașcu, F., Nuță, D. C., Zarafu, I., Ioniță, P., Chifiriuc, M. C., Chiriță, C., Moroșan, A., Popa, M., Bleotu, C., & Limban, C. (2023). New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities. Antibiotics, 12(5), 807. https://doi.org/10.3390/antibiotics12050807










