Decision Challenges for Managing Acute Paediatric Infections: Implications for Antimicrobial Resistance
Abstract
:1. Introduction
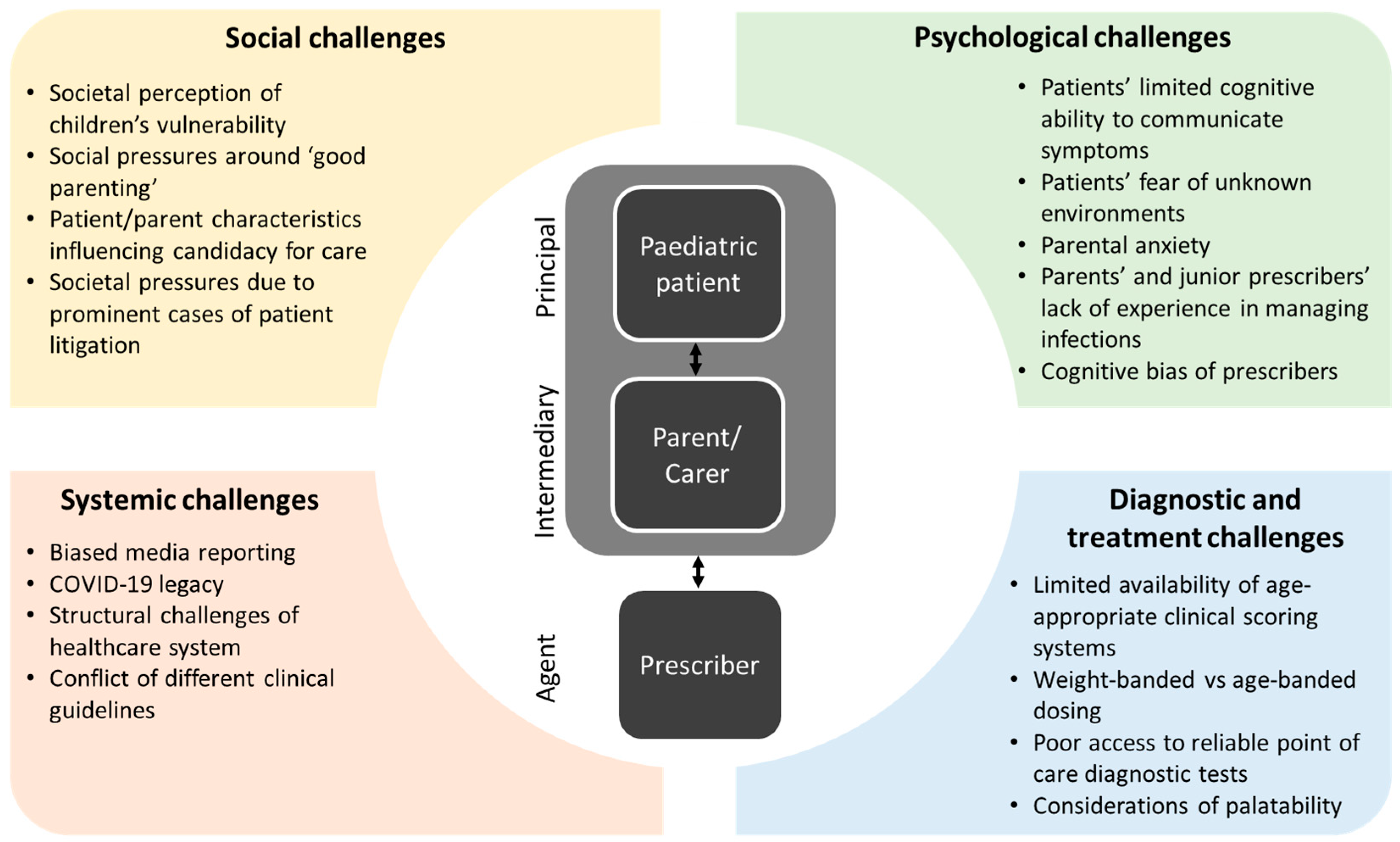
2. Decision Challenges
2.1. The Paediatric Patient (Principal)
2.2. The Parent/Carer (Intermediary)
2.3. The Prescriber (Agent)
3. Addressing Decision Challenges for Managing Infections in Paediatric Care
3.1. Integrate and Educate
3.2. Decision Support
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Rev. Antimicrob. Resist. 2016, 178, 590. [Google Scholar]
- Gerber, J.S.; Prasad, P.A.; Fiks, A.G.; Localio, A.R.; Grundmeier, R.W.; Bell, L.M.; Wasserman, R.C.; Keren, R.; Zaoutis, T.E. Effect of an Outpatient Antimicrobial Stewardship Intervention on Broad-Spectrum Antibiotic Prescribing by Primary Care Pediatricians: A Randomized Trial. JAMA 2013, 309, 2345–2352. [Google Scholar] [CrossRef]
- McMullan, B.J.; Hall, L.; James, R.; Mostaghim, M.; Jones, C.A.; Konecny, P.; Blyth, C.C.; Thursky, K.A. Antibiotic appropriateness and guideline adherence in hospitalized children: Results of a nationwide study. J. Antimicrob. Chemother. 2019, 75, 738–746. [Google Scholar] [CrossRef]
- Williams, M.R.; Greene, G.; Naik, G.; Hughes, K.; Butler, C.C.; Hay, A.D. Antibiotic prescribing quality for children in primary care: An observational study. Br. J. Gen. Pract. 2018, 68, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, C.; Krockow, E.M. Antibiotic overuse: Managing uncertainty and mitigating against overtreatment. BMJ Qual. Amp. Saf. 2022, 31, 163–167. [Google Scholar]
- Krockow, E.M.; Colman, A.M.; Chattoe-Brown, E.; Jenkins, D.R.; Perera, N.; Mehtar, S.; Tarrant, C. Balancing the risks to individual and society: A systematic review and synthesis of qualitative research on antibiotic prescribing behaviour in hospitals. J. Hosp. Infect. 2019, 101, 428–439. [Google Scholar] [CrossRef]
- Tarrant, C.; Colman, A.; Chattoe-Brown, E.; Jenkins, D.; Mehtar, S.; Perera, N.; Krockow, E. Optimizing antibiotic prescribing: Collective approaches to managing a common-pool resource. Clin. Microbiol. Infect. 2019, 25, 1356–1363. [Google Scholar] [CrossRef]
- Blomqvist, Å. The doctor as double agent: Information asymmetry, health insurance, and medical care. J. Health Econ. 1991, 10, 411–432. [Google Scholar] [CrossRef]
- Good Medical Practice. 2019. Available online: https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/good-medical-practice (accessed on 1 March 2023).
- Cabral, C.; Lucas, P.J.; Ingram, J.; Hay, A.D.; Horwood, J. “It's safer to …” parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: An analysis across four qualitative studies. Soc. Sci. Med. 2015, 136, 156–164. [Google Scholar] [CrossRef]
- Brennan, L.; Heal, C.; Brown, S.; Roland, D.; Rowland, A.G. Time to change the reference ranges of children’s physiological observations in emergency care? A prospective study. J. Paediatr. Child Health 2022, 59, 480–486. [Google Scholar] [CrossRef]
- Neill, S.J.; Jones, C.H.; Lakhanpaul, M.; Roland, D.T.; Thompson, M.J. Parents’ help-seeking behaviours during acute childhood illness at home:A contribution to explanatory theory. J. Child Health Care 2016, 20, 77–86. [Google Scholar] [CrossRef]
- Roland, D. Paediatric early warning scores: Holy Grail and Achilles’ heel. Arch. Dis. Child. Educ. Amp Pract. Ed. 2012, 97, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Albut, A.; Roland, D.; Lawton, R.; Connor, M.; O’Hara, J. Capturing parents’ perspectives of child wellness to support identification of acutely unwell children in the Emergency Department. J. Patient Saf. 2022, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Carter, R.; Jones, R.; Roland, D.; Bayes, N.; Tavaré, A.; Hughes, J.; Turner, T.; Chynoweth, J.; Tan, C.; et al. Caring for a sick or injured child during the COVID-19 pandemic lockdown in 2020 in the UK: An online survey of parents’ experiences. Health Expect. 2021, 24, 2036–2046. [Google Scholar] [CrossRef]
- Klever, P. Multigenerational Relationships and Nuclear Family Functioning. Am. J. Fam. Ther. 2015, 43, 339–351. [Google Scholar] [CrossRef]
- Roland, D.; Wolfe, I.; Klaber, R.E.; Watson, M. Final warning on the need for integrated care systems in acute paediatrics. Arch. Dis. Child. 2022, 107, e9. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; Cavers, D.; Agarwal, S.; Annandale, E.; Arthur, A.; Harvey, J.; Hsu, R.; Katbamna, S.; Olsen, R.; Smith, L.; et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med. Res. Methodol. 2006, 6, 35. [Google Scholar] [CrossRef]
- Dyer, C. Paediatrician found guilty of manslaughter after boy’s death from septic shock. BMJ 2015, 351, h5969. [Google Scholar] [CrossRef]
- Cohen, D. Back to blame: The Bawa-Garba case and the patient safety agenda. BMJ 2017, 359, j5534. [Google Scholar] [CrossRef]
- Roland, D.; Snelson, E. “So why didn’t you think this baby was ill?” Decision-making in acute paediatrics. Arch. Dis. Child. Educ. Pract. 2019, 104, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Tarrant, C.; Hamilton, V.; Kiernan, F.M.; Jenkins, D.; Krockow, E.M. Sepsis and antimicrobial stewardship: Two sides of the same coin. BMJ Qual. Saf. 2019, 28, 758–761. [Google Scholar] [CrossRef]
- Little, P.; Hobbs, F.D.R.; Moore, M.; Mant, D.; Williamson, I.; McNulty, C.; Cheng, Y.E.; Leydon, G.; McManus, R.; Kelly, J.; et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: Randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013, 347, f5806. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.A.; Barker, C.; Saxena, S.; Wong, I.C.K.; Long, P.; Sharland, M. Not too little, not too much: Problems of selecting oral antibiotic dose for children. BMJ 2015, 351, h5447. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Leonard, E.; Martin, J.M. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: A meta-analysis. Pediatrics 2010, 126, e557–e564. [Google Scholar] [CrossRef]
- Rose, M.A.; Laurenz, M.; Sprenger, R.; Imöhl, M.; Van der Linden, M. Nasopharyngeal Carriage in Children After the Introduction of Generalized Infant Pneumococcal Conjugate Vaccine Immunization in Germany. Front. Med. 2021, 8, 719481. [Google Scholar] [CrossRef] [PubMed]
- Berkley, J.A.; Munywoki, P.; Ngama, M.; Kazungu, S.; Abwao, J.; Bett, A.; Lassauniére, R.; Kresfelder, T.; Cane, P.A.; Venter, M.; et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA 2010, 303, 2051–2057. [Google Scholar] [CrossRef]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for children—Taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child 2012, 97, 293–297. [Google Scholar] [CrossRef]
- Rajan, S.; Roberts, K.J.; Guerra, L.; Pirsch, M.; Morrell, E. Integrating Health Education in Core Curriculum Classrooms: Successes, Challenges, and Implications for Urban Middle Schools. J. Sch. Health 2017, 87, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Together, H. Healthier Together—A Community Initiative. Available online: https://www.what0-18.nhs.uk/ (accessed on 6 March 2023).
- Ya, K.Z.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar]
- Horner, C.; Cunney, R.; Demirjian, A.; Doherty, C.; Green, H.; Mathai, M.; McMaster, P.; Munro, A.; Paulus, S.; Roland, D.; et al. Paediatric Common Infections Pathways: Improving antimicrobial stewardship and promoting ambulation for children presenting with common infections to hospitals in the UK and Ireland. JAC-Antimicrob. Resist. 2021, 3, dlab029. [Google Scholar] [CrossRef] [PubMed]
- Akhloufi, H.; Van der Sijs, H.; Melles, D.C.; Van der Hoeven, C.P.; Vogel, M.; Mouton, J.W.; Verbon, A. The development and implementation of a guideline-based clinical decision support system to improve empirical antibiotic prescribing. BMC Med. Inform. Decis. Mak. 2022, 22, 127. [Google Scholar] [CrossRef] [PubMed]
| Principal (Paediatric Patient) | Intermediary (Parent/Carer) | Agent (Prescriber) | |
|---|---|---|---|
| Social challenges | Delivering integrated care through greater engagement with vulnerable and disadvantaged groups | Delivering integrated care through greater engagement with vulnerable and disadvantaged groups | Organisational safety-netting to minimise individual prescriber risks for litigation |
| Psychological challenges | Putting “health” on the core curriculum at school | Providing evidence-based suite of resources across a range of languages | Providing targeted decision tools (e.g., treatment algorithms) |
| Systemic challenges | Re-shaping public debates about litigation | Improved testing of national guidance to ensure specificity and sensitivity and minimise unintended consequences | |
| Diagnostic and treatment challenges | Validating diagnostic tools and evidence-based guidelines for paediatrics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krockow, E.M.; Patel, S.; Roland, D. Decision Challenges for Managing Acute Paediatric Infections: Implications for Antimicrobial Resistance. Antibiotics 2023, 12, 828. https://doi.org/10.3390/antibiotics12050828
Krockow EM, Patel S, Roland D. Decision Challenges for Managing Acute Paediatric Infections: Implications for Antimicrobial Resistance. Antibiotics. 2023; 12(5):828. https://doi.org/10.3390/antibiotics12050828
Chicago/Turabian StyleKrockow, Eva M., Sanjay Patel, and Damian Roland. 2023. "Decision Challenges for Managing Acute Paediatric Infections: Implications for Antimicrobial Resistance" Antibiotics 12, no. 5: 828. https://doi.org/10.3390/antibiotics12050828
APA StyleKrockow, E. M., Patel, S., & Roland, D. (2023). Decision Challenges for Managing Acute Paediatric Infections: Implications for Antimicrobial Resistance. Antibiotics, 12(5), 828. https://doi.org/10.3390/antibiotics12050828






