RETRACTED: The Discovery of Novel Antimicrobial Agents Through the Application of Isocyanide-Based Multicomponent Reactions
Abstract
1. Introduction
2. Isocyanides and the Types of Isocyanide-Based Multicomponent Reactions
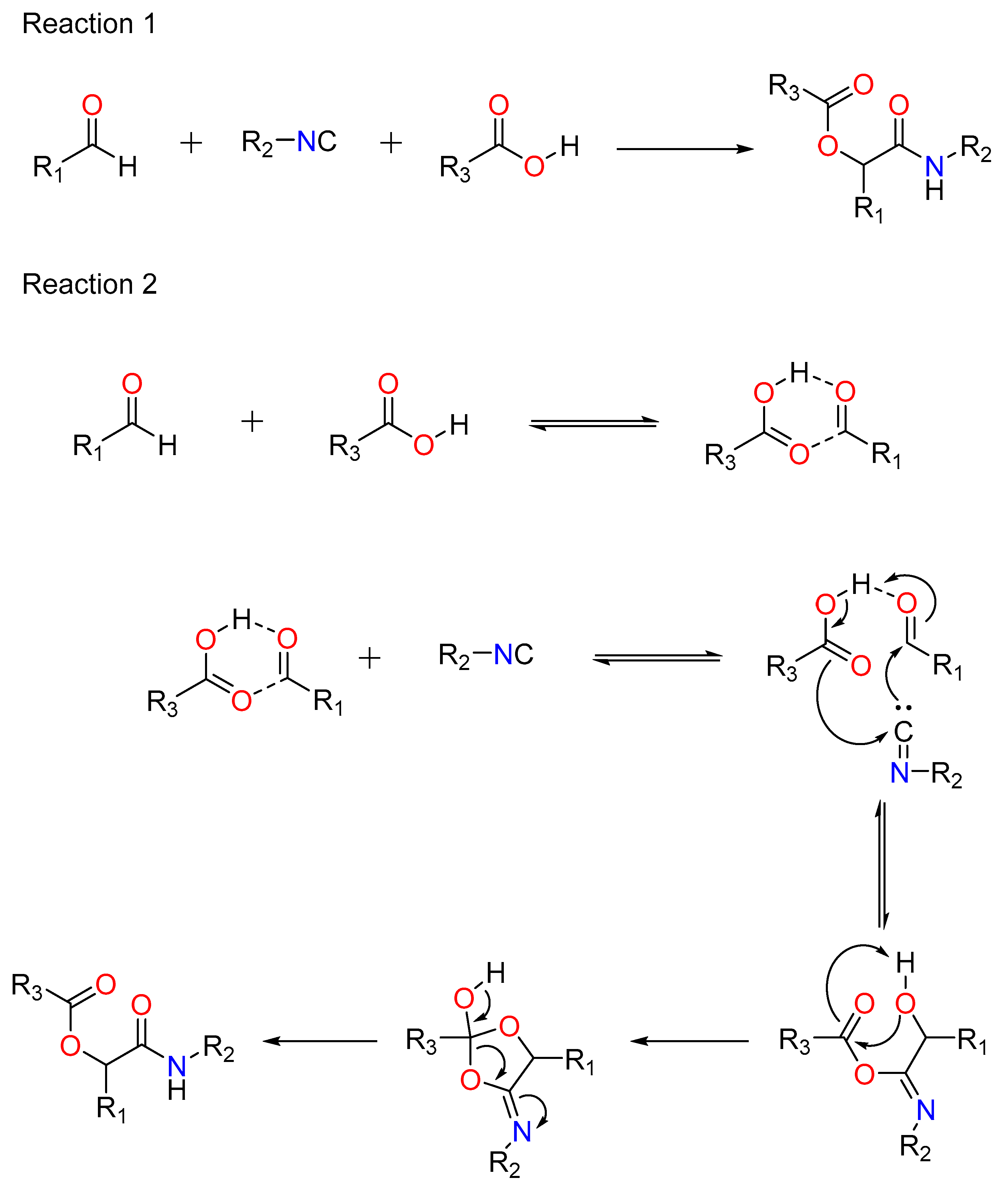
2.1. The Reactions of Passerini
2.2. Ugi-4C Reaction
2.3. Groebke–Blackburn–Bienaymé Reaction
3. Groebke–Blackburn–Bienaymé Reaction in the Discovery of the Modified Antibiotic Trimethoprim
4. The Discovery of Antimicrobial Compounds against Infectious Diseases through the Application of Ugi’s Reaction
5. Advancements in HIV and Cancer Treatment Due to the Application of IMCRs
6. G-Protein-Coupled Receptors (GPCRs) Antagonist by IMCRs for the Treatment of Distinct Diseases
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarganes-Tzitzikas, T.; Chandgude, A.L.; Dömling, A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 2015, 15, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Afshari, R.; Shaabani, A. Materials functionalization with multicomponent reactions: State of the art. ACS Comb. Sci. 2018, 20, 499–528. [Google Scholar] [CrossRef] [PubMed]
- Kruljec, N.; Bratkovič, T. Alternative affinity ligands for immunoglobulins. Bioconjug. Chem. 2017, 28, 2009–2030. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, W.; Sun, T.; Liu, S.; Zhu, Y.; Xie, Z. Rational design of polymeric nanoparticles with tailorable biomedical functions for cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 29612–29622. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Correia, B.E.; Niphakis, M.J.; Cravatt, B.F. Mapping the protein interaction landscape for fully functionalized small-molecule probes in human cells. J. Am. Chem. Soc. 2014, 136, 10777–10782. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.C.; Aguilar-Granda, A.; Zamudio-Medina, A.; Miranda, L.D.; Rodríguez-Molina, B. Synthesis of structurally diverse emissive molecular rotors with four-component ugi stators. J. Org. Chem. 2018, 83, 2570–2581. [Google Scholar] [CrossRef]
- Boukis, A.C.; Reiter, K.; Frölich, M.; Hofheinz, D.; Meier, M.A.R. Multicomponent reactions provide key molecules for secret communication. Nat. Commun. 2018, 9, 1439. [Google Scholar] [CrossRef]
- Domling, A. Recent advances in isocyanide-based multicomponent chemistry. ChemInform 2003, 34. [Google Scholar] [CrossRef]
- Gordon, E.M.; Barrett, R.W.; Dower, W.J.; Fodor, S.P.A.; Gallop, M.A. Applications of combinatorial technologies to drug discovery. 2. combinatorial organic synthesis, library screening strategies, and future directions. J. Med. Chem. 1994, 37, 1385–1401. [Google Scholar] [CrossRef]
- Golisade, A.; Wiesner, J.; Herforth, C.; Joma, H.; Link, A. Anti-Malarial activity of N6-Substituted adenosine derivatives. Part I Med. Bioorg. Med. Chem. 2002, 10, 769–777. [Google Scholar] [CrossRef]
- Teague, S.J.; Davis, A.M.; Leeson, P.D.; Oprea, T. The design of leadlike combinatorial libraries. Angew. Chem. Int. Ed. Engl. 1999, 38, 3743–3748. [Google Scholar] [CrossRef]
- Obert, R.; Armstrong, W.; Combs, A.P.; Tempest, P.A.; Brown, S.D. Multiple-component condensation strategies for combinatorial library synthesis. Acc. Chem. Res. 1996, 29, 123–131. [Google Scholar]
- Orru, R.V.A.; de Greef, M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. ChemInform 2003, 34. [Google Scholar] [CrossRef]
- Li, J.J. Strecker amino acid synthesis. In Name Reactions; Springer: Berlin/Heidelberg, Germany, 2003; pp. 399–400. [Google Scholar]
- Hantzsch, A. Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak. Justus Liebigs Ann. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Biginelli, P. Ueber aldehyduramide des acetessigäthers. II. Ber. Dtsch. Chem. Ges. 1891, 24, 2962–2967. [Google Scholar] [CrossRef]
- Biginelli, P. Aldehydureidderivate des acet-und oxalessigäthers. Ber. Dtsch. Chem. Ges. 1893, 26, 447–450. [Google Scholar]
- Kappe, C.O. 100 Tetrahedron 1993, 49, 6937. (b) kappe, co. Acc. Chem. Res. 2000, 33, 879. [Google Scholar] [CrossRef]
- Kappe, C.O. A reexamination of the mechanism of the biginelli dihydropyrimidine synthesis. Support for an N-Acyliminium ion intermediate1. J. Org. Chem. 1997, 62, 7201–7204. [Google Scholar] [CrossRef]
- Sotiri, K.; Hilgert, S.; Mannich, M.; Bleninger, T.; Fuchs, S. Implementation of comparative detection approaches for the accurate assessment of sediment thickness and sediment volume in the passaúna reservoir. J. Environ. Manag. 2021, 287, 112298. [Google Scholar] [CrossRef]
- Ugi, I. Angewandte Chemie-International Edition; Wiley-VCH Verlag GmbH: Berlin, Germany, 1959. [Google Scholar]
- Ugi, I.; Steinbrückner, C. Über ein neues kondensations-prinzip. Angew. Chem. Weinh. Bergstr. Ger. 1960, 72, 267–268. [Google Scholar] [CrossRef]
- Ugi, I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Gokel, G.; Lüdke, G.; Ugi, I. Isonitrile Chemistry; Ugi, I., Ed.; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Passerini, M. Composto del p-isontril-azobenzolo con acetone ed acido acetico. Gazz. Chim. Ital. 1921, 51, 126–129. [Google Scholar]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef]
- Kakuchi, R. The dawn of polymer chemistry based on multicomponent reactions. Polym. J. 2019, 51, 945–953. [Google Scholar] [CrossRef]
- Quazi, S.; Gavas, S.; Malik, J.A.; Suman, K.S.; Haider, Z. In-silico pharmacophore and molecular docking based drug discovery against marburg virus’s viral protein 35; A potent of MAVD. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Lieke, W. Ueber das cyanallyl. Justus Liebigs Ann. Chem. 1859, 316–321. [Google Scholar] [CrossRef]
- Gautier, A. Ueber die einwirkung des chlorwasserstoffs u. a. Auf das aethyl- und methylcyanür. Ann. Chem. Pharm. 1867, 142, 289–294. [Google Scholar] [CrossRef]
- Ugi, I.; Dömling, A.; Gruber, B.; Almstetter, M. Multicomponent reactions and their libraries—A new approach to preparative organic chemistry. Croat. Chem. Acta 1997, 70, 631–647. [Google Scholar]
- Ugi, I. Neuere methoden der präparativen organischen chemie IV: Mit sekundär-reaktionen gekoppelte α-additionen von immonium-ionen und anionen an isonitrile. Angew. Chem. 1962, 74, 9–22. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit isonitrilen. Angew. Chem. 1959, 71, 386. [Google Scholar]
- Arshady, R.; Ugi, I. Solid phase peptide synthesis by four component condensation: Peptide formation on an isocyano polymer support. Z. Naturforsch. B J. Chem. Sci. 1981, 36, 1202–1203. [Google Scholar] [CrossRef]
- Oertel, K.; Zech, G.; Kunz, H. Stereoselective combinatorial ugi-multicomponent synthesis on solid phase this work was supported by the deutsche forschungsgemeinschaft and by the fonds der chemischen industrie. Angew. Chem. Int. Ed. Engl. 2000, 39, 1431–1433. [Google Scholar] [CrossRef]
- Bienaymé, H.; Bouzid, K. A new heterocyclic multicomponent reaction for the combinatorial synthesis of fused 3-aminoimidazoles. Angew. Chem. Int. Ed 1998, 37, 2234–2237. [Google Scholar] [CrossRef]
- Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Parallel synthesis of 3-aminoimidazo [1,2-a] pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 1998, 39, 3635–3638. [Google Scholar] [CrossRef]
- Groebke, K.; Weber, L.; Mehlin, F. ChemInform abstract: Synthesis of imidazo [1,2-a] annulated pyridines, pyrazines, and pyrimidines by a novel three-component condensation. ChemInform 2010, 29. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Isonitrile, V. Erweiterter anwendungsbereich der passerini-reaktion. Chem. Ber. 1961, 94, 2229–2233. [Google Scholar] [CrossRef]
- Wang, S.-X.; Wang, M.-X.; Wang, D.-X.; Zhu, J. Catalytic enantioselective passerini three-component reaction. Angew. Chem. Int. Ed. Engl. 2008, 47, 388–391. [Google Scholar] [CrossRef]
- Brioche, J.; Masson, G.; Zhu, J. Passerini three-component reaction of alcohols under catalytic aerobic oxidative conditions. Org. Lett. 2010, 12, 1432–1435. [Google Scholar] [CrossRef]
- Yanai, H.; Oguchi, T.; Taguchi, T. Direct alkylative passerini reaction of aldehydes, isocyanides, and free aliphatic alcohols catalyzed by indium (III) triflate. J. Org. Chem. 2009, 74, 3927–3929. [Google Scholar] [CrossRef]
- El Kaim, L.; Gizolme, M.; Grimaud, L. O-arylative passerini reactions. Org. Lett. 2006, 8, 5021–5023. [Google Scholar] [CrossRef]
- Soeta, T.; Kojima, Y.; Ukaji, Y.; Inomata, K. O-silylative passerini reaction: A new one-pot synthesis of α-siloxyamides. Org. Lett. 2010, 12, 4341–4343. [Google Scholar] [CrossRef] [PubMed]
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chemistry 2009, 15, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Paulvannan, K. Preparation of tricyclic nitrogen heterocycles via tandem four-component condensation/intramolecular diels-alder reaction. Tetrahedron Lett. 1999, 40, 1851–1854. [Google Scholar] [CrossRef]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V.A. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed]
- Bonnaterre, F.; Bois-Choussy, M.; Zhu, J. Rapid access to oxindoles by the combined use of an ugi four-component reaction and a microwave-assisted intramolecular buchwald−hartwig amidation reaction. Org. Lett. 2006, 8, 4351–4354. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef]
- Quazi, S.; Jangi, R. Artificial intelligence and machine learning in medicinal chemistry and validation of emerging drug targets. Adv. Control. Drug Deliv. Syst. 2021, 27–43. Available online: https://www.igi-global.com/chapter/artificial-intelligence-and-machine-learning-in-medicinal-chemistry-and-validation-of-emerging-drug-targets/300400 (accessed on 1 March 2023).
- Hulme, C.; Lee, Y.-S. Emerging approaches for the syntheses of bicyclic imidazo [1,2-x]-heterocycles. Mol. Divers. 2008, 12, 1–15. [Google Scholar] [CrossRef]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via groebke–blackburn–bienayme reaction: A review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Akritopoulou-Zanze, I.; Wakefield, B.D.; Gasiecki, A.; Kalvin, D.; Johnson, E.F.; Kovar, P.; Djuric, S.W. Scaffold oriented synthesis. Part 4: Design, synthesis and biological evaluation of novel 5-substituted indazoles as potent and selective kinase inhibitors employing heterocycle forming and multicomponent reactions. Bioorg. Med. Chem. Lett. 2011, 21, 1480–1483. [Google Scholar] [CrossRef]
- Baviskar, A.T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C.; Bharatam, P.V. N-fused imidazoles as novel anticancer agents that inhibit catalytic activity of topoisomerase IIα and induce apoptosis in G1/S phase. J. Med. Chem. 2011, 54, 5013–5030. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.M.; Salunke, D.B.; Yoo, E.; Mutz, C.A.; Balakrishna, R.; David, S.A. Antibacterial activities of groebke–blackburn–bienaymé-derived imidazo [1,2-a] pyridin-3-amines. Bioorg. Med. Chem. 2012, 20, 5850–5863. [Google Scholar] [CrossRef]
- Burchak, O.N.; Mugherli, L.; Ostuni, M.; Lacapère, J.J.; Balakirev, M.Y. Combinatorial discovery of fluorescent pharmacophores by multicomponent reactions in droplet arrays. J. Am. Chem. Soc. 2011, 133, 10058–10061. [Google Scholar] [CrossRef] [PubMed]
- Elleder, D.; Baiga, T.J.; Russell, R.L.; Naughton, J.A.; Hughes, S.H.; Noel, J.P.; Young, J.A.T. Identification of a 3-aminoimidazo [1,2-a] pyridine inhibitor of HIV-1 reverse transcriptase. Virol. J. 2012, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.L.; Gravestock, D.; Moleele, S.S.; van der Westhuyzen, C.W.; Pelly, S.C.; Steenkamp, P.A.; Hoppe, H.C.; Khan, T.; Nkabinde, L.A. Imidazo [1,2-a] pyridin-3-amines as potential HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2011, 19, 4227–4237. [Google Scholar] [CrossRef] [PubMed]
- Urbancic, K.F.; Ierino, F.; Phillips, E.; Mount, P.F.; Mahony, A.; Trubiano, J.A. Taking the challenge: A protocolized approach to optimize pneumocystis pneumonia prophylaxis in renal transplant recipients. Am. J. Transpl. 2018, 18, 462–466. [Google Scholar] [CrossRef]
- Heaslet, H.; Harris, M.; Fahnoe, K.; Sarver, R.; Putz, H.; Chang, J.; Subramanyam, C.; Barreiro, G.; Miller, J.R. Structural comparison of chromosomal and exogenous dihydrofolate reductase from staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins 2009, 76, 706–717. [Google Scholar] [CrossRef]
- Zhou, W.; Scocchera, E.W.; Wright, D.L.; Anderson, A.C. Antifolates as effective antimicrobial agents: New generations of trimethoprim analogs. Medchemcomm 2013, 4, 908. [Google Scholar] [CrossRef]
- Quazi, S.; Malik, J.; Suman, K.S.; Capuzzo, A.M.; Haider, Z. Discovery of potential drug-like compounds against viral protein (VP40) of marburg virus using pharmacophoric based virtual screening from ZINC database. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lombardo, M.N.; G-Dayanandan, N.; Wright, D.L.; Anderson, A.C. Crystal structures of trimethoprim-resistant DfrA1 rationalize potent inhibition by propargyl-linked antifolates. ACS Infect. Dis. 2016, 2, 149–156. [Google Scholar] [CrossRef]
- Rashid, U.; Ahmad, W.; Hassan, S.F.; Qureshi, N.A.; Niaz, B.; Muhammad, B.; Imdad, S.; Sajid, M. Design, synthesis, antibacterial activity and docking study of some new trimethoprim derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 5749–5753. [Google Scholar] [CrossRef] [PubMed]
- Pedrola, M.; Jorba, M.; Jardas, E.; Jardi, F.; Ghashghaei, O.; Viñas, M.; Lavilla, R. Multicomponent reactions upon the known drug trimethoprim as a source of novel antimicrobial agents. Front. Chem. 2019, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, O.; Caputo, S.; Sintes, M.; Revés, M.; Kielland, N.; Estarellas, C.; Luque, F.J.; Aviñó, A.; Eritja, R.; Serna-Gallego, A.; et al. Multiple multicomponent reactions: Unexplored substrates, selective processes, and versatile chemotypes in biomedicine. Chemistry 2018, 24, 14513–14521. [Google Scholar] [CrossRef] [PubMed]
- Dolce, D.; Neri, S.; Grisotto, L.; Campana, S.; Ravenni, N.; Miselli, F.; Camera, E.; Zavataro, L.; Braggion, C.; Fiscarelli, E.V.; et al. Methicillin-resistant staphylococcus aureus eradication in cystic fibrosis patients: A randomized multicenter study. PLoS ONE 2019, 14, e0213497. [Google Scholar] [CrossRef]
- Lo, D.K.; Muhlebach, M.S.; Smyth, A.R. Interventions for the eradication of meticillin-resistant staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst. Rev. 2018, 7, CD009650. [Google Scholar] [CrossRef]
- Xhemali, X.; Smith, J.R.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Compton, M.; Singh, N.B.; Jahanbakhsh, S.; Rybak, M.J. Evaluation of dalbavancin alone and in combination with β-lactam antibiotics against resistant phenotypes of staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 82–86. [Google Scholar] [CrossRef]
- Dömling, A.; Achatz, S.; Beck, B. Novel anti-tuberculosis agents from MCR libraries. Bioorg. Med. Chem. Lett. 2007, 17, 5483–5486. [Google Scholar] [CrossRef]
- Nutt, R.F.; Joullie, M.M. Four-component condensation: A new versatile method for the synthesis of substituted prolyl peptides. J. Am. Chem. Soc. 1982, 104, 5852–5853. [Google Scholar] [CrossRef]
- Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 324–331. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Bories, C.; Loiseau, P.M.; Torrence, P.F. The ugi reaction in the generation of new nucleosides as potential antiviral and antileishmanial agents. Bioorg. Chem. 2007, 35, 121–136. [Google Scholar] [CrossRef]
- Musonda, C.C.; Taylor, D.; Lehman, J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Application of multicomponent reactions to antimalarial drug discovery. Part 1. Parallel synthesis and antiplasmodial activity of new 4-aminoquinoline ugi adducts. Bioorg. Med. Chem. Lett. 2004, 14, 3901–3905. [Google Scholar] [CrossRef] [PubMed]
- Short, K.M.; Ching, B.W.; Mjalli, A.M.M. Exploitation of the ugi 4CC reaction: Preparation of small molecule combinatorial libraries via solid phase. Tetrahedron 1997, 53, 6653–6679. [Google Scholar] [CrossRef]
- Musonda, C.C.; Gut, J.; Rosenthal, P.J.; De Souza, C.; Chibale, R.C. Application of multicomponent reactions to antimalarial drug discovery. Part 2. New antiplasmodial and antitrypanosomal 4-aminoquinoline g-and d-lactams via a ‘catch and release’ protocol. Bioorg. Med. Chem. 2006, 14, 5605–5615. [Google Scholar] [CrossRef] [PubMed]
- Linderman, R.J.; Binet, S.; Petrich, S.R. Enhanced diastereoselectivity in the asymmetric ugi reaction using a new “convertible” isonitrile. J. Org. Chem. 1999, 64, 8058. [Google Scholar] [CrossRef]
- Cohen, E. Chitin biochemistry: Synthesis and inhibition. Annu. Rev. Entomol. 1987, 32, 71–93. [Google Scholar] [CrossRef]
- Plant, A.; Thompson, P.; Williams, D.M. Application of the ugi reaction for the one-pot synthesis of uracil polyoxin C analogues. J. Org. Chem. 2009, 74, 4870–4873. [Google Scholar] [CrossRef]
- Neves Filho, R.A.W.; Stark, S.; Westermann, B.; Wessjohann, L.A. The multicomponent approach to N-methyl peptides: Total synthesis of antibacterial (−)-viridic acid and analogues. Beilstein J. Org. Chem. 2012, 8, 2085–2090. [Google Scholar] [CrossRef]
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef]
- Quazi, S. Role of artificial intelligence and machine learning in bioinformatics: Drug discovery and drug repurposing. Preprints 2021, 2021050346. [Google Scholar] [CrossRef]
- Iarani, G.M.; Moradi, R.; Mahammadkhani, L. Application of multicomponent reactions in the total synthesis of natural peptides. Org. Chem. 2019, 18–40. [Google Scholar] [CrossRef]
- Okandeji, B.O.; Greenwald, D.M.; Wroten, J.; Sello, J.K. Synthesis and evaluation of inhibitors of bacterial drug efflux pumps of the major facilitator superfamily. Bioorg. Med. Chem. 2011, 19, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Sakagami, M.; Feng, F.; Togame, H.; Takemoto, H.; Ichikawa, S.; Matsuda, A. Total synthesis of pacidamycin D by Cu (I)-catalyzed oxy enamide formation. Org. Lett. 2011, 13, 5240–5243. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.; Goss, R.J.; Kimura, K.I.; Bugg, T.D. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure–function studies and nucleoside biosynthesis. Nat. Prod. Rep. 2010, 27, 279–304. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.B.; Swanson, R.N.; Hardy, D.J.; Hanson, C.W.; Coen, L.; Rasmussen, R.R.; Chen, R.H. Pacidamycins, a novel series of antibiotics with anti-pseudomonas aeruginosa activity. III. Microbiologic profile. J. Antibiot. 1989, 42, 521–526. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Rao, Y.; Ding, M.-W. One-pot synthesis of multisubstituted benzimidazoles via sequential ugi and catalytic aza-wittig reaction starting from 2-aminobenzoyl azides. J. Org. Chem. 2016, 81, 1263–1268. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Yancheva, D.; Anastassova, N.; Anichina, K.; Zvezdanovic, J.; Djordjevic, A.; Markovic, D.; Smelcerovic, A. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg. Med. Chem. 2015, 23, 6317–6326. [Google Scholar] [CrossRef]
- Pan, T.; He, X.; Chen, B.; Chen, H.; Geng, G.; Luo, H.; Zhang, H.; Bai, C. Development of benzimidazole derivatives to inhibit HIV-1 replication through protecting APOBEC3G protein. Eur. J. Med. Chem. 2015, 95, 500–513. [Google Scholar] [CrossRef]
- Vasantha, K.; Basavarajaswamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the ugi reaction. ACS Appl. Polym. Mater. 2019, 2, 404–410. [Google Scholar] [CrossRef]
- Reddy, T.S.; Kulhari, H.; Reddy, V.G.; Bansal, V.; Kamal, A.; Shukla, R. Design, synthesis and biological evaluation of 1, 3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur. J. Med. Chem. 2015, 101, 790–805. [Google Scholar] [CrossRef]
- Kesteleyn, B.R.; Schepens, W.B. Assignee. HIV Inhibiting; Inventors; Tibotec Pharmaceuticals Ltd.: Co Cork, Ireland, 2011; Volume 3. [Google Scholar]
- Habashita, H.; Kokubo, M.; Hamano, S.-I.; Hamanaka, N.; Toda, M.; Shibayama, S.; Tada, H.; Sagawa, K.; Fukushima, D.; Maeda, K.; et al. Design, synthesis, and biological evaluation of the combinatorial library with a new spirodiketopiperazine scaffold. discovery of novel potent and selective low-molecular-weight CCR5 antagonists. J. Med. Chem. 2006, 49, 4140–4152. [Google Scholar] [CrossRef]
- Hulme, C.; Morrissette, M.M.; Volz, F.A.; Burns, C.J. ChemInform abstract: The solution phase synthesis of diketopiperazine libraries via the ugi reaction: Novel application of armstrong’ s convertible isonitrile. ChemInform 2010, 29. [Google Scholar] [CrossRef]
- Nishizawa, R.; Nishiyama, T.; Hisaichi, K.; Matsunaga, N.; Minamoto, C.; Habashita, H.; Takaoka, Y.; Toda, M.; Shibayama, S.; Tada, H.; et al. Spirodiketopiperazine-based CCR5 antagonists: Lead optimization from biologically active metabolite. Bioorganic Med. Chem. Lett. 2007, 17, 727–731. [Google Scholar] [CrossRef]
- Maeda, K.; Nakata, H.; Koh, Y.; Miyakawa, T.; Ogata, H.; Takaoka, Y.; Shibayama, S.; Sagawa, K.; Fukushima, D.; Moravek, J.; et al. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 2004, 78, 8654–8662. [Google Scholar] [CrossRef]
- ACS Medical Content and News Staff. 2022 Cancer Facts & Figures Cancer|Cancer Death Rate Drops. Available online: https://www.cancer.org/latest-news/facts-and-figures-2022.html (accessed on 31 March 2022).
- Mullica, D.F.; Pinney, K.G.; Mocharla, V.P.; Dingeman, K.M.; Bounds, A.D.; Sappenfield, E.L. Characterization and structural analyses of trimethoxy and triethoxybenzo[b]thiophene. J. Chem. Crystallogr. 1998, 28, 289–295. [Google Scholar] [CrossRef]
- Flynn, B.L.; Hamel, E.; Jung, M.K. ChemInform abstract: One-pot synthesis of benzo[B]furan and indole inhibitors of tubulin polymerization. ChemInform 2010, 33. [Google Scholar] [CrossRef]
- Pulley, S.R. CCR5 antagonists: From discovery to clinical efficacy. In Chemokine Biology—Basic Research and Clinical Application; Birkhäuser Basel: Basel, Switzerland, 2007; pp. 145–163. [Google Scholar]
- Crabb, C. GlaxoSmithKline ends aplaviroc trials. AIDS 2006, 20, 641. [Google Scholar] [CrossRef]
- Szardenings, A.K.; Burkoth, T.S.; Lu, H.H.; Tien, D.W.; Campbell, D.A. A simple procedure for the solid phase synthesis of diketopiperazine and diketomorpholine derivatives. Tetrahedron 1997, 53, 6573–6593. [Google Scholar] [CrossRef]
- Wyatt, P.G.; Allen, M.J.; Borthwick, A.D.; Davies, D.E.; Exall, A.M.; Hatley, R.; Irving, W.R.; Livermore, D.G.; Miller, N.D.; Nerozzi, F. 5-diketopiperazines as potent and selective oxytocin antagonists. 1. Identifification, stereochemistry and initial SAR. Bioorg. Med. Chem. Lett. 2005, 15, 2579–2582. [Google Scholar] [CrossRef]
- Borthwick, A.D.; Davies, D.E.; Exall, A.M.; Livermore, D.G.; Sollis, S.L.; Nerozzi, F.; Allen, M.J.; Perren, M.; Shabbir, S.S.; Woollard, P.M. Wyatt PG: 2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists. 2. Synthesis, chirality and pharmacokinetics. J. Med. Chem. 2005, 48, 6956–6969. [Google Scholar] [CrossRef]
- Liddle, J.; Allen, M.J.; Borthwick, A.D.; Brooks, D.P.; Davies, D.E.; Edwards, R.M.; Exall, A.M.; Hamlett, C.; Irving, W.R.; Mason, A.M.; et al. The discovery of GSK221149A: A potent and selective oxytocin antagonist. Bioorg. Med. Chem. Lett. 2008, 18, 90–94. [Google Scholar] [CrossRef]
- Filosa, R.; Marinozzi, M.; Constantino, G.; Hermit, M.B.; Thomsen, C.; Pellicciari, R. Synthesis and biological evaluation of (2S)-and (2R)-2-(30-phosphonobicyclo [1.1.1] pentyl) glycines as novel group III selective metabotropic glutamate receptor ligands. Bioorg. Med. Chem. 2006, 14, 3811–3817. [Google Scholar] [CrossRef]
- Kühnert, S.; Zemolka, S.; Haurand, M.; Schiene, K. Substituted imidazo [2, 1-b] thiazole compounds and their use for producing drugs. PCT Int. Appl. 2020, 95, 103496. [Google Scholar] [CrossRef]











| Names of Researcher | Invention Year | Reaction | Reaction Litterateur |
|---|---|---|---|
| Strecker | 1850 |  | An aldehyde is condensed with ammonium chloride in the presence of potassium cyanide to generate an a-amino nitrile, which can be hydrolyzed to give the a-amino acid [13]. |
| Hantzsch | 1882 |  | An ammonia, two equivalents of ethyl acetoacetate and an aldehyde has developed a dihydropyridine from this synthesis [14]. |
| Biginelli | 1891 |  | Synthesis of an ethyl acetoacetate, urea, and an aryl aldehyde (benzaldehyde) has developed 3,4-dihydropyrimidin-2(1H) [15,16,17,18]. |
| Mannich | 1912 |  | An amino alkylation of an enol with an amine and an aldehyde is used in this reaction [19]. |
| Ugi | 1960 |  | A ketone or aldehyde, an amine, an isocyanide, and a carboxylic acid are used in this multicomponent process to generate a bis-amide [20,21,22,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quazi, S.; Rashid, M.T.; Malik, J.A.; Gavas, S. RETRACTED: The Discovery of Novel Antimicrobial Agents Through the Application of Isocyanide-Based Multicomponent Reactions. Antibiotics 2023, 12, 849. https://doi.org/10.3390/antibiotics12050849
Quazi S, Rashid MT, Malik JA, Gavas S. RETRACTED: The Discovery of Novel Antimicrobial Agents Through the Application of Isocyanide-Based Multicomponent Reactions. Antibiotics. 2023; 12(5):849. https://doi.org/10.3390/antibiotics12050849
Chicago/Turabian StyleQuazi, Sameer, Maliha Tabassum Rashid, Javid Ahmad Malik, and Shreelaxmi Gavas. 2023. "RETRACTED: The Discovery of Novel Antimicrobial Agents Through the Application of Isocyanide-Based Multicomponent Reactions" Antibiotics 12, no. 5: 849. https://doi.org/10.3390/antibiotics12050849
APA StyleQuazi, S., Rashid, M. T., Malik, J. A., & Gavas, S. (2023). RETRACTED: The Discovery of Novel Antimicrobial Agents Through the Application of Isocyanide-Based Multicomponent Reactions. Antibiotics, 12(5), 849. https://doi.org/10.3390/antibiotics12050849






