Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity
Abstract
1. Introduction
1.1. Bacterial Kill Characteristics of the Beta-Lactams
1.2. The Current Debate on Beta-Lactam PK/PD Targets in Critically Ill Patients
1.3. Challenges among Critically Ill Patients
1.3.1. Pharmacokinetic (PK) Alterations in the ICU
1.3.2. Pharmacodynamic (PD) Challenges in the ICU
1.3.3. The Need for Beta-Lactam Dose Optimisation
2. Overview of Evidence for Usefulness of Beta-Lactam TDM in the ICU
3. Excessive Exposure and Evidence of What Constitutes ‘Beta-Lactam-Associated Toxicity’
3.1. Cytopenia
3.2. Neurotoxicity
3.3. Hepatoxicity
3.4. Nephrotoxicity
3.5. Other Adverse Drug Reactions
| Drug | Maximum Unbound Trough Concentration | Toxicity with Reported Associated with Supratherapeutic Concentration |
|---|---|---|
| Flucloxacillin | 20 mg/L | neurotoxicity [75] * |
| Cefepime | 20 mg/L | neurotoxicity [100] |
| Piperacillin | 130 mg/L | neurotoxicity, nephrotoxicity [74] |
| Meropenem | 44 mg/L | neurotoxicity, nephrotoxicity [74] |
4. The Ideal Service for Beta-Lactam TDM in the ICU
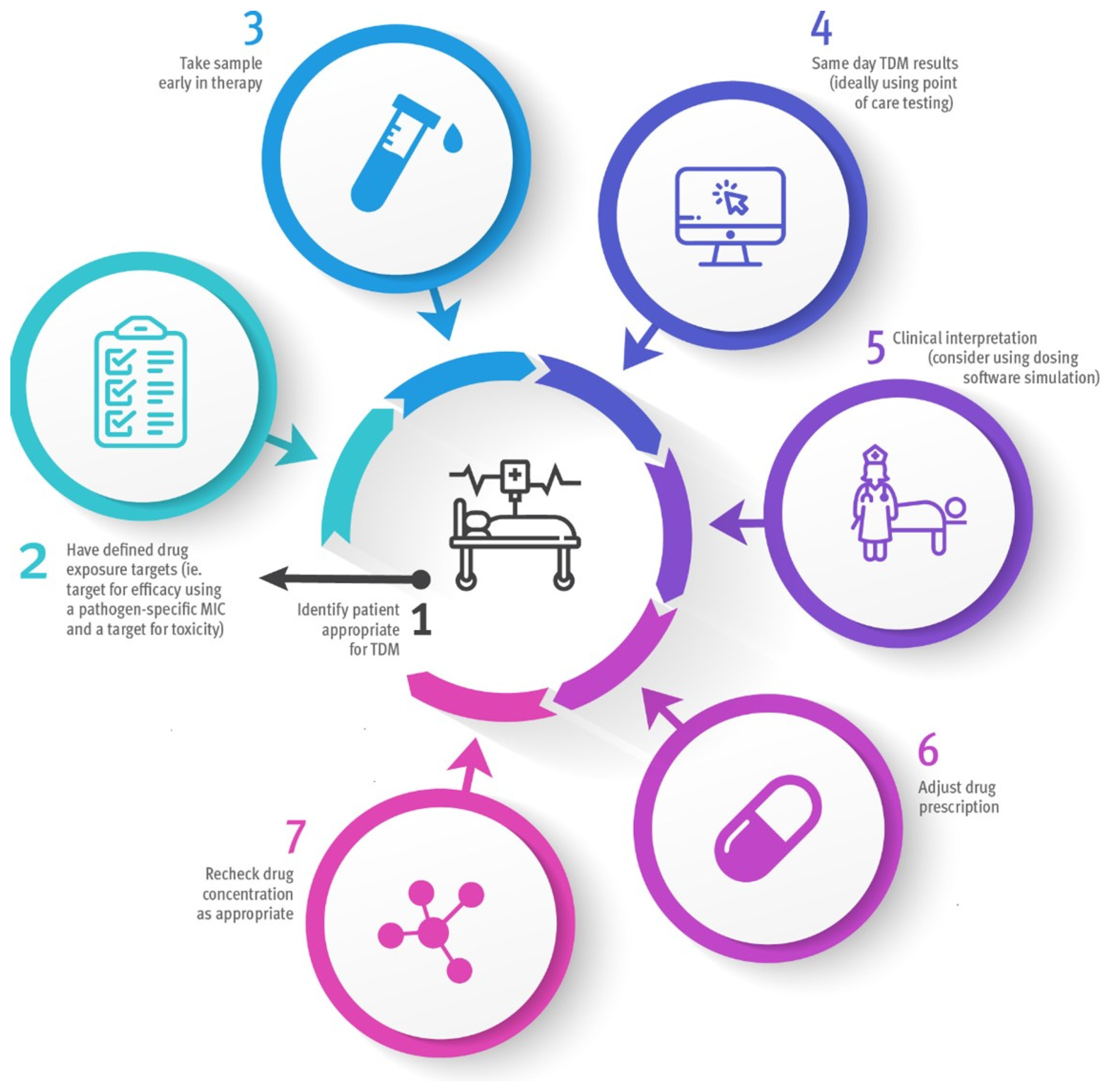
5. Areas for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eagle, H.; Fleischman, R.; Musselman, A.D. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am. J. Med. 1950, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H.; Fleischman, R.; Levy, M. “Continuous” vs. “discontinuous” therapy with penicillin; the effect of the interval between injections on therapeutic efficacy. N. Engl. J. Med. 1953, 248, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Ebert, S.C. Killing and regrowth of bacteria in vitro: A review. Scand. J. Infect. Dis. Suppl. 1990, 74, 63–70. [Google Scholar] [PubMed]
- Eagle, H. The kinetics of the bactericidal action of penicillin and the therapeutic significance of the blood penicillin level. J. Bacteriol. 1947, 54, 6. [Google Scholar] [PubMed]
- McDonald, P.J.; Craig, W.A.; Kunin, C.M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J. Infect. Dis. 1977, 135, 217–223. [Google Scholar] [CrossRef]
- Bundtzen, R.W.; Gerber, A.U.; Cohn, D.L.; Craig, W.A. Postantibiotic suppression of bacterial growth. Rev. Infect. Dis. 1981, 3, 28–37. [Google Scholar] [CrossRef]
- Eagle, H.; Fleischman, R.; Musselman, A.D. The effective concentrations of penicillin in vitro and in vivo for streptococci, pneumococci, and Treponema pallidum. J. Bacteriol. 1950, 59, 625–643. [Google Scholar] [CrossRef]
- Ebert, S.C.; Craig, W.A. Pharmacodynamic properties of antibiotics: Application to drug monitoring and dosage regimen design. Infect. Control Hosp. Epidemiol. 1990, 11, 319–326. [Google Scholar] [CrossRef]
- Eagle, H.; Musselman, A.D. The Slow Recovery of Bacteria from the Toxic Effects of Penicillin. J. Bacteriol. 1949, 58, 475–490. [Google Scholar] [CrossRef]
- Tam, V.H.; Chang, K.T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Bambeke, F.V.; Sanchez-Diaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining beta-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Craig, W.A. Does the dose matter? Clin. Infect. Dis. 2001, 33 (Suppl. 3), S233–S237. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Craig, W.A. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: Identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 2006, 50, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Keel, R.A.; Crandon, J.L.; Nicolau, D.P. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2011, 55, 4028–4032. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J.D. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 1998, 27, 10–22. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Andes, D.; Craig, W.A. Animal model pharmacokinetics and pharmacodynamics: A critical review. Int. J. Antimicrob. Agents 2002, 19, 261–268. [Google Scholar] [CrossRef]
- Tam, V.H.; McKinnon, P.S.; Akins, R.L.; Rybak, M.J.; Drusano, G.L. Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 2002, 50, 425–428. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Schilling, A.N.; Neshat, S.; Poole, K.; Melnick, D.A.; Coyle, E.A. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 4920–4927. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hafiz, A.A.; Staatz, C.E.; Kirkpatrick, C.M.; Lipman, J.; Roberts, J.A. Continuous infusion vs. bolus dosing: Implications for beta-lactam antibiotics. Minerva. Anestesiol. 2012, 78, 94–104. [Google Scholar] [PubMed]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.; Wallemacq, P.E.; Bambeke, F.V.; Tulkens, P.M. Optimizing beta-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. Anti-Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; Shen, C. Augmented Renal Clearance in Critical Illness: An Important Consideration in Drug Dosing. Pharmaceutics 2017, 9, 36. [Google Scholar] [CrossRef]
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol. Infect. Dis. 2014, 78, 443–448. [Google Scholar] [CrossRef]
- Valenza, G.; Seifert, H.; Decker-Burgard, S.; Laeuffer, J.; Morrissey, I.; Mutters, R. Comparative Activity of Carbapenem Testing (COMPACT) study in Germany. Int. J. Antimicrob. Agents 2012, 39, 255–258. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Schoenenberger-Arnaiz, J.A.; Ahmad-Diaz, F.; Miralbes-Torner, M.; Aragones-Eroles, A.; Cano-Marron, M.; Palomar-Martinez, M. Usefulness of therapeutic drug monitoring of piperacillin and meropenem in routine clinical practice: A prospective cohort study in critically ill patients. Eur. J. Hosp. Pharm. 2020, 27, e30–e35. [Google Scholar] [CrossRef]
- Roberts, J.A.; Ulldemolins, M.; Roberts, M.S.; McWhinney, B.; Ungerer, J.; Paterson, D.L.; Lipman, J. Therapeutic drug monitoring of beta-lactams in critically ill patients: Proof of concept. Int. J. Antimicrob. Agents 2010, 36, 332–339. [Google Scholar] [CrossRef]
- Nikolas, S.; Thorsten, R.; Max, K.; Patrick, M.; Markus, K.; Guzin, S.; Olivetr, S.-C.; Alexander, S.; Andreas, P.; Kerstin, H. Personalized Antibiotic Therapy for the Critically Ill: Implementation Strategies and Effects on Clinical Outcome of Piperacillin Therapeutic Drug Monitoring-A Descriptive Retrospective Analysis. Antibiotics 2021, 10, 1452. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Pea, F.; Della Siega, P.; Cojutti, P.; Sartor, A.; Crapis, M.; Scarparo, C.; Basetti, M. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int. J. Antimicrob. Agents 2017, 49, 255–258. [Google Scholar] [CrossRef]
- Carrie, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early therapeutic monitoring of beta-lactams and associated therapy outcomes in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef]
- Roberts, J.A.; De Waele, J.J.; Dimopoulos, G.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; Rhodes, A.; Starr, T.; Wallis, S.C.; et al. DALI: Defining Antibiotic Levels in Intensive care unit patients: A multi-centre point of prevalence study to determine whether contemporary antibiotic dosing for critically ill patients is therapeutic. BMC Infect. Dis. 2012, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Mulletr, A.E.; Van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.C.; Frey, O.; Rohr, A.; Roberts, J.A.; Koberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Jacobs, A.; Taccone, F.S.; Roberts, J.A.; Jacobs, F.; Cotton, F.; Wolff, F.; Creteur, J.; Vincent, J.; Hites, M. Beta-Lactam Dosage Regimens in Septic Patients with Augmented Renal Clearance. Antimicrob. Agents Chemother. 2018, 62, e02534-17. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Carrette, S.; Roberts, J.A.; Stove, V.; Verstraete, A.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; Lipman, J.; Wallis, S.; et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: Does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit. Care 2013, 17, R84. [Google Scholar] [CrossRef]
- Roberts, J.A.; Udy, A.A.; Jarrett, P.; Wallis, S.C.; Hope, W.W.; Sharma, R.; Kirkpatrick, C.M.J.; Kruger, P.; Roberts, M.S.; Lipman, J. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J. Antimicrob. Chemother. 2015, 70, 1495–1502. [Google Scholar] [CrossRef]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.J.; Kruger, P.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015, 19, 28. [Google Scholar] [CrossRef]
- Scharf, C.; Paal, M.; Schroeder, I.; Vogeser, M.; Draenert, R.; Irlbeck, M.; Zölletr, M.; Liebchen, U. Therapeutic Drug Monitoring of Meropenem and Piperacillin in Critical Illness-Experience and Recommendations from One Year in Routine Clinical Practice. Antibiotics 2020, 9, 131. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Day, R.; Gentili, S.; Jones, G.R.D.; Marriott, D.; Norris, R.; Sandaradura, I. An evaluation of risk factors to predict target concentration non-attainment in critically ill patients prior to empiric beta-lactam therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2171–2175. [Google Scholar] [CrossRef]
- Roberts, D.M.; Roberts, J.A.; Roberts, M.S.; Liu, X.; Nair, P.; Cole, L.; Lipman, J.; Bellomo, R. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit. Care Med. 2012, 40, 1523–1528. [Google Scholar] [CrossRef]
- Miglis, C.; Rhodes, N.J.; Kuti, J.L.; Nicolau, D.P.; Van Wart, S.A.; Scheetz, M.H. Defining the impact of severity of illness on time above the MIC threshold for cefepime in Gram-negative bacteraemia: A ‘Goldilocks’ window. Int. J. Antimicrob. Agents 2017, 50, 487–490. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; Pannatietr, A.; Voirol, P.; Que, Y.-A. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Eggimann, P.; Pagani, J.L.; Revelly, J.P.; Decosterd, L.A.; Marchetti, O.; Pannatier, A.; Voirol, P.; Que, Y. Impact of the introduction of real-time therapeutic drug monitoring on empirical doses of carbapenems in critically ill burn patients. Burns 2015, 41, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Smekal, A.K.; Furebring, M.; Eliasson, E.; Lipcsey, M. Low attainment to PK/PD-targets for beta-lactams in a multi-center study on the first 72 h of treatment in ICU patients. Sci. Rep. 2022, 12, 21891. [Google Scholar] [CrossRef] [PubMed]
- Seyler, L.; Cotton, F.; Taccone, F.S.; De Backer, D.; Macours, P.; Vincent, J.L.; Jacobs, F. Recommended beta-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care 2011, 15, R137. [Google Scholar] [CrossRef]
- Henderson, A.; Paterson, D.L.; Chatfield, M.D.; Tambyah, P.A.; Lye, D.C.; De, P.P.; Lin, R.T.P.; Chew, K.L.; Yin, M.; Lee, T.H.; et al. Association Between Minimum Inhibitory Concentration, Beta-lactamase Genes and Mortality for Patients Treated With Piperacillin/Tazobactam or Meropenem From the MERINO Study. Clin. Infect. Dis. 2021, 73, e3842–e3850. [Google Scholar] [CrossRef]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Fournier, A.; Eggimann, P.; Pantet, O.; Pagani, J.L.; Dupuis-Lozeron, E.; Pannatier, A.; Sadetghipour, F.; Voirol, P.; Que, Y.-A. Impact of Real-Time Therapeutic Drug Monitoring on the Prescription of Antibiotics in Burn Patients Requiring Admission to the Intensive Care Unit. Antimicrob. Agents Chemother. 2018, 62, e01818-17. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.; Haringman, J. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Lagace-Wiens, P.; Rubinstein, E. Adverse reactions to beta-lactam antimicrobials. Expert Opin. Drug Saf. 2012, 11, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Olaison, L.; Belin, L.; Hogevik, H.; Alestig, K. Incidence of beta-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Arch. Intern. Med. 1999, 159, 607–615. [Google Scholar] [CrossRef]
- Olaison, L.; Alestig, K. A prospective study of neutropenia induced by high doses of beta-lactam antibiotics. J. Antimicrob. Chemother. 1990, 25, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadramy, M.S.; Aman, H.; Omer, A.; Khan, M.A. Benzylpenicillin-induced neutropenia. J. Antimicrob. Chemother. 1986, 17, 251–253. [Google Scholar] [CrossRef]
- Ho, W.K.; Martinelli, A.; Duggan, J.C. Severe immune haemolysis after standard doses of Penicillin. Clin. Lab. Haematol. 2004, 26, 153–156. [Google Scholar] [CrossRef]
- Scheetz, M.H.; McKoy, J.M.; Parada, J.P.; Djulbegovic, B.; Raisch, D.W.; Yarnold, P.R.; Zagory, J.; Trifilio, S.; Jakiche, R.; Palella, F.; et al. Systematic review of piperacillin-induced neutropenia. Drug Saf. 2007, 30, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Neftel, K.A.; Hauser, S.P.; Muller, M.R. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J. Infect. Dis. 1985, 152, 90–98. [Google Scholar] [CrossRef]
- Neftel, K.A.; Hubscher, U. Effects of beta-lactam antibiotics on proliferating eucaryotic cells. Antimicrob. Agents Chemother. 1987, 31, 1657–1661. [Google Scholar] [CrossRef]
- Neftel, K.A.; Walti, M.; Schulthess, H.K.; Gubler, J. Adverse reactions following intravenous penicillin-G relate to degradation of the drug in vitro. Klin Wochenschr. 1984, 62, 25–29. [Google Scholar] [CrossRef]
- Chow, K.M.; Hui, A.C.; Szeto, C.C. Neurotoxicity induced by beta-lactam antibiotics: From bench to bedside. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Schliamser, S.E.; Cars, O.; Norrby, S.R. Neurotoxicity of beta-lactam antibiotics: Predisposing factors and pathogenesis. J. Antimicrob. Chemother. 1991, 27, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Neuville, M.; El-Helali, N.; Magalhaes, E.; Radjou, A.; Smonig, R.; Soubirou, J.F.; Voiriot, G.; Le Monnier, A.; Ruckly, S.; Bouadma, L.; et al. Systematic overdosing of oxa- and cloxacillin in severe infections treated in ICU: Risk factors and side effects. Ann. Intensive Care 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of beta-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef]
- Moser, S.; Rehm, S.; Guertler, N.; Hinic, V.; Drager, S.; Bassetti, S.; Rentsch, K.M.; Sendi, P.; Osthoff, M. Probability of pharmacological target attainment with flucloxacillin in Staphylococcus aureus bloodstream infection: A prospective cohort study of unbound plasma and individual MICs. J. Antimicrob. Chemother. 2021, 76, 1845–1854. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.-S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Boschung-Pasquier, L.; Atkinson, A.; Kastner, L.K.; Banholzer, S.; Haschke, M.; Buetti, N.; Furrer, D.; Hauser, C.; Jent, P.; Que, Y.; et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 333–339. [Google Scholar] [CrossRef]
- Huwyler, T.; Lenggenhager, L.; Abbas, M.; Ing Lorenzini, K.; Hughes, S.; Huttner, B.; Karmime, A.; Uçkay, I.; von Dach, E.; Lescuyer, P.; et al. Cefepime plasma concentrations and clinical toxicity: A retrospective cohort study. Clin. Microbiol. Infect. 2017, 23, 454–459. [Google Scholar] [CrossRef]
- Lau, C.; Marriott, D.; Gould, M.; Andresen, D.; Reuter, S.E.; Penm, J. A retrospective study to determine the cefepime-induced neurotoxicity threshold in hospitalized patients. J. Antimicrob. Chemother. 2020, 75, 718–725. [Google Scholar] [CrossRef]
- Vercheval, C.; Sadzot, B.; Maes, N.; Denooz, R.; Damas, P.; Frippiat, F. Continuous infusion of cefepime and neurotoxicity: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.J.; Kuti, J.L.; Nicolau, D.P.; Neely, M.N.; Nicasio, A.M.; Scheetz, M.H. An exploratory analysis of the ability of a cefepime trough concentration greater than 22 mg/L to predict neurotoxicity. J. Infect. Chemother. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Buclin, T.; Pascual, A.; Vora, S.; Bolay, S.; Decosterd, L.A.; Calandra, T.; Marchetti, O. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob. Agents Chemother. 2010, 54, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Marriott, D.; Schultz, H.B.; Gould, M.; Andresen, D.; Wicha, S.G.; Alffenaar, J.-W.; Penm, J.; Reuter, S.E. Assessment of cefepime toxicodynamics: Comprehensive examination of pharmacokinetic/pharmacodynamic targets for cefepime-induced neurotoxicity and evaluation of current dosing guidelines. Int. J. Antimicrob. Agents 2021, 58, 106443. [Google Scholar] [CrossRef]
- Zeller, V.; Durand, F.; Kitzis, M.D.; Lhotellier, L.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous cefazolin infusion to treat bone and joint infections: Clinical efficacy, feasibility, safety, and serum and bone concentrations. Antimicrob. Agents Chemother. 2009, 53, 883–887. [Google Scholar] [CrossRef]
- Bora, I.; Demir, A.B.; Uzun, P. Nonconvulsive status epilepticus cases arising in connection with cephalosporins. Epilepsy Behav. Case Rep. 2016, 6, 23–27. [Google Scholar] [CrossRef]
- Grill, M.F.; Maganti, R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharmacol. 2011, 72, 381–393. [Google Scholar] [CrossRef]
- Barreto, E.F.; Webb, A.J.; Pais, G.M.; Rule, A.D.; Jannetto, P.J.; Scheetz, M.H. Setting the Beta-Lactam Therapeutic Range for Critically Ill Patients: Is There a Floor or Even a Ceiling? Crit. Care Explor. 2021, 3, e0446. [Google Scholar] [CrossRef]
- Lheureux, O.; Trepo, E.; Hites, M.; Cotton, F.; Wolff, F.; Surin, R.; Creteur, J.; Vincent, J.; Gustot, T.; Jacobs, F.; et al. Serum beta-lactam concentrations in critically ill patients with cirrhosis: A matched case-control study. Liver Int. 2016, 36, 1002–1010. [Google Scholar] [CrossRef]
- Leise, M.D.; Poterucha, J.J.; Talwalkar, J.A. Drug-induced liver injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef]
- Donaldson, P.T.; Daly, A.K.; Henderson, J.; Graham, J.; Pirmohamed, M.; Bernal, W.; Day, C.P.; Aithal, G.P. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J. Hepatol. 2010, 53, 1049–1053. [Google Scholar] [CrossRef]
- Teixeira, M.; Macedo, S.; Batista, T.; Martins, S.; Correia, A.; Matos, L.C. Flucloxacillin-Induced Hepatotoxicity—Association with HLA-B*5701. Rev. Assoc. Med. Bras. 2020, 66, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Wiholm, B.E.; Sand, C.; Zettergren, L.; Hultcrantz, R.; Myrhed, M. Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J. Hepatol. 1992, 15, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cephalosporins, P. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; Bethesda: Rockville, MD, USA, 2012. [Google Scholar]
- Fairley, C.K.; McNeil, J.J.; Desmond, P.; Smallwood, R.; Young, H.; Forbes, A.; Purcell, P.; Boyd, I. Risk factors for development of flucloxacillin associated jaundice. BMJ 1993, 306, 233–235. [Google Scholar] [CrossRef]
- Linton, A.L.; Clark, W.F.; Driedger, A.A.; Turnbull, D.I.; Lindsay, R.M. Acute interstitial nephritis due to drugs: Review of the literature with a report of nine cases. Ann. Intern. Med. 1980, 93, 735–741. [Google Scholar] [CrossRef]
- Tune, B.M. Nephrotoxicity of beta-lactam antibiotics: Mechanisms and strategies for prevention. Pediatr. Nephrol. 1997, 11, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, A.; Vigneau, C.; Polard, E.; Triquet, L.; Rioux-Leclercq, N.; Tattevin, P.; Golbin, L. Acute kidney injury during treatment with high-dose cloxacillin: A report of 23 cases and literature review. Int. J. Antimicrob. Agents 2018, 52, 344–349. [Google Scholar] [CrossRef]
- Miano, T.A.; Hennessy, S.; Yang, W.; Dunn, T.G.; Weisman, A.R.; Oniyide, O.; Agyekum, R.S.; Turner, A.P.; Ittner, C.A.G.; Anderson, B.J.; et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: A prospective cohort study. Intensive Care Med. 2022, 48, 1144–1155. [Google Scholar] [CrossRef]
- Maan, G.; Keitoku, K.; Kimura, N.; Sawada, H.; Pham, A.; Yeo, J.; Hagiya, H.; Nishimural, Y. Cefepime-induced neurotoxicity: Systematic review. J. Antimicrob. Chemother. 2022, 77, 2908–2921. [Google Scholar] [CrossRef]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of beta-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef]
- Dilworth, T.J.; Schulz, L.T.; Micek, S.T.; Kollef, M.H.; Rose, W.E. beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients: Weighing the Challenges and Opportunities to Assess Clinical Value. Crit. Care Explor. 2022, 4, e0726. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Trono, C.; Berneschi, S.; Berrettoni, C.; Giannetti, A.; Bernini, R.; Persichetti, G.; Testa, G.; Orellana, G.; Salis, F.; et al. An integrated device for fast and sensitive immunosuppressant detection. Anal. Bioanal. Chem. 2022, 414, 3243–3255. [Google Scholar] [CrossRef] [PubMed]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 791–797. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Mohd Sazlly Lim, S.; Lipman, J.; Roberts, J.A. A personalised approach to antibiotic pharmacokinetics and pharmacodynamics in critically ill patients. Anaesth Crit. Care Pain Med. 2021, 40, 100970. [Google Scholar] [CrossRef]
- Kadambari, S.; Heath, P.T.; Sharland, M.; Lewis, S.; Nichols, A.; Turner, M.A. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J. Antimicrob. Chemother. 2011, 66, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Paterson, D.; Webb, S.A.; Lipman, J. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth Intensive Care 2011, 39, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.-F.; Roberts, J.A. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Kantasiripitak, W.; Van Daele, R.; Gijsen, M.; Ferrante, M.; Spriet, I.; Dreesen, E. Software Tools for Model-Informed Precision Dosing: How Well Do They Satisfy the Needs? Front. Pharmacol. 2020, 11, 620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legg, A.; Carmichael, S.; Chai, M.G.; Roberts, J.A.; Cotta, M.O. Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity. Antibiotics 2023, 12, 870. https://doi.org/10.3390/antibiotics12050870
Legg A, Carmichael S, Chai MG, Roberts JA, Cotta MO. Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity. Antibiotics. 2023; 12(5):870. https://doi.org/10.3390/antibiotics12050870
Chicago/Turabian StyleLegg, Amy, Sinead Carmichael, Ming G. Chai, Jason A. Roberts, and Menino O. Cotta. 2023. "Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity" Antibiotics 12, no. 5: 870. https://doi.org/10.3390/antibiotics12050870
APA StyleLegg, A., Carmichael, S., Chai, M. G., Roberts, J. A., & Cotta, M. O. (2023). Beta-Lactam Dose Optimisation in the Intensive Care Unit: Targets, Therapeutic Drug Monitoring and Toxicity. Antibiotics, 12(5), 870. https://doi.org/10.3390/antibiotics12050870






