Anti-Candidal Activity of Reboxetine and Sertraline Antidepressants: Effects on Pre-Formed Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Antifungal and Antidepressant Drugs
2.2. Candida Strains
2.3. Biofilm Formation
2.4. Biofilm Biomass Quantification
2.5. Biofilm Metabolic Viability
2.6. Antifungal Activities of REB and SER in Combination with FLC and ITR on Candidal Biofilms
2.7. Data Analysis
3. Results
3.1. Reduction in Biomass
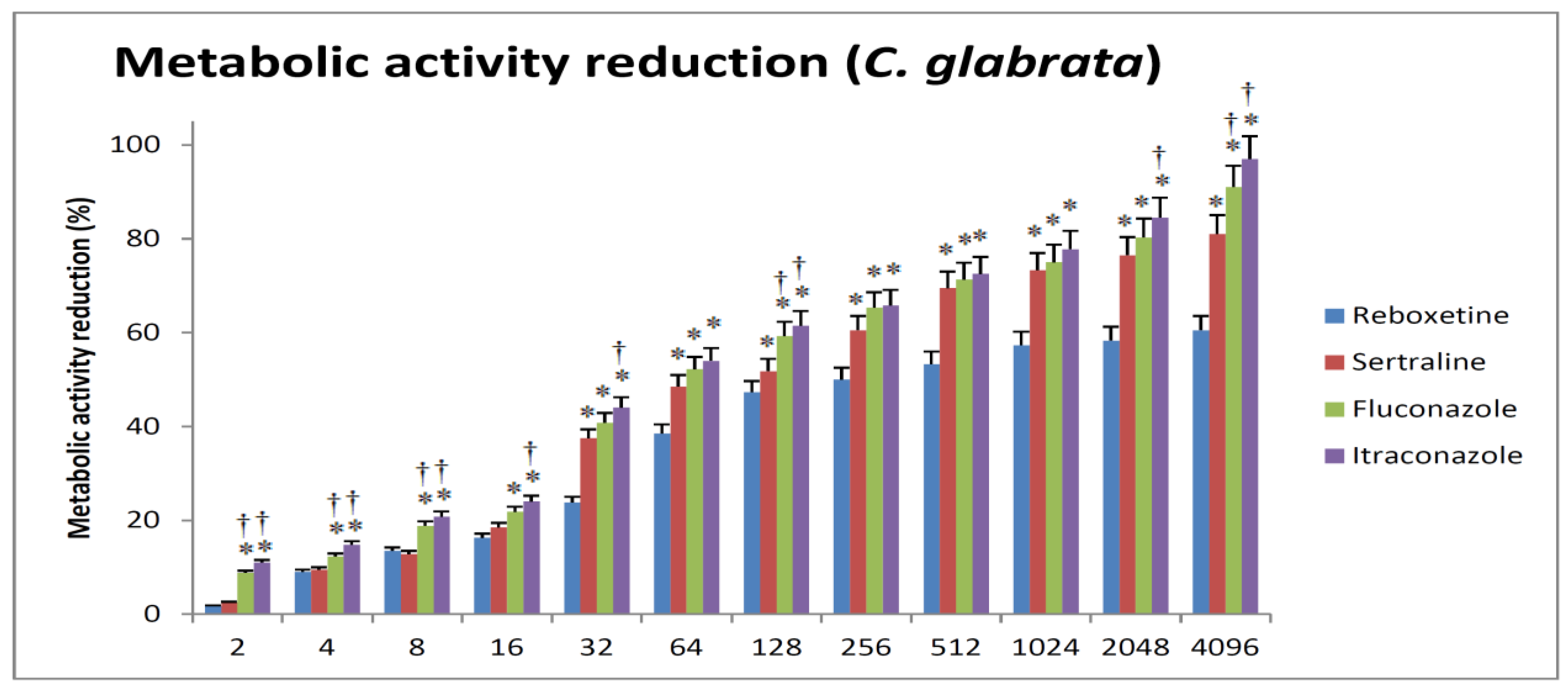
3.2. Reduction in Metabolic Activity
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slavin, M.; van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Mycology 2015, 21, 490.e1–490.e10. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, A. Emerging Invasive Fungal Infections: Clinical Features and Controversies in Diagnosis and Treatment Processes. Infect. Drug Resist. 2020, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Guo, D.; Zhang, L.; Xu, D.; Sun, S. The synergistic effect of azoles and fluoxetine against resistant Candida albicans strains is attributed to attenuating fungal virulence. Antimicrob. Agents Chemother. 2016, 60, 6179–6188. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Kadry, A.A.; El-Ganiny, A.M.; El-Baz, A.M. Relationship between Sap prevalence and biofilm formation among resistant clinical isolates of Candida albicans. Afr. Health Sci. 2018, 18, 1166–1174. [Google Scholar] [CrossRef]
- El-Telbany, M.; Mohamed, A.A.; Yahya, G.; Abdelghafar, A.; Abdel-Halim, M.S.; Saber, S.; Alfaleh, M.A.; Mohamed, A.H.; Abdelrahman, F.; Fathey, H.A.; et al. Combination of Meropenem and Zinc Oxide Nanoparticles; Antimicrobial Synergism, Exaggerated Antibiofilm Activity, and Efficient Therapeutic Strategy against Bacterial Keratitis. Antibiotics 2022, 11, 1374. [Google Scholar] [CrossRef]
- Abdel-Halim, M.S.; Askoura, M.; Mansour, B.; Yahya, G.; El-Ganiny, A.M. In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates. J. Antibiot. 2022, 75, 679–690. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Borecka-Melkusova, S.; Moran, G.P.; Sullivan, D.J.; Kucharíková, S.; Chorvát, D., Jr.; Bujdáková, H. The expression of genes involved in the ergosterol biosynthesis pathway in Candida albicans and Candida dubliniensis biofilms exposed to fluconazole. Mycoses 2009, 52, 118–128. [Google Scholar] [CrossRef]
- Samanta, A.; Debprasad, C.; Sinha, C.; Jana, A.D.; Ghosh, S.; Mandal, A.; Banerjee, A.; Hendricks, O.; Christensen, J.B.; Kristiansen, J.E. Evaluation of in vivo and in vitro antimicrobial activities of a selective serotonin reuptake inhibitor sertraline hydrochloride. Anti-Infect. Agents 2012, 10, 95–104. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gaspar, C.A.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Anti-Candida activity of fluoxetine alone and combined with fluconazole: A synergistic action against fluconazole-resistant strains. Antimicrob. Agents Chemother. 2014, 58, 4224–4226. [Google Scholar] [CrossRef]
- Rossato, L.; Loreto, E.S.; Zanette, R.A.; Chassot, F.; Santurio, J.M.; Alves, S.H. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol. 2016, 61, 399–403. [Google Scholar] [CrossRef]
- Costa Silva, R.A.; da Silva, C.R.; de Andrade Neto, J.B.; da Silva, A.R.; Campos, R.S.; Sampaio, L.S.; do Nascimento, B.S.A.; Gaspar, B.D.S.; Fonseca, S.G.D.C.; Josino, M.A.A.; et al. In vitro anti-Candida activity of selective serotonin reuptake inhibitors against fluconazole-resistant strains and their activity against biofilm-forming isolates. Microb. Pathog. 2017, 107, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Rainey, M.M.; Korostyshevsky, D.; Lee, S.; Perlstein, E.O. The antidepressant sertraline targets intracellular vesiculogenic membranes in yeast. Genetics 2010, 185, 1221–1233. [Google Scholar] [CrossRef]
- Spitzer, M.; Griffiths, E.; Blakely, K.M.; Wildenhain, J.; Ejim, L.; Rossi, L.; De Pascale, G.; Curak, J.; Brown, E.; Tyers, M.; et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 2011, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic Cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Xue, W.; Wang, P.; Yang, F.; Li, B.; Li, X.; Li, Y.; Yao, X.; Zhu, F. Exploring the Inhibitory Mechanism of Approved Selective Norepinephrine Reuptake Inhibitors and Reboxetine Enantiomers by Molecular Dynamics Study. Sci. Rep. 2016, 6, 26883. [Google Scholar] [CrossRef]
- Weiss, J.; Dormann, S.G.; Martin-Facklam, M.; Kerpen, C.J.; Ketabi-Kiyanvash, N.; Haefeli, W. Inhibition of P-glycoprotein by newer antidepressants. J. Pharmacol. Exp. Ther. 2003, 305, 197–204. [Google Scholar] [CrossRef]
- Kalayci, S.; Demirci, S.; Şahin, F. Antimicrobial properties of various psychotropic drugs against broad range microorganisms. Curr. Psychopharmacol. 2014, 3, 195–202. [Google Scholar] [CrossRef]
- Brown, G.; Denning, D.W.; Gow, N.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 2, 165rv13. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Martinez-de-Oliveira, J.; Donders, G.G.G.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A. Anti-Candida activity of antidepressants sertraline and fluoxetine: Effect upon pre-formed biofilms. Med. Microbiol. Immunol. 2018, 207, 195–200. [Google Scholar] [CrossRef]
- Ramage, G.; VandeWalle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.H.; Montanari, L.B.; Martins, C.G.; Zaia, J.E.; Almeida, A.M.F.; Matsumoto, M.T.; Mendes-Giannini, M.J.S. AntiCandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 2011, 172, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Wan, T.; Shao, J.; Da, W.; Li, Q.; Shi, G.; Wu, D.; Wang, C. Strong Synergism of Palmatine and Fluconazole/Itraconazole Against Planktonic and Biofilm Cells of Candida Species and Efflux-Associated Antifungal Mechanism. Front. Microbiol. 2018, 9, 2892. [Google Scholar] [CrossRef]
- Shao, J.; Wang, T.M.; Yan, Y.Y.; Shi, G.X.; Cheng, H.J.; Wu, D.Q.; Wang, C. Matrine reduces yeast-to-hypha transition and resistance of a fluconazole resistant strain of Candida albicans. J. Appl. Microbiol. 2014, 117, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, B.A.; Bulatova, N.R.; abuRokba, W.; Darwish, R.M. Serotonin reuptake inhibitors effect on fluconazole activity against resistant Candida glabrata strains. J. Glob. Antimicrob. Resist. 2022, 29, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tekintaş, Y.; Temel, A.; Ateş, A.; Eraç, B.; Metin, D.Y.; Hilmioğlu Polat, S.; Hoşgör Limoncu, M. Antifungal and Antibiofilm Activities of Selective Serotonin Reuptake Inhibitors Alone and in Combination with Fluconazole. Turk. J. Pharm. Sci. 2020, 23, 17, 667–672. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Ledochowski, M. Antifungal activity against Candida species of the selective serotonin reuptake inhibitor, sertraline. Clin. Infect. Dis. 2001, 33, 135–136. [Google Scholar] [CrossRef]
- Sertraline Hydrochloride. Available online: https://go.drugbank.com/salts/DBSALT000808 (accessed on 21 January 2023).
- Reboxetine. Available online: https://go.drugbank.com/drugs/DB00234 (accessed on 21 January 2023).
- Podunavac-Kuzmanović, S.O.; Velimirović, S.D. Correlation between the lipophilicity and antifungal activity of some benzoxazole derivatives. Acta Period. Technol. 2010, 41, 177–185. [Google Scholar] [CrossRef]
- Comer, A.M.; Figgitt, D.P. Sertraline. CNS Drugs 2000, 14, 391–407. [Google Scholar] [CrossRef]
- Fleishaker, J.C. Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin. Pharmacokinet. 2000, 39, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Foye, W.; Lemke, T.; Williams, D. FOYE’S Principles of Medicinal Chemistry, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Poggesi, I.; Pellizzoni, C.; Fleishaker, J.C. Pharmacokinetics of reboxetine in elderly patients with depressive disorders. Int. J. Clin. Pharmacol. Ther. 2000, 38, 254–259. [Google Scholar] [CrossRef] [PubMed]



| Strains | SMIC80 Alone (µg/mL) 1 | SMIC80 in Combination (µg/mL) 1 | sFICI 2 of REB + FLC (Interpretation) | sFICI 2 of REB + ITR (Interpretation) | |||
|---|---|---|---|---|---|---|---|
| REB | FLC | ITR | REB/FLC | REB/ITR | |||
| Candida albicans (ATCC 10231) | >1024 | >1024 | >1024 | 64/256 | 64/256 | 0.3125 (synergism) | 0.3125 (synergism) |
| Candida krusei (ATCC 6258) | >1024 | 1024 | 1024 | 512/512 | 64/128 | 1 (indifference) | 0.1875 (synergism) |
| Candida glabrata (ATCC 14053) | >1024 | >1024 | >1024 | 512/512 | 512/512 | 1 (indifference) | 1 (indifference) |
| Strains | SMIC80 Alone (µg/mL) 1 | SMIC80 in Combination (µg/mL) 1 | sFICI 2 of SER + FLC (Interpretation) | sFICI 2 of SER + FLC (Interpretation) | |||
|---|---|---|---|---|---|---|---|
| SER | FLC | ITR | SER/FLC | SER/ITR | |||
| Candida albicans (ATCC 10231) | >1024 | >1024 | >1024 | 64/256 | 64/256 | 0.3125 (synergism) | 0.3125 (synergism) |
| Candida krusei (ATCC 6258) | >1024 | 1024 | 1024 | 64/128 | 64/256 | 0.1875 (synergism) | 0.3125 (synergism) |
| Candida glabrata (ATCC 14053) | >1024 | >1024 | >1024 | 256/128 | 256/128 | 0.375 (synergism) | 0.375 (synergism) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, E.I.; Alhuwaydi, A.M.; Taha, A.E.; Abouelkheir, M. Anti-Candidal Activity of Reboxetine and Sertraline Antidepressants: Effects on Pre-Formed Biofilms. Antibiotics 2023, 12, 881. https://doi.org/10.3390/antibiotics12050881
Ahmed EI, Alhuwaydi AM, Taha AE, Abouelkheir M. Anti-Candidal Activity of Reboxetine and Sertraline Antidepressants: Effects on Pre-Formed Biofilms. Antibiotics. 2023; 12(5):881. https://doi.org/10.3390/antibiotics12050881
Chicago/Turabian StyleAhmed, Eman Ibrahim, Ahmed M. Alhuwaydi, Ahmed E. Taha, and Mohamed Abouelkheir. 2023. "Anti-Candidal Activity of Reboxetine and Sertraline Antidepressants: Effects on Pre-Formed Biofilms" Antibiotics 12, no. 5: 881. https://doi.org/10.3390/antibiotics12050881
APA StyleAhmed, E. I., Alhuwaydi, A. M., Taha, A. E., & Abouelkheir, M. (2023). Anti-Candidal Activity of Reboxetine and Sertraline Antidepressants: Effects on Pre-Formed Biofilms. Antibiotics, 12(5), 881. https://doi.org/10.3390/antibiotics12050881





