Abstract
The nature of microorganisms and the efficiency of antimicrobials have witnessed a huge co-dependent change in their dynamics over the last few decades. On the other side, metals and metallic compounds have gained popularity owing to their effectiveness against various microbial strains. A structured search of both research and review papers was conducted via different electronic databases, such as PubMed, Bentham, Springer, and Science Direct, among others, for the present review. Along with these, marketed products, patents, and Clinicaltrials.gov were also referred to for our review. Different microbes such as bacteria, fungi, etc., and their diverse species and strains have been reviewed and found to be sensitive to metal-carrying formulations. The products are observed to restrict growth, multiplication, and biofilm formation effectively and adequately. Silver has an apt use in this area of treatment and recovery, and other metals like copper, gold, iron, and gallium have also been observed to generate antimicrobial activity. The present review identified membrane disruption, oxidative stress, and interaction with proteins and enzymes to be the primary microbicidal processes. Elaborating the action, nanoparticles and nanosystems are shown to work in our favor in well excelled and rational ways.
1. Introduction
Several kinds of microorganisms lead to the initiation and further development of microbial infections. Such infections primarily and solely manifest in many pathological conditions with variant degrees of severity. Their pathologies precipitate numerous mild symptoms (fever, fatigue, nausea, headache) to serious symptoms (cyanosis, tissue necrosis, lymphadenopathy, respiratory effects) [1,2]. These have also evolved to be one of the major secondary factors in various diseased conditions, while in some, they ultimately cause death [3,4]. Microbial infections and their manifestations interfere at every step of medical methodologies from concluding misleading and erroneous diagnoses to resulting in deleterious surgeries as well as unsuccessful and incomplete treatments [5]. The severity of the condition worsens when there is any kind of additional or major infection. All of this has made antimicrobials a very important and fundamental part of therapeutics and pharmacology [6,7].
The last century has seen excessive use of antibiotics for various kinds of infections. They work by targeting bacterial cell components and altering necessary processes like DNA replication, cell wall synthesis, etc. [8,9,10]. However, they do have certain drawbacks that make them insufficient and problematic in various ways:
- They may affect healthy bacteria present in the body [11,12].
- Monitoring its effectiveness is difficult and challenging [13,14].
- Inconsistency in therapeutic security and the number of associated side-effects [15].
Along with these deficiencies, increasing antibiotic resistance has hinted at the clinical need for newer antimicrobials to tackle microbial growth and biofilm production effectively. Continuous mutations, irrational use of antibiotics, and the production of enzymes that inactivate the bacterial cells have contributed to this increasing resistance to the agents [16,17].
Barring antibiotics, metal compounds were largely in use and practice before the 1920s, after which antibiotics took over [18,19,20]. The potential of metals to conveniently restrict biofilm production make them the best possible alternative in present times [21,22]. Antimicrobial properties of metals have been used since ancient times for disinfecting food and water, managing plant diseases in agriculture, and in medical areas as well [23,24]. Certain metals are necessary for cell functioning and cell membrane formation but their presence in excess amounts can be lethal, whereas specific other metals like non-essential groups, such as mercury, silver, etc., are found to be microbicidal even at very low concentrations [25].
Antimicrobial-metallic agents are now being explored due to their capability to tackle multi-drug resistant bacteria, biofilms, and resistant biofilms, as well as to inhibit important metabolic pathways, plus their suitability with other biocides [26,27,28]. This review compiled and presented the applications of various metal-based nanoparticles as potential antimicrobial agents, including their mechanism of antimicrobial activities. For this work, we have considered a total of 200 papers from electronic databases like PubMed, Bentham, Springer, and Science Direct. We have selected 126 papers covering research and review articles published over the past 10 years, and we have selected keywords such as “metal-based nanoparticles”, “antimicrobial drug-resistance”, and “metal oxides”. Along with these, marketed products, patents, and clinical trials data were also searched in order to write this review article.
2. Metals as Antimicrobials
Metals are abundant in the earth’s crust and ecosphere. The Great Oxidation Event (GOE), which took place 2.3–2.4 billion years ago, exposed bacteria to a wide range of metal ions. The earth’s crust contains a variety of oxidized forms of metal compounds as a result of the atmosphere’s rising oxygen level. Enzymes used metals like copper, iron, and zinc for their redox reactions. Metals are necessary for the process of life but are toxic at high intracellular concentrations, and, thus, cells need a homeostasis mechanism to keep the intracellular concentration constant. Zinc and copper share a similar pathogen-killing mechanism in eukaryotes, where oxidative stress is used to destroy the encapsulated bacterium. Metals like gold, silver, and mercury are extremely poisonous to microorganisms at low concentrations [29].
Metals were once utilized as antibacterial agents, but their industrial usage can harm the ecological system, although they do have a medical use. Infections were treated with arsenic, mercury, silver, copper, zinc, and other elements. Antimony and arsenic are employed as fungicides, rodenticides, insecticides, and to treat protozoal illnesses. While zinc salts can be used to treat diarrhea, copper salts are used to make the Bordeaux and Burgundy mixture, which is used to prevent bacterial and fungal problems in plants and to promote animal growth. Burns can be relieved with silver. Organic mercury compounds are utilized to keep eye drops in good condition. Mercury was utilized as a disinfectant and a syphilis infection treatment. In dental restorations, mercury is combined with copper, silver, and tin [30].
2.1. Metal-Based Nanoparticles as Antimicrobials
Metallic NPs of sizes ranging from 1 nm to 100 nm can be synthesized by two approaches, i.e., top-down and bottom-up. The top-down approach involves beginning with the material in bulk, which is then broken down into the size of a nanoscopic scale via ball milling or attrition etc. It is an easy method to employ, but increased accommodated impurities and non-uniform sizes of particles limit its use [31,32]. On the other hand, the bottom-up nanofabrication approach includes variant techniques such as the colloidal synthesis, the sol-gel method, the chemical vapor decomposition process, and the atomic layer deposition among others. The process, though time consuming and tedious, has the benefit of uniform-sized and uniform-shaped smaller particles bearing the least number of defects and controlled surface properties [32,33,34].
The use of metal-based nanoparticles as components in the creation of antibacterial agents has been made possible by nanotechnologies. Metal-based nanoparticles (NPs) demonstrate an effective role in locating and eliminating bacteria through a variety of mechanisms, including attraction to the surface of the bacteria, disruption of the cell wall and membrane, and induction of a toxic mechanism mediated by an increase in oxidative stress (e.g., the production of reactive oxygen species (ROS)) [35,36,37,38]. The creation of oxidative stress is a valuable and effective antibacterial method to combat MDR bacteria, given the absence of new antimicrobial medicines with unique mechanisms of action. Therefore, it is important to identify and properly characterize whether NPs might cause oxidative stress in these bacteria [39,40,41]. Metal-based NPs physically interact with bacterial cell surfaces, disrupt their membrane, and, ultimately, restrict the formation of biofilms [42]. The formation of biofilms also leads to the development of resistance against antimicrobial agents, so their hindrance ultimately restricts the modulation of resistant mutants, too [43,44,45]. The shape of metal-based NPs along with their ultra-small, compliantly controllable size, and resultant greater surface area to mass ratio all contribute to the prevention of biofilm formation [46,47,48]. The target microorganisms and their mechanisms of action for a few metal-based nanoparticles are provided in Table 1.

Table 1.
Antimicrobial activity mechanisms of different metal-based NPs.
The targeted drug delivery approach of metal-based NPs can be achieved through either active or passive targeting. Active targeting involves the modification of surfaces of metal-based NPs and processes like magnetic targeting, receptor targeting, and other approaches such as temperature-dependent cell death patterned targeting. Passive targeting, meanwhile, is generally accomplished by improving permeation and enhanced retention at the site of infection [55].
Within a single metal-based NP, multiple drugs can be accommodated to bring an elaborative action. Different drugs that have different kinds of mechanisms will exert a joint action and will subsequently result in higher efficiency. The microbe being either resistant or a multi-drug resistant mutant will probably be successfully affected via one or the other of the variant processes [56,57].
2.2. Mechanisms Involved in Antimicrobial Activity of Metal and Metal-Based NPs
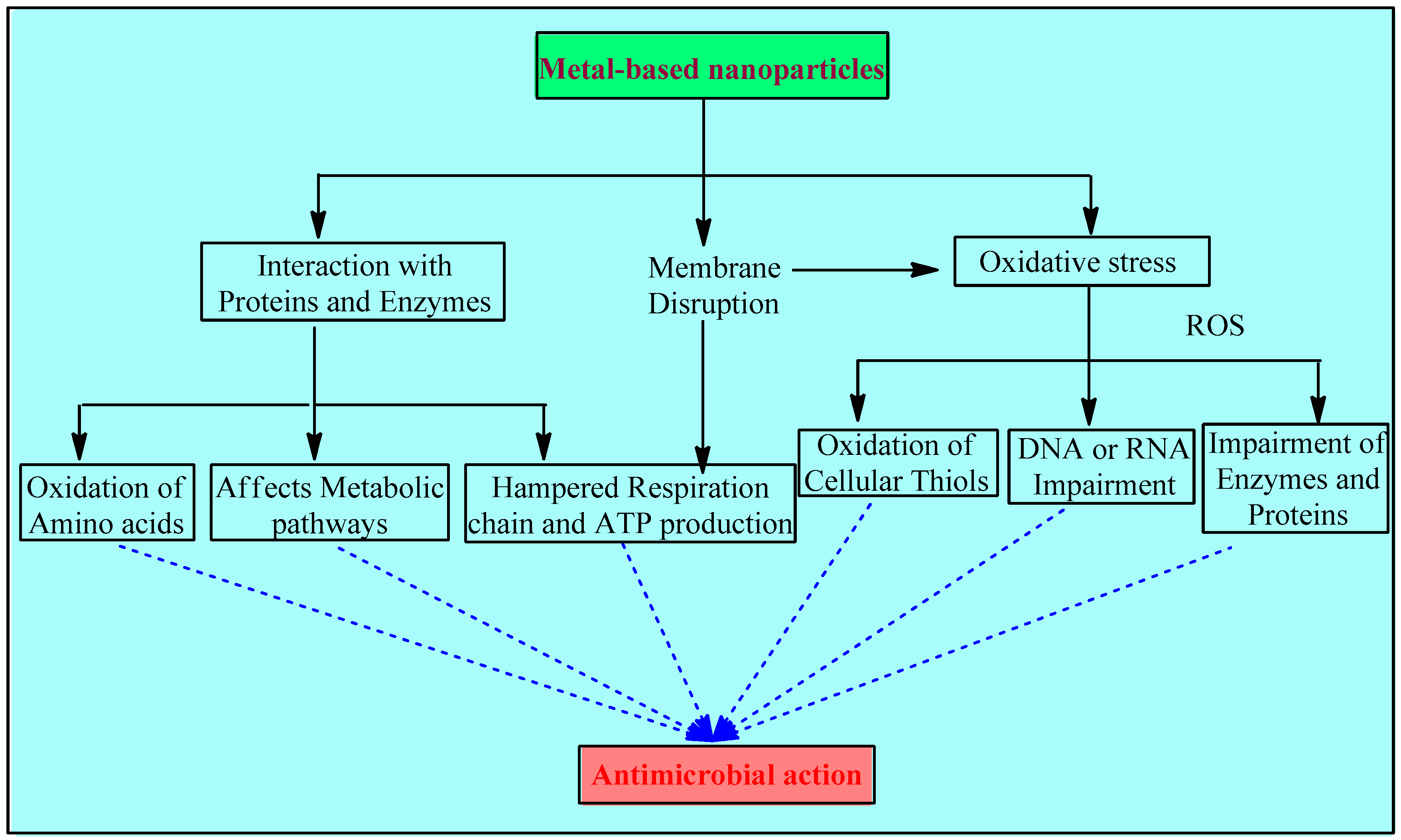
Metal and metal NPs interfere with bacteria’s hemostasis in 3 major ways: disruption of the membrane, oxidative stress, and interaction with proteins and enzymes (Figure 1).
Figure 1.
Mechanisms involved in the antimicrobial activity of metals and metal-based NPs.
2.2.1. Disruption of the Membrane
Associating metal nanoparticles with conducting polymers that have positive charges on their surfaces is essential for stabilizing solutions with high nanoparticle concentrations. According to the bacterial eradication mechanism, cell death is caused by electrostatic contact between the negatively charged bacteria (electronegative groups of the polysaccharides in the membrane) and the positively charged compound, such as metal and metal oxide nanoparticles with a variety of forms, roughness, and positive zeta potentials. Positively charged NPs interact with electro-negatively charged bacterial membranes, directly causing bactericidal toxicity in the membrane and targeting cell integrity The surface charge is an important factor for the antibacterial activity of the metal-based NPs. Positively charged NPs are more effective than neutral or negatively charged ones. As reported in [45], NPs with a positive charge display higher toxicity due to their electrostatic interaction with the negative charge of the bacterial cell wall. A comparative study showed that magnetic NPs with a positive charge (NP+) efficiently attracted over 90% of E. coli, while negatively charged magnetic NPs (NP−) did not show any affinities. These results suggest that NPs+ have good potential to capture bacteria via electrostatic attraction. In another study, the antibacterial efficiency of three different AgNPs that were positively, negatively, and neutrally charged were compared, and it was found that positively charged NPs showed the highest bactericidal activity, while the negative ones showed the lowest [58,59,60].
Electron microscopic studies over S. aureus and E. coli also indicate the compromised cytoplasmic membrane’s integrity due to toxic doses of certain metals such as aluminum and silver. A few agents, especially silver, impede the electron transport chain [ETC] [61,62]. Cell wall synthesis is also interrupted due to the interaction between the sulfur-containing constituents of the membrane and the silver nanomaterials. Apart from this, toxic doses of copper and cadmium have also been thought to cause lipid peroxidation [60,63,64].
All such disruptions lead to oxidative stress and, subsequently, to further damage, such as interrupted energy homeostasis, impeded respiration, and, ultimately, cell death [65].
2.2.2. Oxidative Stress
The metal-based NP system provides the benefit of controlling the particle specifications, including its shape, size, and even the charge on the surface, and it also provides the option to manage the release of metal ions [66]. The mode of their action is generally linked with the disruption of the membrane initially and the generation of ROS in large amounts gradually. The driving force of their action is associated with the controlled release of metal ions, while some research suggests that this release is through the leaching of metal complexes with amino acids of bacteria [67]. Metal ions in solution, ROS, and ROS-mediated machinery may all play a role in the toxicity of metal oxide nanoparticles. The potential of CuO NPs for toxicity may be related to problems with DNA synthesis and repair as well as an increase in the production of reactive oxygen species [68,69]. Higher antimicrobial activity of smaller metallic NPs is reported compared to larger NPs, and this is due to their larger surface area to volume ratio, which increases the production of ROS [70].
The introduction of metal-based NPs initiates intracellular ROS production, which has been confirmed by the electron paramagnetic resonance technique [41]. Such oxidation reactions are catalyzed by numerous metal ions, such as copper, iron, chromium, arsenic, etc., which upregulate genes and also cause other damage [71]. Iron brings in auto-oxidation through aerobic respiration, which leads to the production of oxygen radicals and hydrogen peroxide [72].
Moreover, the consumption of cellular anti-oxidants may also begin during this redox cycling phase of metals. Oxidative imbalance causes oxidation of cellular thiols as well, which, in turn, develops covalent bonds with sulfur. Ultimately, this results in the formation of protein disulfides and the reduction of anti-oxidant sources [70,73,74,75]. Oxidative stress led by ROS impairs the DNA or RNA, attacks enzymes and proteins, and thereby damages macromolecules [76].
2.2.3. Interaction with Proteins and Enzymes
Metallic NPs initiate an antimicrobial response by binding to cytosolic proteins. The interaction of these NPs with enzymes and DNA hampers the whole homeostasis. Since metabolic pathways are affected, the respiratory chain, ATP production, and replication processes are impaired and ultimately inhibited [77,78].
2.3. Bio-Medical Antimicrobial Applications of Metal-Based NPs
Multi-drug resistant organisms are often resistant to commonly used antibiotics. The lack of and the great need for effective antimicrobial agents have led to the development of novel strategies to address this growing public health issue. A growing number of drug-resistant mutants and the inability to cure infective conditions completely has fueled the production of NPs, and this has now found several applications:
Dental products—Microbes tend to settle on teeth, leading to the development of plaque, thereby increasing the chance of mouth infections. NPs not only potently restrict the growth of bacteria but nano-crystallization also improves their performance and inhibits the formation of biofilms as well [79].
Coating of implantable devices—Coating of implantable devices like heart valves prevents adhesion and further growth of bacteria like E. coli, reducing the risk of inflammation and infections [80,81]. Devices that are commonly prone to the colonization of bacteria, such as dental implants, catheters, etc., are generally subjected to NP coatings [56,62].
Wound dressings—Several microbes like Streptococcus, E. coli, and Staphylococcus, among others, can cause wound infections, inflammation, and other complications that can be precipitated. To significantly prevent this, broad-spectrum antimicrobial NPs are an option [82,83].
Other than these, NPs can often be used along with bone cement and also in certain drug delivery systems [84]. It has been stated that we are on the verge of the ‘post-antibiotic era’ by the Centre for Disease Control and Prevention (CDC). The prediction of more deaths due to antimicrobial resistance than cancers by 2050 has also been made by many, and all of these predictions and statistics have led to the desire for newer molecule options and drug delivery methods as well as post-numerous research and studies, and the use of metal-based nanoparticles seems to be an effective lead here [85]. Indeed, the latter have been spotted to target resistance and biofilms via different mechanisms and pathways, depending on the metal and its potential.
3. Metal-Based Nanoparticles Mediated Antimicrobial Effects
Metal is precipitated by bacteria as protein aggregates, sulfides, oxides, or elemental crystals. The formed precipitates then interact with the membrane and move into the cell, initiating antimicrobial effects via different mechanisms and gradually, terminating the planktonic growth.
3.1. Silver Ions and Silver Nanoparticles
Silver [Ag] has been in use for water purification, preservation, anti-fouling, anti-fungal, antiseptic, and antibiotic effects for many ages now, and it has the potential to mitigate or even solve considerable medical burdens in our societies [86]. Indeed, silver-impregnated bandages, devices, and other equipment have shown effective antimicrobial action, and this effect is commonly witnessed in practice [87].
Silver requires the formation of silver ions to develop bactericidal action. The release of these ions is through retarded oxidation, which is a slow process that gradually results in lower efficient silver levels. Thus, since the 1st century BC, silver salts have been used [88]. Silver ions tend to interact with the membrane, and later pursue microbicidal action. Silver NPs are known to be more effective, and they strongly anchor to the membrane and keep on releasing biochemically active silver ions in small amounts. As the ions are released, pit formation is initiated by the metallic silver in the cell wall of bacteria. Pit formation gradually increases the permeation of the membrane, which impedes necessary transport processes. Slowly, lysis is precipitated, since vital membrane proteins and cellular components begin to leak out of the pits [89,90,91].
Along with this, silver ions bind to reduced thiol groups and interfere with iron-sulfur [Fe-S] clusters, which are vital for biochemical processes such as electron transfer and respiration [92,93].
Electron donor groups of proteins and silver ions share an extremely firm and powerful electrostatic interaction, which indirectly gives access to Ag ions to interfere with the proteins [94]. Metal chelation sites inside the enzymes are occupied by Ag ions, causing enzyme inactivation [95]. This not only hampers necessary cellular processes but also impairs the cell transport system and cell wall integrity. In the later stages, the cell undergoes necrosis led by silver ion-induced DNA condensation and the lack of replication capacity [96].
Apart from bacteria, silver NPs are seen to be active even against certain viruses––namely, murine norovirus and HIV-1 among others. They tend to inactivate certain bacteriophages and restrict the virus’ capability to infect the host cells by targeting disulfide groups present in surface proteins [97,98].
One of the first found and used silver salt-silver nitrates has high solubility, and consequently leads to a higher concentration of silver in the cell, while silver sulfides and silver halides are sparingly soluble agents that release silver slowly. Another salt that has been in use for decades for its antibiotic effects is silver sulfadiazine. It is a sulfonamide as well, bringing out a more elaborative action. The use of silver nanoparticles in combination with fluconazole and with other clinically approved marketed drugs showed a better resistance to fungal infection [99,100].
Ag+ ion, the most stable ion of silver, bears a moderate oxidizing ability, while the higher oxidation states’ species- Ag2+ and Ag3+ are extremely strong oxidants but have not been explored as much, owing to their instability in solution. A potential approach to the instability of the higher oxidation states can be Silver Oxysalts [Ag(Ag3O4)2X]. The most stable of all oxysalts of silver is the nitrate-coordinated moiety silver oxynitrate [101].
NPs with domains of silver and covering shells of gold were tested over separate strains and in combination strains of S. aureus, P. aeruginosa, E. coli, and Enterococcus faecalis. The combination NPs were seen to produce dose-dependent effects while inhibiting biofilm formation. They also permitted specific and faster leaching and interaction of silver ions into cellular components. The damaging process was observed to be immediate and quicker at every stage of the initiation of cellular damage, disintegration, and lysis, owing to the increased affinity of silver to molecules precipitating the inactivation of enzymes and certain proteins, which hindered ATP production [102].
Silver Oxynitrate [Ag(Ag3O4)2NO3 or Ag7NO11]
Silver oxynitrate is a silver oxysalt, containing stable higher oxidation state ion species of silver, and it is now being explored because of excellent effects observed in various types of research. It is not only effective against antibiotic-resistant and multi-drug-resistant mutants but has also been seen to target biofilms. Biofilms are one of the prominent reasons for resistance development and they also slow the healing process, gradually creating a chronic non-healing infection [103].
Doherty et al. reported that the new silver dressing promises to fight wound infection, although its anti-biofilm effectiveness and benefits on healing apart from infection are sometimes ambiguous. The dressings exhibited a distinct impact on the healing of biofilm-infected and uninfected wounds, with Ag Oxysalts dressings having a more favorable effect than the control therapy and the other silver dressings on re-epithelialization, wound size, and inflammation. The study was carried out by using in vitro and in vivo S. sureus and P. aeruginosa biofilm models [104].
This involved a comparison between Ag7NO11, AgNO3, and CuSO4 to tackle dual-species planktonic biofilm population, where MIC of silver oxynitrate was found to be remarkably lower than that of CuSO4 and AgNO [105]. A list of a few antimicrobial marketed products containing silver is provided in Table 2. Apart from the listed ones, certain other dressings and medical products exist using silver metal for its antimicrobial potential, such as Mepilex® Ag. This is a silicone foam-type dressing, capable of action within 30 min, providing an effect on wounds, burns, and certain ulcers [106].

Table 2.
List of select antimicrobial-marketed products containing silver.
3.2. Copper and Copper Nanoparticles
Copper was the first metal element to have been used by mankind, and it was used for various reasons. It is also mentioned in one of the oldest books ever written, Smith Papyrus. Egyptian, Roman, and Greek medical literature have noted its benefit in treating ear infections, burns, and intestinal worm-caused infections and headaches, as well as its use for sterilizing water and wounds. The nineteenth and twentieth centuries witnessed the increased use of copper in treating ailments such as syphilis, tuberculosis, anemia, lupus, facial neuralgia, eczema, adenitis, and gastric ulcers [111].
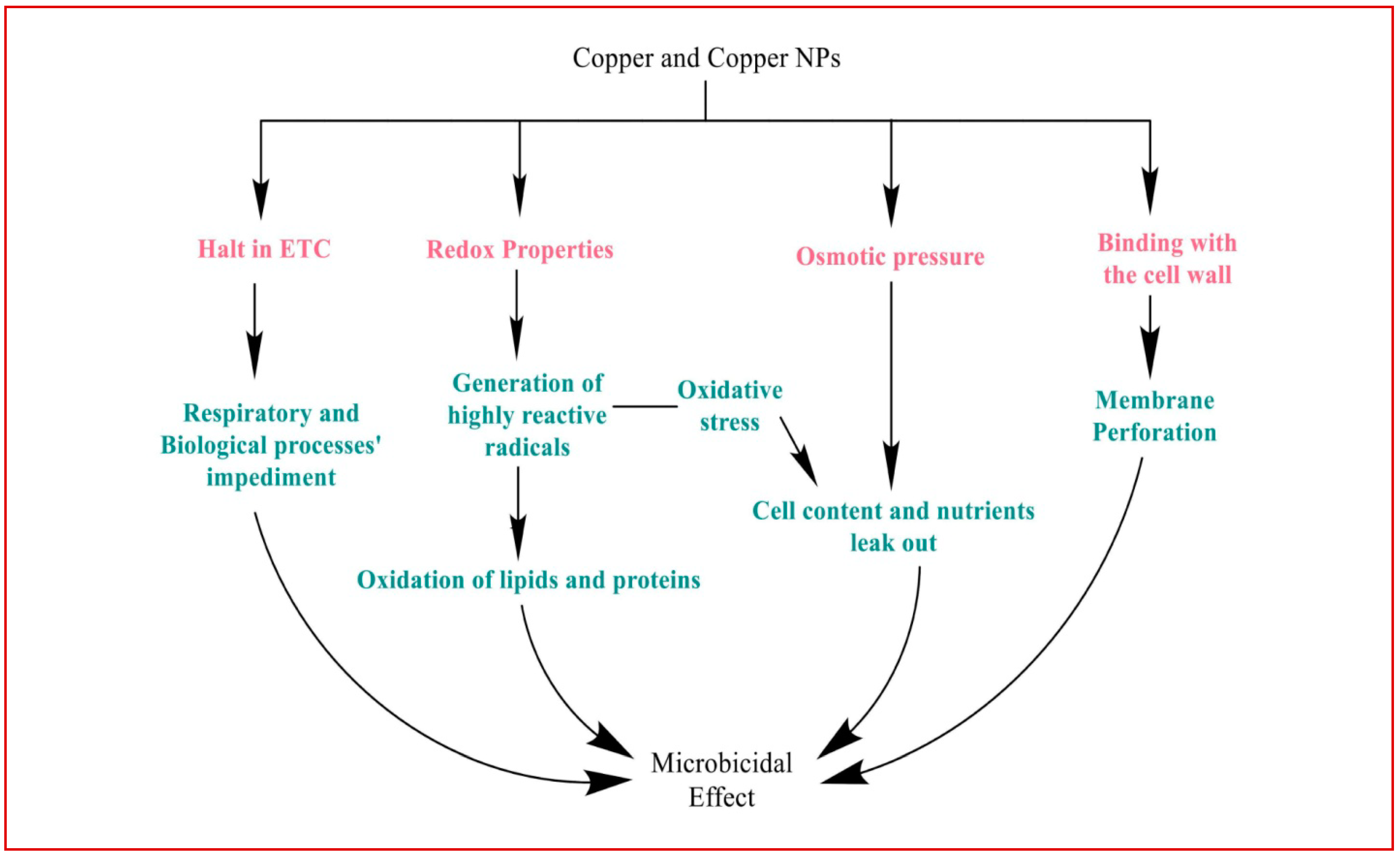
Copper binds to the cell wall of bacteria, directly hampering membrane proteins and initiating membrane perforation. Ions, here, begin interacting with sulfur and phosphorus-containing moieties such as certain proteins, enzymes, and DNA (Figure 2) [112].
Figure 2.
Various mechanisms mediated the antimicrobial action of copper on bacteria and its homeostasis.
Cellular damage by copper generally begins because of its redox properties. A fenton-type reaction involving copper ions and hydrogen peroxide leads to the generation of highly reactive hydroxyl radicals. These radicals, in turn, target lipids and proteins, and oxidize them. Subsequently, electron transport is interrupted along with the peroxidation of lipids, impeding biological and respiratory processes in the end [113,114,115].
Copper damages the membrane of the cell through disturbed osmotic pressure and oxidative stress, causing cell contents and nutrients to leak out, and precipitating cell death. Another known mechanism of damage is through displacing iron from clusters of iron and sulfur. The competition of copper ions with other metal ions such as zinc on proteins for the binding sites, as well as inappropriate binding, leads to the loss of function of proteins, which gradually breaks apart the protein into many non-functional segments [113,116].
Copper is generally seen to be effective against both gram-negative and gram-positive bacteria. Abbas et al. reported that copper nanoparticles have antibacterial activity, where copper nanoparticles have an effect on gram-negative microorganisms by showing an impact on the cell film of microscopic organisms, i.e., S. aureus and E. coli [117]. Another study carried out by Sharma et al. shows that colloidal copper nanoparticles have an antibacterial activity against gram-negative bacteria (E. coli and Proteus vulgaris) under culture conditions. By using the chemical reduction method, three sets of Cu nanoparticles were synthesized and antibacterial activity was calculated by minimum inhibitory concentration and minimum bactericidal concentration, reactive oxygen species, and cytoplasmic leakage assays. Copper nanoparticles exhibit good antibacterial activity [118].
As is the case with silver, copper also gained importance in the past due to its effectiveness against a broad spectrum of species and multi-drug resistant species, and it also tends to be a cheaper and more easily available than silver and gold products. The synthesis and fabrication of these NPs has given us products with better physical and chemical properties, owing to their potential to undergo oxidation and form copper oxide NPs [114].
3.3. Gold and Gold Nanoparticles
Metal gold [Au] carries properties for cytotoxic and genotoxic effects, supported by its characteristics of being inert and highly stable, though it requires a high concentration to initiate the antimicrobial effect. The potential is intensified when its size is reduced to formulate a nanomaterial. Similar to the enzyme glucose oxidase-catalyzed hydrogen peroxide generation, gold NPs increase ROS production. They tend to upregulate the oxidative enzymes and, on the other side, downregulate reductive enzymes, thus creating a state of metabolic imbalance. The generated oxidative imbalance gradually impedes cell functioning [119,120].
Along with this, these nanoclusters bring about irreversible damage to the membrane by downregulating genes associated with the proteins that bind to the surface of the cell wall and subsequently upregulate membrane stability and integrity. Theories also suggest that Au NPs impede vital processes such as transcription and translation, and that they promote their collapse [121].
The antifungal effect of gold and its NPs was seen to be dependent on size and concentration. A study by Salehi et al. reported excellent antifungal action against 58 Candida spp., for example. For the experiment, the polymerase chain reaction fragment length polymorphism and HWP1 gene amplification approach methods were employed to identify Candida spp. The synthesized CAS-AuNPs were characterized using transmission electron microscopy (TEM) and the Zetasizer system, and they were evaluated by SEM. Results were found to be beneficial with a low MIC value of ≥4 µg/mL for CAS. A TEM result was also found with an average size of 20 nm, and the Zeta potential of CAS-AuNPs was found to be −38.2 mV. The statistical analyses showed that CAS-AuNPs reduce the min. IC50 against C. albicans with a p value of 0.0005 and non-albicans Candida with a value of p < 0.0001. SEM confirms the effect of AuNPs on the C. globrata cell wall structure by the formation of pores. Conclusively, thereof, the better antifungal action of CAS-Au NPs has observed the encapsulation of antifungal drugs in combination with NPs [122].
3.4. Iron and Iron-Based Nanoparticles
The use of iron for conditions like iron deficiency is well known, but the need for novel therapeutic products has led to the identification of various other indications for administering iron and iron NPs, which have worked wonders for patients. They have been found to have the potential to be anti-cancer, immunosuppressive, anticonvulsant, anti-inflammatory, antibiotic, and antifungal agents [123].
The effectiveness of iron as an antimicrobial has been observed to be tremendously good, generating toxicity via several mechanisms involving membrane depolarization, hampering membrane and cell integrity, and mediating damage through oxidative stress that, in turn, affects the homeostasis of bacteria [124].
Iron tends to enter the membrane through diffusion or endocytosis, and it facilitates the production of ROS. The generated oxidative stress and ROS lead to the direct interference of electron transport at the stage of oxidation of bacterial NAD, which is the essential co-factor involved in various cellular processes. ETC is stimulated in a way that generates superoxide ions, which subsequently target Fe-S clusters. This damage affects all necessary biochemical pathways, impeding survival [125,126].
Superparamagnetic iron oxide, through the Fenton reaction, not only accelerates the production of ROS and oxidative stress but also targets protein, lipids, and DNA. Ultimately, lipid peroxidation terminates into ferroptosis [127].
When iron enters the cell via endocytosis, the NPs accumulate in lysosomes and initiate lysosomal destabilization. From this cell, destruction is followed as growth is arrested, leading to gradual autophagy and cell death. Along with this, the NPs potentiate biocidal activity by serving as antibiotic-carrier nanosystems. This kind of physical mixture shows rapid and elaborative activity [123].
3.5. Gallium and Gallium Nanoparticles
Gallium [Ga], though bearing no natural function of its own, takes advantage of bacteria’s disability to differentiate between iron and gallium ions. Iron is required by bacteria for most of its processes of growth, maintaining metabolic balance, and replication. The reduction of ferric ions to ferrous ions helps the bacterial enzymes to avoid the state of increased ROS. Gallium uses the uptake mechanism of ferric ions to make its entry into the cell. Gallium ions, though mimicking iron, cannot be reduced as ferric ions, thus terminating redox reactions and disturbing the whole hemostasis. This hampers normal electron transfer, and it inhibits the generation of ATP, thereby disrupting the respiratory process. The halt in redox reactions impairs DNA synthesis and the ability to replicate, causing cell death [77].
Gallium can be further grafted, along with other metals, for variant applications. It can be implied in some forms like Ga-mesoporphyrin, Ga-hematoporphyrin, Ga-octaethylporphyrin, and Ga-protoporphyrin, etc. [128]. A coordination complex between maltol and gallium–gallium maltolate is demonstrated to be an anti-biofilm formation agent, which terminates the formation of biofilms by decreasing bacterial colony-forming units significantly, and, along with this, also reduces pain and inflammation at low doses [129].
Apart from bacteria, gallium also impedes and gradually halts HIV growth. Ga NPs tend to inhibit the release of Interleukins- IL-6 and Il-8, which are essential in propagating HIV. Gallium possesses the capability of inhibiting both bacterial and viral co-infection of HIV, and M. tuberculosis is the first reported formulation to bring about such an effect with great success [130].
3.6. Patent Products and Clinical Status of Metals as Antimicrobials
Several metals and metal ions, or their combinations, are in trials. Silver, extensively used for antimicrobial purposes, has more than 100 recruited trials in process. One of the interventional-randomized-single blind trials, initiated in 2018, comparing Silver NPs to an approved antimicrobial topical gel for fungal infection, expects rational, positive results by 2021 [131]. Another much-awaited result is for copper’s activity. This trial aims to appraise the efficacy of copper oxide-bearing dressing for wounds, such as for cases of diabetic foot ulcers and pressure ulcers [132]. Studies on other metals are also in trials for proper and safe application, but they still need to be concluded. Some patents relating to such combinations have been approved and issued claiming effectiveness, and a few of these are listed and elaborated in Table 3.

Table 3.
List of patents of formulations containing different metals or metal ions registered for their antimicrobial action.
4. Conclusions and Future Prospective
It is becoming increasingly difficult to treat patients and combat infectious diseases in an era of rising MDR, in which bacteria are developing resistance to many different antibiotics and leading to major morbidity and mortality consequences. Metal alternatives for addressing the microbe-mediated infectious burden have been supported by numerous reported investigations and ideas. A better knowledge of microbial metal toxicity heralds a new age for the rational design of metal-based antimicrobial therapies. Nanotechnology might be useful for treating bacterial infections in particular. Metallic NPs are an effective substitute for antibiotics, and show great promise in addressing the issue of the emergence of bacterial MDR. The applications of metallic NPs, whether in antibacterial vaccines to prevent bacterial infections, in antibiotic delivery systems to treat disease, in bacterial detection systems to produce microbial diagnostics, or in antibacterial coatings for implantable devices and medicinal materials to prevent infection and promote wound healing, are just a few of these applications. Bimetallic NPs have also shown additive and elaborative action in addition to metals and their salt oxides.
These molecules have grown in popularity as suitable, apt, and efficient candidate options to formulate into conventional therapies for patients taking proper knowledge, rational use, and suitable implications for technology, especially nanotechnology, on account of their antimicrobial effects. Recent technological advancements include the use of siderophores, antibacterial metal nanoparticles, and abiotic metal surfaces and coatings. Metal-based antimicrobial therapies have a lot of potential as antibiotic substitutes and can be considered as the lead for the development of safer, non-resistant antimicrobials. Further and extensive studies are required to explore and improve the toxicity limits for their usage, though.
Author Contributions
R.K. and K.K.: Conceived the study and wrote the first draft of the paper; M.H.A.: Funding acquisition and visualization; D.K.L., B.S. and R.K.: Data compilation; M.F.B. and B.C.: Figure Work, Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Najran University under the General Research Funding program grant code (NU/RG/MRC/12/20). The authors would also like to thank Chitkara College of Pharmacy, Chitkara University, Punjab, India, and the Faculty of Pharmacy, Philadelphia University, Amman, Jordan for the facilities and support, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work, under the General Research Funding program grant code (NU/RG/MRC/12/20). The authors would also like to thank Chitkara College of Pharmacy, Chitkara University, Punjab, India and the Faculty of Pharmacy, Philadelphia University, Amman, Jordan for the facilities and support, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azimi, S.; Lewin, G.R.; Whiteley, M. The biogeography of infection revisited. Nat. Rev. Microbiol. 2022, 20, 579–592. [Google Scholar] [CrossRef] [PubMed]
- El-Radhi, A.S. Fever in Common Infectious Diseases. In Clinical Manual of Fever in Children; El-Radhi, A.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 85–140. [Google Scholar]
- Hilton, B.; Wilson, D.J.; O’Connell, A.-M.; Ironmonger, D.; Rudkin, J.K.; Allen, N.; Oliver, I.; Wyllie, D.H. Laboratory diagnosed microbial infection in English UK Biobank participants in comparison to the general population. Sci. Rep. 2023, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Taebnia, N.; Römling, U.; Lauschke, V.M. In vitro and ex vivo modeling of enteric bacterial infections. Gut Microbes 2023, 15, 2158034. [Google Scholar] [CrossRef]
- Patil, S.A.; Nesaragi, A.R.; Rodríguez-Berrios, R.R.; Hampton, S.M.; Bugarin, A.; Patil, S.A. Coumarin Triazoles as Potential Antimicrobial Agents. Antibiotics 2023, 12, 160. [Google Scholar] [CrossRef]
- Abe, T.; Tokuda, Y.; Shiraishi, A.; Fujishima, S.; Mayumi, T.; Sugiyama, T.; Deshpande, G.A.; Shiino, Y.; Hifumi, T.; Otomo, Y. In-hospital mortality associated with the misdiagnosis or unidentified site of infection at admission. Crit. Care 2019, 23, 202. [Google Scholar] [CrossRef]
- Rosas, N.C.; Lithgow, T. Targeting bacterial outer-membrane remodelling to impact antimicrobial drug resistance. Trends Microbiol. 2022, 30, 544–552. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Thomas, S.P.; Denizer, E.; Zuffa, S.; Best, B.M.; Bode, L.; Chambers, C.D.; Dorrestein, P.C.; Liu, G.Y.; Momper, J.D.; Nizet, V. Transfer of antibiotics and their metabolites in human milk: Implications for infant health and microbiota. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022. [Google Scholar] [CrossRef]
- Mmbando, G.S. Recent Advances in Antibiotic-Free Markers; Novel Technologies to Enhance Safe Human Food Production in the World. Mol. Biotechnol. 2022, 1–12. [Google Scholar] [CrossRef]
- Madhav, A.; Will, R.C.; Mutreja, A. The Evolution of Microbial Defence Systems Against Antimicrobial Agents. Antimicrob. Resist. Glob. Chall. Future Interv. 2020, 1–31. [Google Scholar] [CrossRef]
- Gopal, A.; Yan, L.; Kashif, S.; Munshi, T.; Roy, V.A.; Voelcker, N.H.; Chen, X. Biosensors and Point-of-Care Devices for Bacterial Detection: Rapid Diagnostics Informing Antibiotic Therapy. Adv. Healthc. Mater. 2022, 11, 2101546. [Google Scholar] [CrossRef]
- Mendes, A.I.; Rebelo, R.; Aroso, I.; Correlo, V.M.; Fraga, A.G.; Pedrosa, J.; Marques, A.P. Development of an antibiotics delivery system for topical treatment of the neglected tropical disease Buruli ulcer. Int. J. Pharm. 2022, 623, 121954. [Google Scholar] [CrossRef]
- Dubey, A.; Ghosh, N.; Saxena, G.K.; Purohit, D.; Patel, S.; Singh, S. Management implications for neurotoxic effects associated with antibiotic use. Neuro-Quantology 2022, 20, 304–328. [Google Scholar]
- Xu, J.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Short-term dietary antibiotics altered the intestinal microbiota and improved the lipid metabolism in hybrid grouper fed medium and high-lipid diets. Aquaculture 2022, 547, 737453. [Google Scholar] [CrossRef]
- Mboya, E.A.; Sanga, L.A.; Ngocho, J.S. Irrational use of antibiotics in the Moshi Municipality Northern Tanzania: A cross sectional study. Pan Afr. Med. J. 2018, 31, 165. [Google Scholar] [CrossRef] [PubMed]
- Vala, A.K.; Andhariya, N.; Chudasama, B.K. Silver and gold nanoparticles: Promising candidates as antimicrobial nanomedicines. In Gold and Silver Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 329–354. [Google Scholar]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Seena, S.; Rai, A. Nanoengineering Approaches to Fight Multidrug-Resistant Bacteria. In Non-Traditional Approaches to Combat Antimicrobial Drug Resistance; Springer: Berlin/Heidelberg, Germany, 2023; pp. 221–248. [Google Scholar]
- Sharma, M.; Bassi, H.; Chauhan, P.; Thakur, P.; Chauhan, A.; Kumar, R.; Kollarigowda, R.H.; Thakur, N.K. Inhibition of the bacterial growth as a consequence of synergism of Ag and ZnO: Calendula officinalis mediated green approach for nanoparticles and impact of altitude. Inorg. Chem. Commun. 2022, 136, 109131. [Google Scholar] [CrossRef]
- Marji, S.M.; Bayan, M.F.; Jaradat, A. Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy. Biomimetics 2022, 7, 242. [Google Scholar] [CrossRef]
- Manzoor, A.; Khan, S.; Dar, A.H.; Pandey, V.K.; Shams, R.; Ahmad, S.; Jeevarathinam, G.; Kumar, M.; Singh, P.; Pandiselvam, R. Recent insights into green antimicrobial packaging towards food safety reinforcement: A review. J. Food Saf. 2023, e13046. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyam, R.; Manickaraj, S.; Anusuyadevi, P.R. Bioinspired Metal Nanoparticles for Microbicidal Activity. Bioinspired Nanomater. Synth. Emerg. Appl. 2021, 111, 36–62. [Google Scholar]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New antimicrobial strategies based on metal complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Ghezzi, D.; Boi, M.; Sassoni, E.; Valle, F.; Giusto, E.; Boanini, E.; Baldini, N.; Cappelletti, M.; Graziani, G. Customized biofilm device for antibiofilm and antibacterial screening of newly developed nanostructured silver and zinc coatings. J. Biol. Eng. 2023, 17, 18. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Mittapally, S.; Taranum, R.; Parveen, S. Metal ions as antibacterial agents. J. Drug Deliv. Ther. 2018, 8, 411–419. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front Chem 2020, 8, 412. [Google Scholar] [CrossRef]
- Andreo, J.; Ettlinger, R.; Zaremba, O.; Pena, Q.; Lächelt, U.; de Luis, R.F.; Freund, R.; Canossa, S.; Ploetz, E.; Zhu, W. Reticular nanoscience: Bottom-up assembly nanotechnology. J. Am. Chem. Soc. 2022, 144, 7531–7550. [Google Scholar] [CrossRef]
- Indiarto, R.; Indriana, L.P.A.; Andoyo, R.; Subroto, E.; Nurhadi, B. Bottom–up nanoparticle synthesis: A review of techniques, polyphenol-based core materials, and their properties. Eur. Food Res. Technol. 2022, 248, 1–24. [Google Scholar] [CrossRef]
- Chellathurai, B.J.; Anburose, R.; Alyami, M.H.; Sellappan, M.; Bayan, M.F.; Chandrasekaran, B.; Chidambaram, K.; Rahamathulla, M. Development of a Polyherbal Topical Gel for the Treatment of Acne. Gels 2023, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Paesa, M.; de Ganuza, C.R.; Alejo, T.; Yus, C.; Irusta, S.; Arruebo, M.; Sebastian, V.; Mendoza, G. Elucidating the mechanisms of action of antibiotic-like ionic gold and biogenic gold nanoparticles against bacteria. J. Colloid Interface Sci. 2023, 633, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Ayipo, Y.O.; Osunniran, W.A.; Babamale, H.F.; Ayinde, M.O.; Mordi, M.N. Metalloenzyme mimicry and modulation strategies to conquer antimicrobial resistance: Metal-ligand coordination perspectives. Coord. Chem. Rev. 2022, 453, 214317. [Google Scholar] [CrossRef]
- Salazar-Alemán, D.A.; Turner, R.J. Metal Based Antimicrobials: Uses and Challenges. In Microbial Metabolism of Metals and Metalloids; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–106. [Google Scholar]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Social behavior of antibiotic resistant mutants within Pseudomonas aeruginosa biofilm communities. Front. Microbiol. 2019, 10, 570. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent Nanotechnologies to Overcome the Bacterial Biofilm Matrix Barriers. Small 2023, 19, 2206220. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Stauber, R.H.; Siemer, S.; Becker, S.; Ding, G.-B.; Strieth, S.; Knauer, S.K. Small meets smaller: Effects of nanomaterials on microbial biology, pathology, and ecology. ACS Nano 2018, 12, 6351–6359. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Alsaleh, N.B.; Aljasham, A.T.; Tawfik, E.A.; Almutairi, M.M.; Assiri, M.A.; Alkholief, M.; Almutairi, M.M. Silver Nanoparticle-Based Combinations with Antimicrobial Agents against Antimicrobial-Resistant Clinical Isolates. Antibiotics 2022, 11, 1219. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef]
- Sarker, S.R.; Polash, S.A.; Karim, M.N.; Saha, T.; Dekiwadia, C.; Bansal, V.; Sabri, Y.; Kandjani, A.E.; Bhargava, S.K. Functionalized concave cube gold nanoparticles as potent antimicrobial agents against pathogenic bacteria. ACS Appl. Bio Mater. 2022, 5, 492–503. [Google Scholar] [CrossRef]
- Vitta, Y.; Figueroa, M.; Calderon, M.; Ciangherotti, C. Synthesis of iron nanoparticles from aqueous extract of Eucalyptus robusta Sm and evaluation of antioxidant and antimicrobial activity. Mater. Sci. Energy Technol. 2020, 3, 97–103. [Google Scholar] [CrossRef]
- Qu, C.-C.; Liang, Y.-T.; Wang, X.-Q.; Gao, S.; He, Z.-Z.; Sun, X.-Y. Gallium-Based Liquid Metal Materials for Antimicrobial Applications. Bioengineering 2022, 9, 416. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial applications and its prospects. Adv. Nanostruct. Mater. Environ. Remediat. 2019, 25, 321–355. [Google Scholar]
- Liu, J.-Q.; Li, M.; Yin, S.; Chen, X.; Li, M.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jandaik, S.; Kumar, S.; Chitkara, M.; Sandhu, I.S. Synthesis, characterisation and antimicrobial activity of manganese-and iron-doped zinc oxide nanoparticles. J. Exp. Nanosci. 2016, 11, 54–71. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Gupta, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Conducting Polymers and Their Composites. Macromol 2022, 2, 78–99. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.; Yahya, R.; Bakri, M.M.; Al Abboud, M.A.; Yahya, R.; Qanash, H.; Bazaid, A.S.; Salem, S.S. Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Convers. Biorefin. 2023, 13, 417–430. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Carrapiço, A.; Martins, M.R.; Caldeira, A.T.; Mirão, J.; Dias, L. Biosynthesis of Metal and Metal Oxide Nanoparticles Using Microbial Cultures: Mechanisms, Antimicrobial Activity and Applications to Cultural Heritage. Microorganisms 2023, 11, 378. [Google Scholar] [CrossRef]
- Vellingiri, B.; Suriyanarayanan, A.; Selvaraj, P.; Abraham, K.S.; Pasha, M.Y.; Winster, H.; Gopalakrishnan, A.V.; Singaravelu, G.; Reddy, J.K.; Ayyadurai, N. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 2022, 301, 134625. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Sokolova, I.; Stoika, R. Uptake, biodistribution, and mechanisms of toxicity of metal-containing nanoparticles in aquatic invertebrates and vertebrates. In Biomedical Nanomaterials: From Design and Synthesis to Imaging, Application and Environmental Impact; Springer: Cham, Switzerland, 2022; pp. 227–263. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Doma, A.S.; Abo-Zaid, G.A.; Badawi, M.S.; Mohamed, M.M.; Mohamed, A.S. Antibacterial activity of some nanoparticles prepared by double arc discharge method. Nano-Struct. Nano-Objects 2020, 23, 100473. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.; Islam, F.; Emran, T.B.; et al. Green metallic nanoparticles: Biosynthesis to applications. Front. Bioeng. Biotechnol. 2022, 10, 548. [Google Scholar] [CrossRef]
- Mang, C.; Li, G.; Rao, M.; Zhang, X.; Luo, J.; Jiang, T. Transition metal ions-modified birnessite toward highly efficiency photocatalytic formaldehyde oxidation under visible light irradiation. Environ. Sci. Pollut. Res. 2022, 29, 49739–49751. [Google Scholar] [CrossRef]
- Van Mieghem, T.; Delvaux, F.; Dekleermaeker, S.; Britton, S.J. Top of the Ferrous Wheel–The Influence of Iron Ions on Flavor Deterioration in Beer. J. Am. Soc. Brew. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Metryka, O.; Wasilkowski, D.; Adamczyk-Habrajska, M.; Mrozik, A. Undesirable consequences of the metallic nanoparticles action on the properties and functioning of Escherichia coli, Bacillus cereus and Staphylococcus epidermidis membranes. J. Hazard. Mater. 2023, 446, 130728. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.; Arraiano, C.M.; Andrade, J.M. Bacterial response to oxidative stress and RNA oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Busi, S.; Rajkumari, J. Chapter 15—Microbially synthesized nanoparticles as next generation antimicrobials: Scope and applications. In Nanoparticles in Pharmacotherapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 485–524. [Google Scholar]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, L.; Huang, X.; Lu, Y.-G.; Zheng, D.-L.; Feng, Y. Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology. J. Nanomater. 2022, 2022, 2063265. [Google Scholar] [CrossRef]
- Billings, C.; Anderson, D.E. Role of implantable drug delivery devices with dual platform capabilities in the prevention and treatment of bacterial osteomyelitis. Bioengineering 2022, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdam, M.; Shaabani, E.; Asghari, F.; Faridi-Majidi, R. Chitosan coated metallic nanoparticles with stability, antioxidant, and antibacterial properties: Potential for wound healing application. J. Appl. Polym. Sci. 2022, 139, 51766. [Google Scholar] [CrossRef]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2022, 7, e10254. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2022, 19, 666–678. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Wei, M.; Xiang, Q.; Wang, P.; Chen, L.; Ren, M. Ambivalent Effects of Dissolved Organic Matter on Silver Nanoparticles/Silver Ions Transformation: A Review. J. Hazard. Mater. 2022, 445, 130533. [Google Scholar] [CrossRef]
- Sarviya, N.; Mahanta, U.; Dart, A.; Giri, J.; Deshpande, A.S.; Khandelwal, M.; Bhave, M.; Kingshott, P. Biocompatible and antimicrobial multilayer fibrous polymeric wound dressing with optimally embedded silver nanoparticles. Appl. Surf. Sci. 2023, 612, 155799. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. Therapeutic Effect of Green Synthesized Silver Nanoparticles Using Erodium glaucophyllum Extract against Oral Candidiasis: In Vitro and In Vivo Study. Molecules 2022, 27, 4221. [Google Scholar] [CrossRef] [PubMed]
- Falcão, C.M.C.; Andrade, A.; Holanda, V.N.; de Figueiredo, R.C.B.Q.; Ximenes, E.A.; Gomes, A.S.L. Activity of poly (methacrylic acid)-silver nanoparticles on fluconazole-resistant Candida albicans strains: Synergistic and cytotoxic effects. J. Appl. Microbiol. 2022, 132, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.Y.; Akter, S.; Huq, M.A. Probiotic-Mediated Biosynthesis of Silver Nanoparticles and Their Antibacterial Applications against Pathogenic Strains of Escherichia coli O157:H7. Polymers 2022, 14, 1834. [Google Scholar] [CrossRef]
- Vernis, L.; El Banna, N.; Baille, D.; Hatem, E.; Heneman, A.; Huang, M.-E. Fe-S clusters emerging as targets of therapeutic drugs. Oxidative Med. Cell. Longev. 2017, 2017, 3647657. [Google Scholar] [CrossRef]
- Bayaraa, T.; Gaete, J.; Sutiono, S.; Kurz, J.; Lonhienne, T.; Harmer, J.R.; Bernhardt, P.V.; Sieber, V.; Guddat, L.; Schenk, G. Dihydroxy-Acid Dehydratases from Pathogenic Bacteria: Emerging Drug Targets to Combat Antibiotic Resistance. Chem. A Eur. J. 2022, 28, e202200927. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Sajjad, A.; Bhatti, S.H.; Zia, M. Photo excitation of silver ions during the synthesis of silver nanoparticles modify physiological, chemical, and biological properties. Part. Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Reina, G.; Peng, S.; Jacquemin, L.; Andrade, A.F.; Bianco, A. Hard Nanomaterials in Time of Viral Pandemics. ACS Nano 2020, 14, 9364–9388. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Lee, S.Y.; Siddiqi, M.Z.; Balusamy, S.R. Ecofriendly Synthesis of Silver Nanoparticles by Terrabacter humi sp. nov. and Their Antibacterial Application against Antibiotic-Resistant Pathogens. Int. J. Mol. Sci. 2020, 21, 9746. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Sun, W. Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. 2021, 93, 104937. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Ahmed, D.; Anwar, A.; Perveen, S.; Ahmed, S.; Anis, I.; Shah, M.R.; Khan, N.A. Combination therapy of clinically approved antifungal drugs is enhanced by conjugation with silver nanoparticles. Int. Microbiol. 2019, 22, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Hoang, S.A.; Bolan, N.S.; Kirkham, M.B.; Liu, J.; Xia, X.; Li, Y. Silver nanoparticles in aquatic sediments: Occurrence, chemical transformations, toxicity, and analytical methods. J. Hazard. Mater. 2021, 418, 126368. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Potent antimicrobial and antibiofilm activities of bacteriogenically synthesized gold–silver nanoparticles against pathogenic bacteria and their physiochemical characterizations. J. Biomater. Appl. 2016, 31, 366–378. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Hoang Thi, T.T. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Doherty, C.; Byrne, C.V.; Baqader, S.; El-Chami, C.; McBain, A.J.; Thomason, H.A. Anti-biofilm effects and healing promotion by silver oxynitrate-based dressings. Sci. Rep. 2023, 13, 2014. [Google Scholar] [CrossRef]
- Lemire, J.A.; Kalan, L.; Gugala, N.; Bradu, A.; Turner, R.J. Silver oxynitrate—An efficacious compound for the prevention and eradication of dual-species biofilms. Biofouling 2017, 33, 460–469. [Google Scholar] [CrossRef]
- Davies, P.; McCarty, S.; Hamberg, K. Silver-containing foam dressings with Safetac: A review of the scientific and clinical data. J. Wound Care 2017, 26, S1–S32. [Google Scholar] [CrossRef]
- Available online: https://pharmeasy.in/online-medicine-order/silverex-ionic-gel-20gm-22672 (accessed on 20 December 2022).
- Available online: https://www.rxlist.com/silvadene-drug.htm (accessed on 22 December 2022).
- Available online: https://www.1mg.com/otc/megaheal-gel-otc120932 (accessed on 25 December 2022).
- Available online: https://www.smartmedicalbuyer.com/products/silvel?variant=32458981408867¤cy=INR&utm_medium=product_sync&utm_source=google&utm_content=sag_organic&utm_campaign=sag_organic&gclid=EAIaIQobChMIuL-t3YvH7wIV-pZLBR1kcAKPEAYYAiABEgLfpPD_BwE (accessed on 25 December 2022).
- Hyder, A.; Buledi, J.A.; Nawaz, M.; Rajpar, D.B.; Orooji, Y.; Yola, M.L.; Karimi-Maleh, H.; Lin, H.; Solangi, A.R. Identification of heavy metal ions from aqueous environment through gold, Silver and Copper Nanoparticles: An excellent colorimetric approach. Environ. Res. 2022, 205, 112475. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper nanoparticles: Synthesis and characterization, physiology, toxicity and antimicrobial applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon 2020, 6, e03123. [Google Scholar] [PubMed]
- Wang, W.B.; Clapper, J.C. Antibacterial Activity of Electrospun Polyacrylonitrile Copper Nanoparticle Nanofibers on Antibiotic Resistant Pathogens and Methicillin Resistant Staphylococcus aureus (MRSA). Nanomaterials 2022, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, S.; Han, A.; Yang, Y.; Fang, G.; Liu, J.; Wang, S. Intracellular Fenton reaction based on mitochondria-targeted copper (ii)–peptide complex for induced apoptosis. J. Mater. Chem. B 2019, 7, 4008–4016. [Google Scholar] [CrossRef]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef]
- Abbas, A.H.; Fairouz, N.Y. Characterization, biosynthesis of copper nanoparticles using ginger roots extract and investigation of its antibacterial activity. Mater. Today Proc. 2022, 61, 908–913. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, D.; Chudasama, B. Antibacterial activity of colloidal copper nanoparticles against Gram-negative (Escherichia coli and Proteus vulgaris) bacteria. Lett. Appl. Microbiol. 2022, 74, 695–706. [Google Scholar] [CrossRef]
- Carmo, P.H.F.d.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.d.; Junqueira, J.C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Judan Cruz, K.G.; Alfonso, E.D.; Fernando, S.I.D.; Watanabe, K. Candida albicans biofilm inhibition by ethnobotanicals and ethnobotanically-synthesized gold nanoparticles. Front. Microbiol. 2021, 12, 665113. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Fattahi, A. Susceptibility Pattern of Caspofungin-Coated Gold Nanoparticles Against Clinically Important Candida Species. Adv. Pharm. Bull. 2021, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Mandić-Rajčević, S.; Pantic, I. Iron-based nanoparticles and their potential toxicity: Focus on oxidative stress and apoptosis. Chem. Biol. Interact. 2020, 316, 108935. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Larrea, G.; Osial, M.; Jackowska, K.; Krysinski, P. Photocatalytic degradation of antibiotics by superparamagnetic iron oxide nanoparticles. Tetracycline case. Catalysts 2021, 11, 1243. [Google Scholar] [CrossRef]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of Gallium and Gallium-Based Compounds as Antimicrobial Agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Cereceres, S.; Lan, Z.; Bryan, L.; Whitely, M.; Wilems, T.; Greer, H.; Alexander, E.R.; Taylor, R.J.; Bernstein, L.; Cohen, N. Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng. 2019, 3, 026102. [Google Scholar] [CrossRef]
- Alamri, H.M. Synthesis, Characterization and Biological Applications of Iron and Gallium Nanomaterials as New-Generation Antibacterial Drugs. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2020. [Google Scholar]
- Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT03752424?term=silver+as+antimicrobial&draw=2 (accessed on 15 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04634838 (accessed on 15 January 2023).
- Yang, W.-D. Medical Needles Having Antibacterial and Painless Function. WO2006088288A1, 5 January 2006. [Google Scholar]
- Willoughby, A.J.M. Dental Uses of Silver Hydrosol. WO2010143075A2, 10 June 2010. [Google Scholar]
- Jiang, X.; Zhao, Y.; Tian, Y.; Liu, W.; Zhang, W. Gold Nanoparticles Modified by Aminopyrimide, Preparation Method and Use Thereof. WO2011106993A1, 9 September 2011. [Google Scholar]
- Seville, S.; Gottardello, P.; Fitzgerald Daniel, J. Antimicrobial Formulation Containing Quinone and A Copper Salt. GB2435419A, 20 February 2007. [Google Scholar]
- Holladay, R.J.; Christensen, H.; Moeller, W.D. Treatment of Humans with Colloidal Silver Composition. CA2526150C, 15 August 2003. [Google Scholar]
- Bradley, E.B.; Pradeep, S. Gallium Inhibits Biofilm Formation. EP1691614B1, 3 December 2004. [Google Scholar]
- Gan, L.; Scott, M.L.; Jani, S.C.; Whitsitt, L.S. Gradient Antimicrobial Coating for Medical Implants. CA2716896C, 9 August 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).