Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database
Abstract
:1. Introduction
2. Results
2.1. Descriptive Analysis
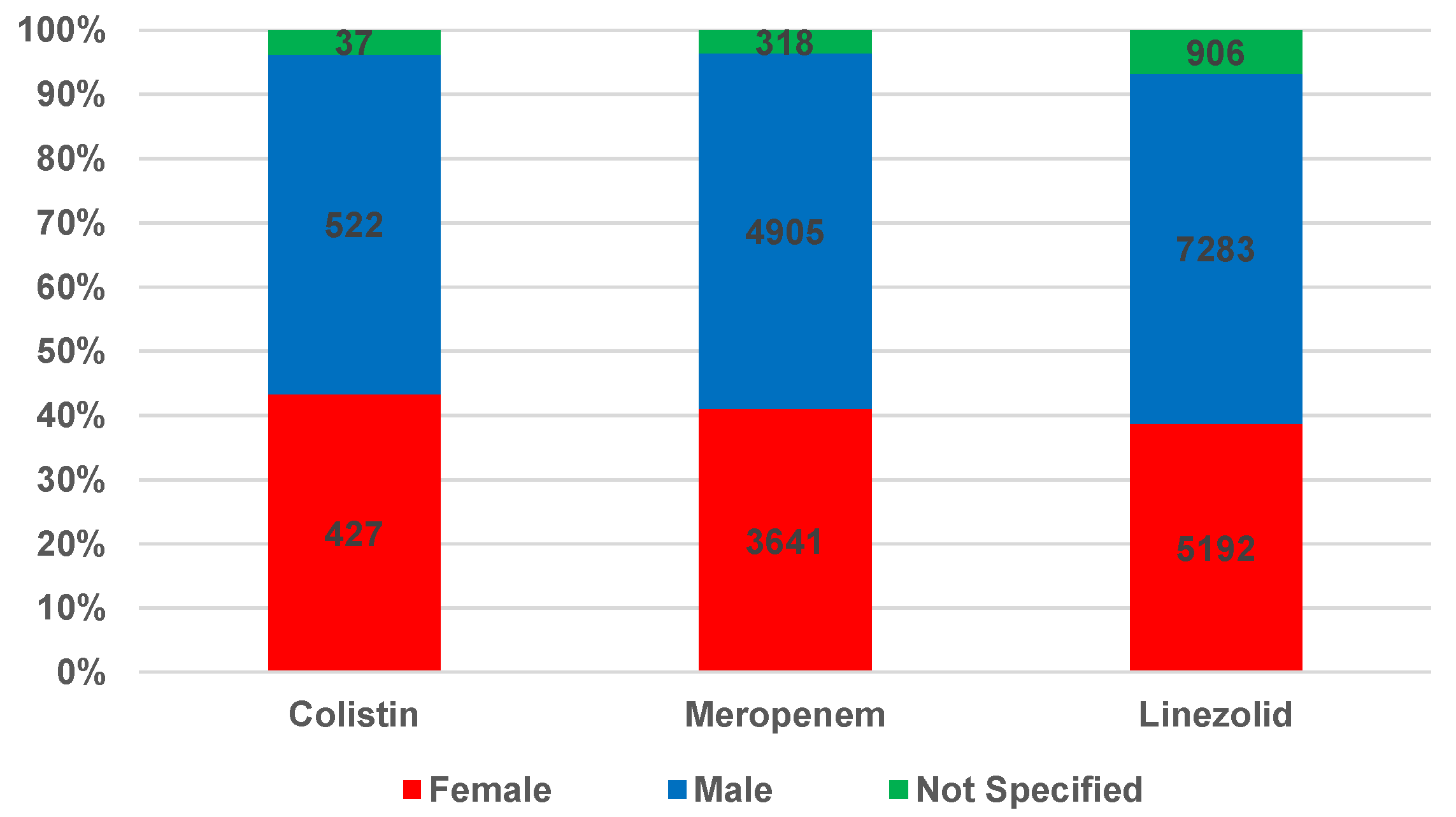
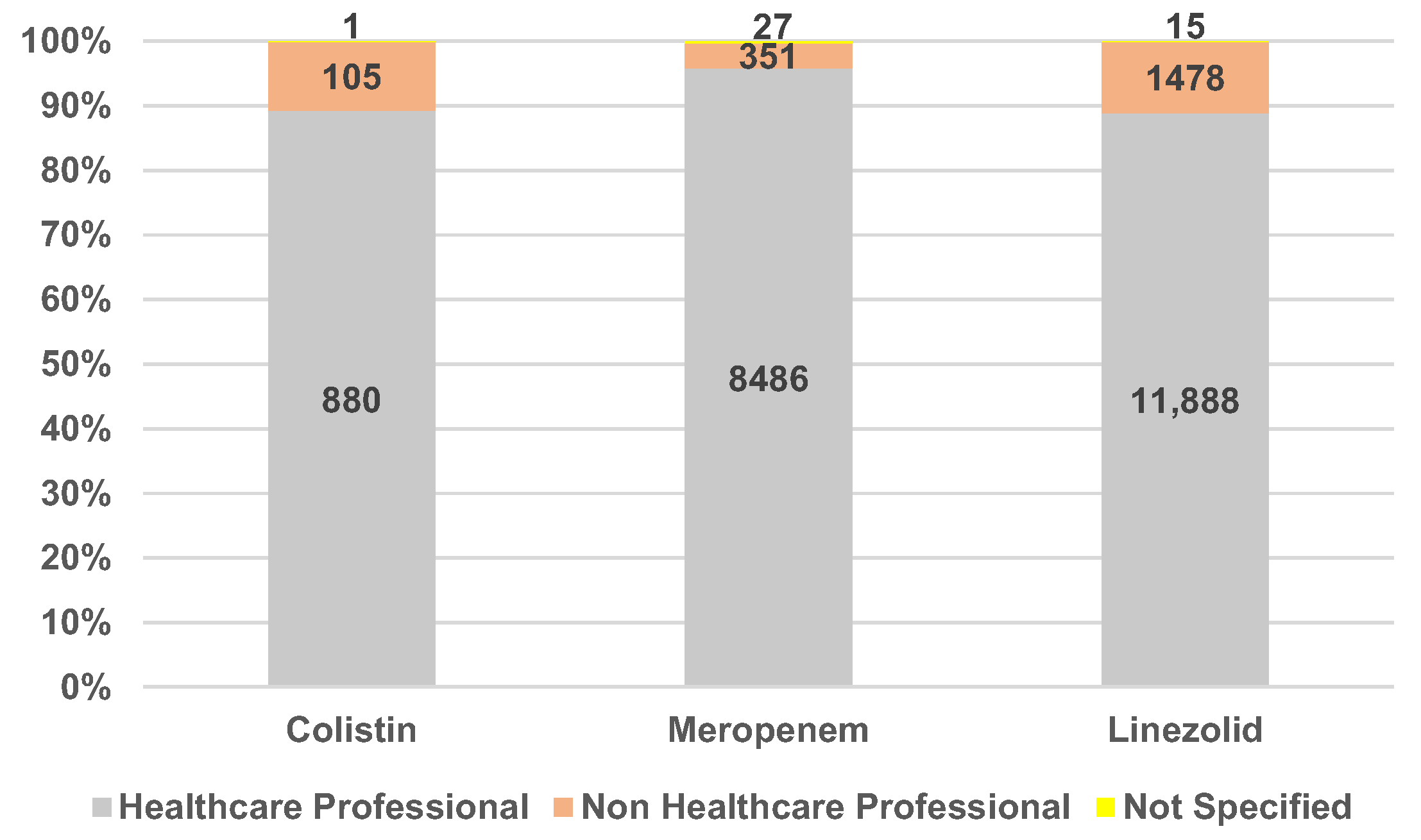
2.1.1. Characteristics of Individual Case Safety Reports
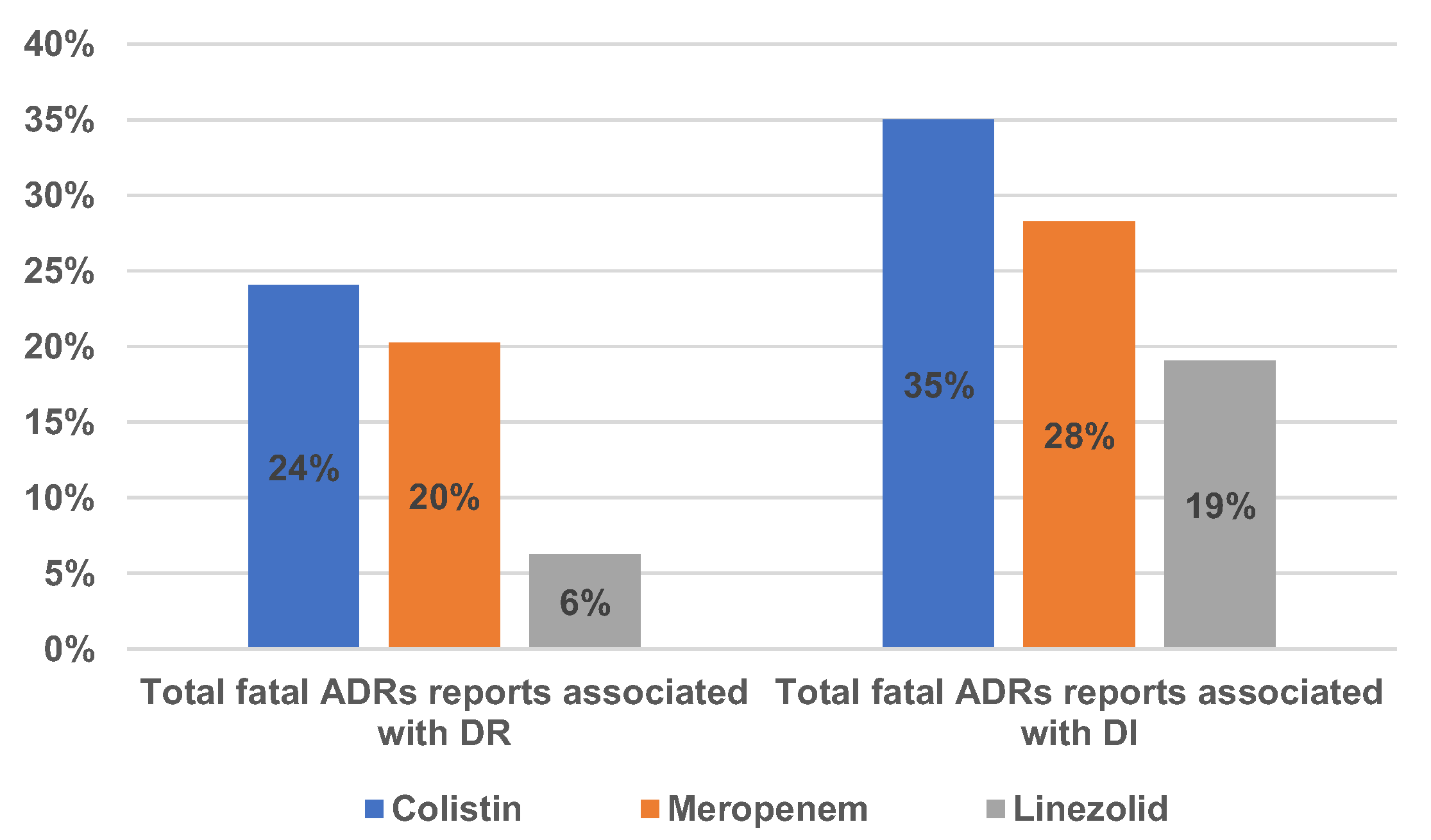
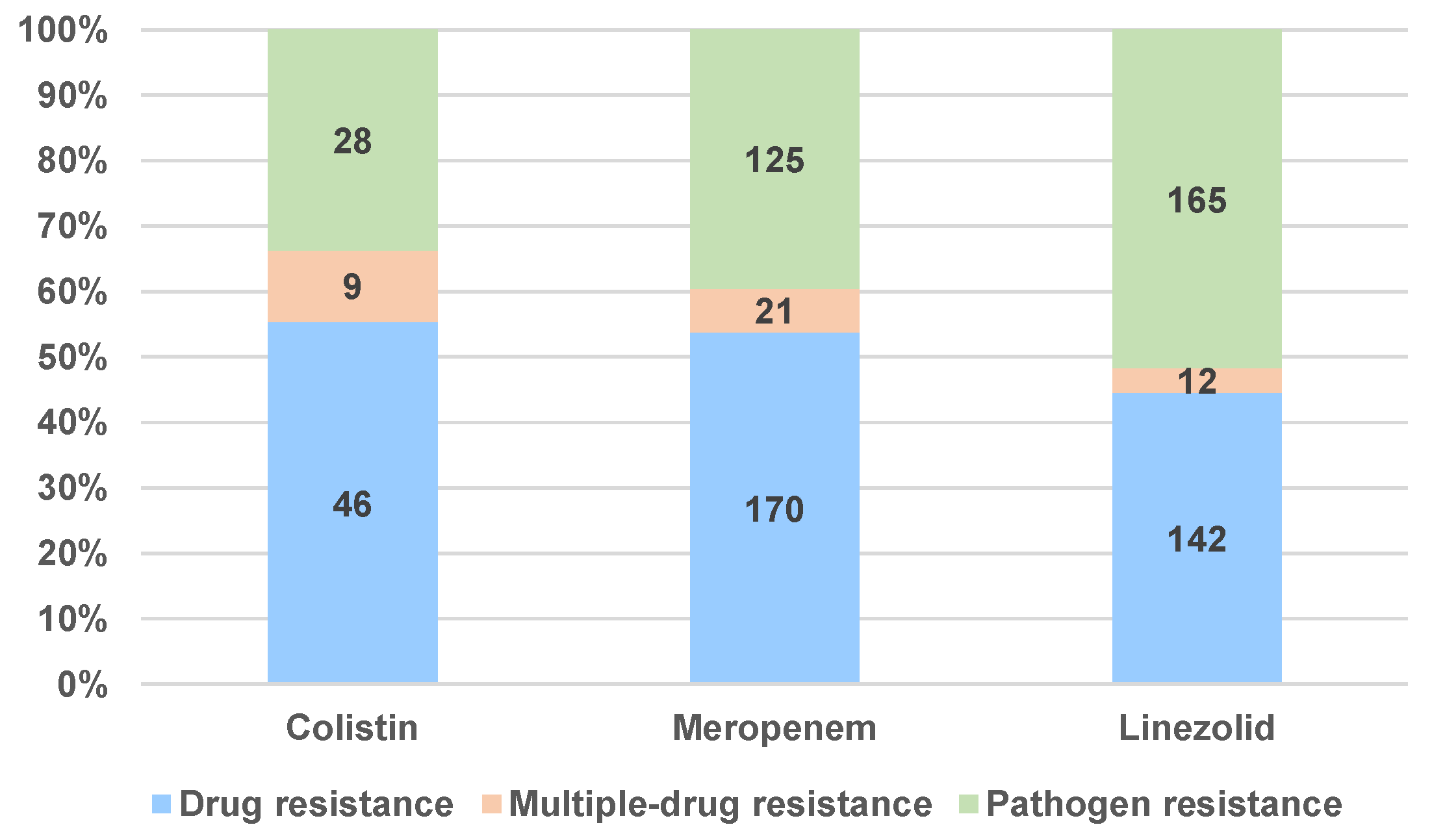
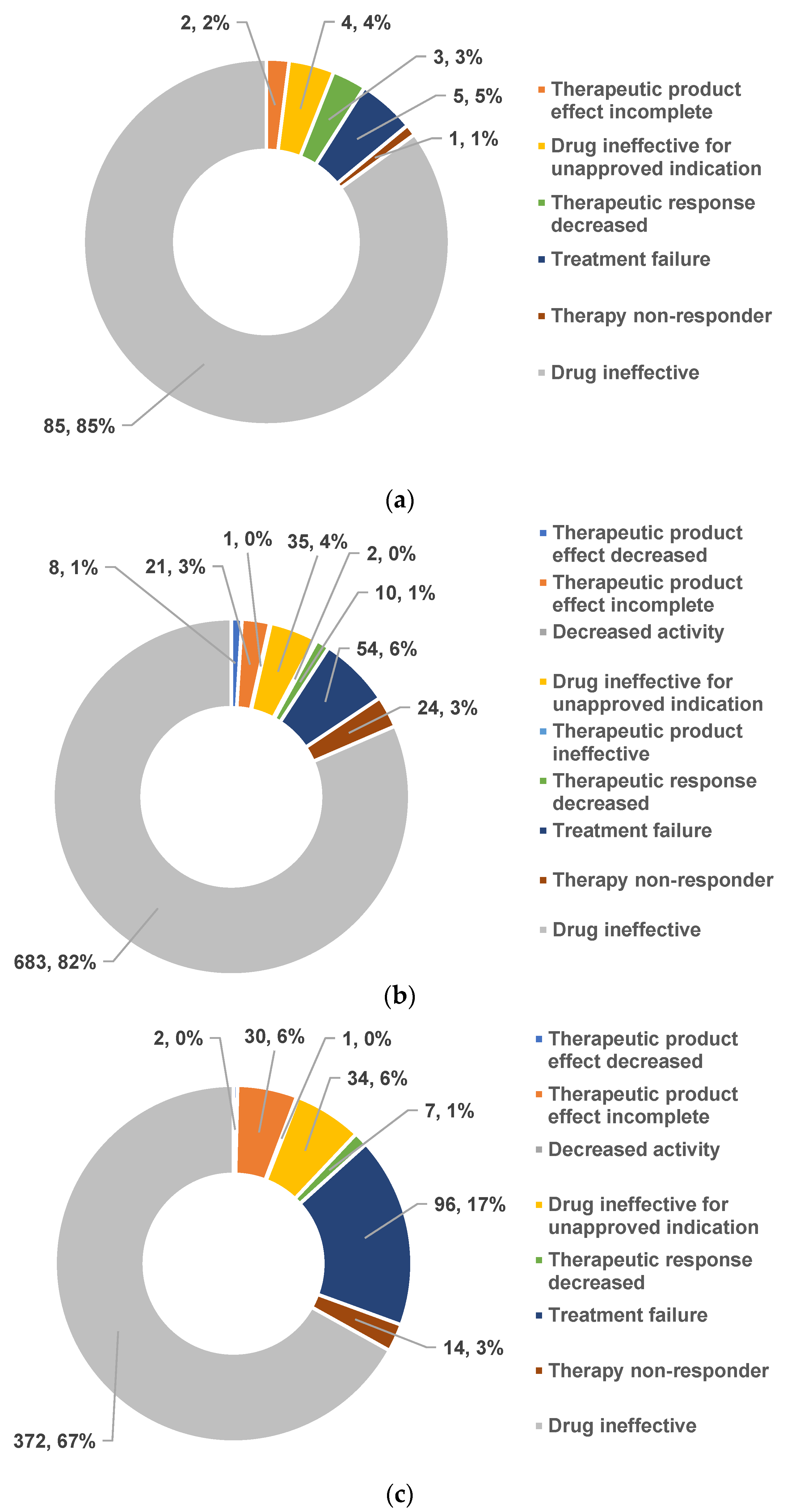
2.1.2. Drug Resistance and Drug Ineffectiveness
2.2. Disproportionality Analysis
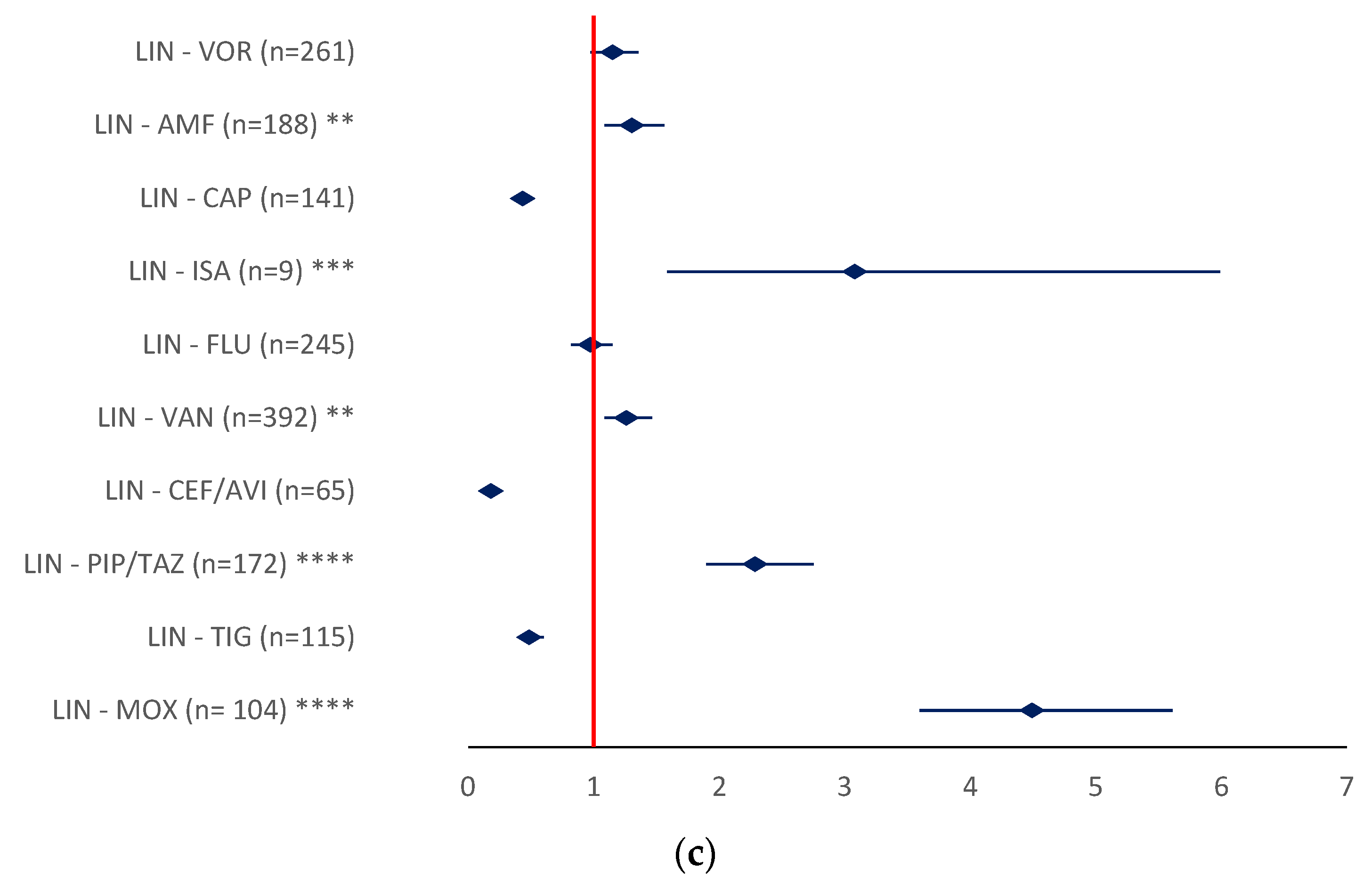
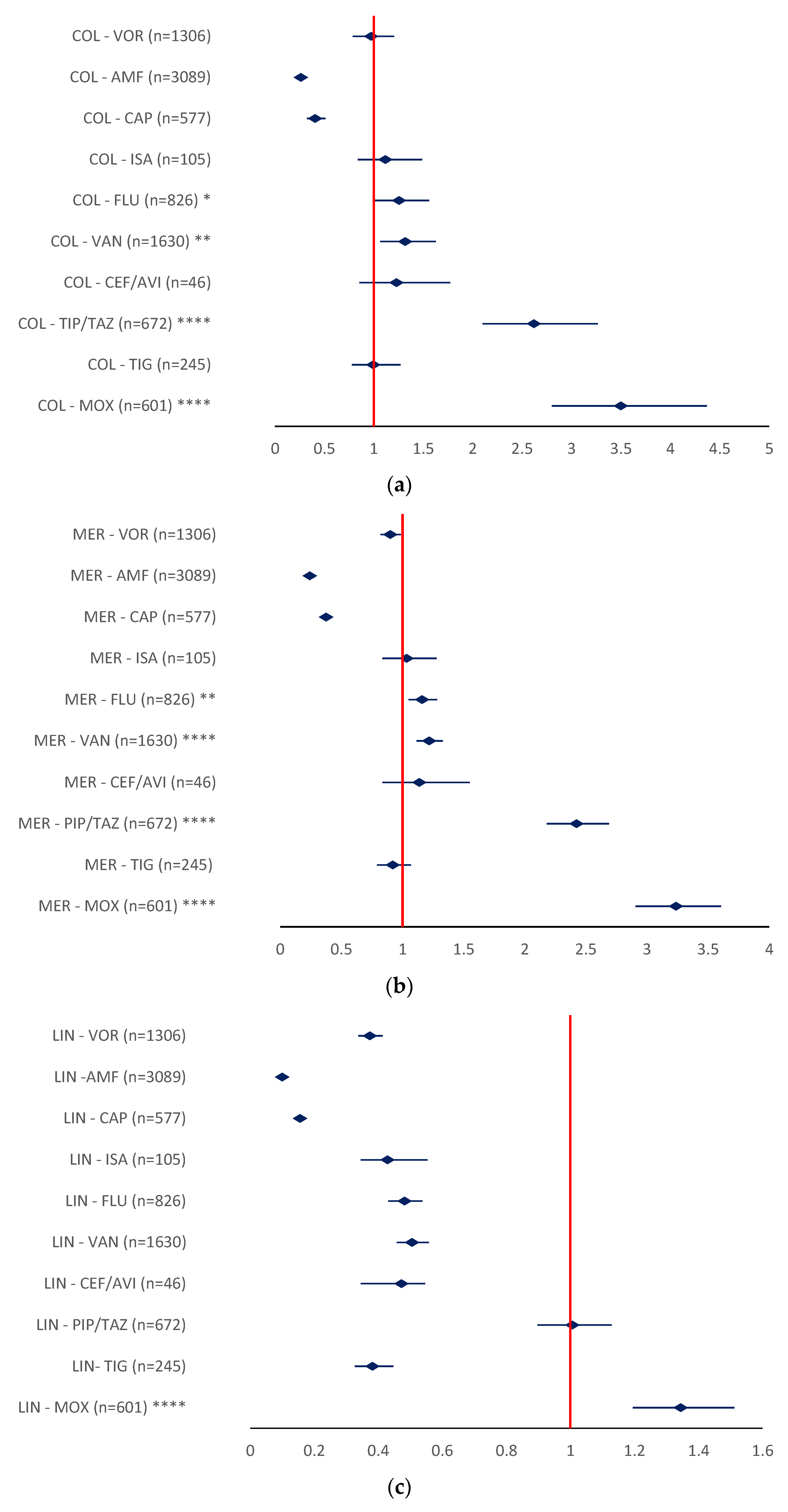
2.2.1. Drug Resistance
- MER-MOX (ROR 6.8006, 95% CI 5.4411–8.4999), MER-PIP/TAZ (ROR 3.4591, 95% CI 2.8675–4.1727), and MER-ISA (ROR 4.662, 95% CI 2.3897–9.0695) (Figure 10b).
- LIN-MOX (ROR 4.927, 95% CI 3.5967–5.6119), LIN-PIP/TAZ (ROR 2.2852, 95%CI 1.8957–2.7546), and LIN-ISA (ROR 3.0799, 95% CI 1.5835–5.9903) (Figure 10c).
2.2.2. Drug Ineffectiveness
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design
4.2. Materials
4.3. Data Analysis
- ROR = reporting odds ratio
- a = evaluated ADR for targeted drug
- b = other ADRs for targeted drug
- c = evaluated ADR for the drug used for comparison
- d = other ADRs for the drug used for comparison
- CI = confidence interval
- SE = standard error
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | adverse reaction |
| AMF | amphotericin |
| CAP | caspofungin |
| CEF/AVI | ceftazidime/avibactam |
| CI | confidence interval |
| COL | colistin |
| DI | drug ineffectiveness |
| DR | drug resistance |
| EEA | European Economic Area |
| EMA | European Medicines Agency |
| EV | EudraVigilance |
| FLU | fluconazole |
| ICSR | Individual Case Safety Report |
| ICU | intensive care unit |
| ISA | isavuconazole |
| LIN | linezolid |
| LPS | lipopolysaccharide |
| MER | meropenem |
| MOX | moxifloxacin |
| PBP | penicillin-binding proteins |
| PIP/TAZ | piperacillin/tazobactam |
| PTs | preferred terms |
| ROR | reporting odds ratio |
| TIG | tigecycline |
| VAN | vancomycin |
| VOR | voriconazole |
References
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic Changes in Critical Illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Morales Castro, D.; Dresser, L.; Granton, J.; Fan, E. Pharmacokinetic Alterations Associated with Critical Illness. Clin. Pharmacokinet. 2023, 62, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Vlasselaers, D.; Spriet, I.; Allegaert, K. Pharmacokinetics of Antibiotics in Pediatric Intensive Care: Fostering Variability to Attain Precision Medicine. Antibiotics 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Barton, G.; Fischer, A. Pharmacokinetic Considerations and Dosing Strategies of Antibiotics in the Critically Ill Patient. J. Intensive Care Soc. 2015, 16, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic Issues for Antibiotics in the Critically Ill Patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Coculescu, B.I. Antimicrobial Resistance Induced by Genetic Changes. J. Med. Life 2009, 2, 114. [Google Scholar]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef]
- Chis, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Tincu, A.L.; Gligor, F.G.; Mures, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Mohd Sazlly Lim, S.; Lipman, J.; Roberts, J.A. A Personalised Approach to Antibiotic Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. Anaesth. Crit. Care Pain Med. 2021, 40, 100970. [Google Scholar] [CrossRef] [PubMed]
- Bue, M.; Sou, T.; Okkels, A.S.L.; Hanberg, P.; Thorsted, A.; Friberg, L.E.; Andersson, T.L.; Öbrink-Hansen, K.; Christensen, S. Population Pharmacokinetics of Piperacillin in Plasma and Subcutaneous Tissue in Patients on Continuous Renal Replacement Therapy. Int. J. Infect. Dis. 2020, 92, 133–140. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic Resistance--What’s Dosing Got to Do with It? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Mahadevaiah, S. Healthcare-Associated Infection in Intensive Care Units: Overall Analysis of Patient Criticality by Acute Physiology and Chronic Health Evaluation IV Scoring and Pathogenic Characteristics. Indian J. Crit. Care Med. 2020, 24, 252–257. [Google Scholar] [CrossRef]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Rosso-Fernández, C.; Montero, J.G.; Antonelli, M.; Dimopoulos, G.; Cisneros, J.M.; Gallmore, P.R.; Bonastre, J.; González, J.C.M.; Díaz-Miguel, R.O.; García, A.E.; et al. Safety and Efficacy of Colistin versus Meropenem in the Empirical Treatment of Ventilator-Associated Pneumonia as Part of a Macro-Project Funded by the Seventh Framework Program of the European Commission Studying off-Patent Antibiotics: Study Protocol for a Randomized Controlled Trial. Trials 2015, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des. Devel. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, J.M.; Rosso-Fernández, C.M.; Roca-Oporto, C.; De Pascale, G.; Jiménez-Jorge, S.; Fernández-Hinojosa, E.; Matthaiou, D.K.; Ramírez, P.; Díaz-Miguel, R.O.; Estella, A.; et al. Colistin versus Meropenem in the Empirical Treatment of Ventilator-Associated Pneumonia (Magic Bullet Study): An Investigator-Driven, Open-Label, Randomized, Noninferiority Controlled Trial. Crit. Care 2019, 23, 383. [Google Scholar] [CrossRef]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem Use and Therapeutic Drug Monitoring in Clinical Practice: A Literature Review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of Action of Carbapenem Resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef]
- Meletis, G. Carbapenem Resistance: Overview of the Problem and Future Perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef]
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM Profile: Carbapenems: A Broad-Spectrum Antibiotic. J. Med. Microbiol. 2021, 70, 001462. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef]
- Roman, M.D.; Bocea, B.A.; Ion, N.I.C.; Vorovenci, A.E.; Dragomirescu, D.; Birlutiu, R.M.; Birlutiu, V.; Fleaca, S.R. Are There Any Changes in the Causative Microorganisms Isolated in the Last Years from Hip and Knee Periprosthetic Joint Infections? Antimicrobial Susceptibility Test Results Analysis. Microorganisms 2023, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Jones, R.N. Oxazolidinones A Review. Drugs 2000, 59, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.G.; Gold, H.S. Antimicrobial Resistance to Linezolid. Clin. Infect. Dis. 2004, 39, 1010–1015. [Google Scholar] [CrossRef]
- Long, K.S.; Vester, B. Resistance to Linezolid Caused by Modifications at Its Binding Site on the Ribosome. Antimicrob. Agents Chemother. 2012, 56, 603–612. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Justo, J.A.; Bosso, J.A. Adverse Reactions Associated with Systemic Polymyxin Therapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 28–33. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Boyce, J.D. Mechanisms of Polymyxin Resistance. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1145, pp. 55–71. ISBN 9783030163730. [Google Scholar]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.T.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the Last-Resort Antibiotics: Mode of Action, Resistance Emergence, and Potential Solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef] [PubMed]
- Upsala Monitoring Center—Members of the WHO Programme for International Drug Monitoring. Available online: https://who-umc.org/about-the-who-programme-for-international-drug-monitoring/member-countries/ (accessed on 25 February 2023).
- Habarugira, J.M.V.; Figueras, A. Pharmacovigilance Network as an Additional Tool for the Surveillance of Antimicrobial Resistance. Pharmacoepidemiol. Drug Saf. 2021, 30, 1123–1131. [Google Scholar] [CrossRef]
- Truong, C.B.; Durham, S.H.; Qian, J. Comparisons of Adverse Event Reporting for Colistin versus Polymyxin B Using the US Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin. Drug Saf. 2021, 20, 603–609. [Google Scholar] [CrossRef]
- Delcher, C.; Moga, D.; Li, Y.; Muñoz, M.; Sohn, M.; Bae, J. Pharmacoepidemiology and Pharmacovigilance. In Remington; Elsevier: Amsterdam, The Netherlands, 2021; pp. 899–913. ISBN 9780128200070. [Google Scholar]
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action; World Health Organization: Geneva, Switzerland, 2012; pp. 1–119.
- EudraVigilance—European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/ (accessed on 3 January 2023).
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Ferreira, J.; Placido, A.I.; Afreixo, V.; Ribeiro-Vaz, I.; Roque, F.; Herdeiro, M.T. Descriptive Analysis of Adverse Drug Reactions Reports of the Most Consumed Antibiotics in Portugal, Prescribed for Upper Airway Infections. Antibiotics 2022, 11, 477. [Google Scholar] [CrossRef]
- Elshamy, A.A.; Aboshanab, K.M. A Review on Bacterial Resistance to Carbapenems: Epidemiology, Detection and Treatment Options. Futur. Sci. OA 2020, 6, FSO438. [Google Scholar] [CrossRef]
- WHO Access, Watch, Reserve (AWaRe). Classification of Antibiotics for Evaluation and Monitoring of Use. 2021. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 25 February 2023).
- Thirot, H.; Briquet, C.; Frippiat, F.; Jacobs, F.; Holemans, X.; Henrard, S.; Tulkens, P.M.; Spinewine, A.; Van Bambeke, F. Clinical Use and Adverse Drug Reactions of Linezolid: A Retrospective Study in Four Belgian Hospital Centers. Antibiotics 2021, 10, 530. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Palmer, K.; Vetrano, D.L.; Carfì, A.; Onder, G. Adverse Drug Reactions in Older Adults: A Narrative Review of the Literature. Eur. Geriatr. Med. 2021, 12, 463–473. [Google Scholar] [CrossRef]
- Creagh-Brown, B.; Green, S. Increasing Age of Patients Admitted to Intensive Care, and Association between Increased Age and Greater Risk of Post-ICU Death. Crit. Care 2014, 18, P56. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Lat, T.I.; McGraw, M.K.; White, H.D. Gender Differences in Critical Illness and Critical Care Research. Clin. Chest Med. 2021, 42, 543–555. [Google Scholar] [CrossRef]
- Habarugira, J.M.V.; Härmark, L.; Figueras, A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics 2021, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Uzairue, L.I.; Rabaan, A.A.; Adewumi, F.A.; Okolie, O.J.; Folorunso, J.B.; Bakhrebah, M.A.; Garout, M.; Alfouzan, W.A.; Halwani, M.A.; Alamri, A.A.; et al. Global Prevalence of Colistin Resistance in Klebsiella Pneumoniae from Bloodstream Infection: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Emergence of Colistin-Resistant Bacteria in Humans without Colistin Usage: A New Worry and Cause for Vigilance. Int. J. Antimicrob. Agents 2016, 47, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The Negative Impact of Antibiotic Resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.-M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef]
- Medicines Agency, E. Guidelines on Good Pharmacovigilance Practices (GVP)—Introductory Cover Note, Last Updated with Chapter P.IV on Pharmacovigilance for the Paediatric Population Finalised Post-Public Consultation. 2018. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guidelines-good-pharmacovigilance-practices-gvp-introductory-cover-note-last-updated-chapter-piv_en.pdf (accessed on 8 January 2023).
- Screening for Adverse Reactions in EudraVigilance. Available online: www.ema.europa.eu/contact (accessed on 4 February 2023).
- MedCalc Software Ltd. Odds Ratio Calculator, Version 22.001. Available online: https://www.medcalc.org/calc/odds_ratio.php (accessed on 14 May 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. https://doi.org/10.3390/antibiotics12050918
Vintila BI, Arseniu AM, Butuca A, Sava M, Bîrluțiu V, Rus LL, Axente DD, Morgovan C, Gligor FG. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics. 2023; 12(5):918. https://doi.org/10.3390/antibiotics12050918
Chicago/Turabian StyleVintila, Bogdan Ioan, Anca Maria Arseniu, Anca Butuca, Mihai Sava, Victoria Bîrluțiu, Luca Liviu Rus, Dan Damian Axente, Claudiu Morgovan, and Felicia Gabriela Gligor. 2023. "Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database" Antibiotics 12, no. 5: 918. https://doi.org/10.3390/antibiotics12050918
APA StyleVintila, B. I., Arseniu, A. M., Butuca, A., Sava, M., Bîrluțiu, V., Rus, L. L., Axente, D. D., Morgovan, C., & Gligor, F. G. (2023). Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics, 12(5), 918. https://doi.org/10.3390/antibiotics12050918








