Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species
Abstract
1. Introduction
2. Results
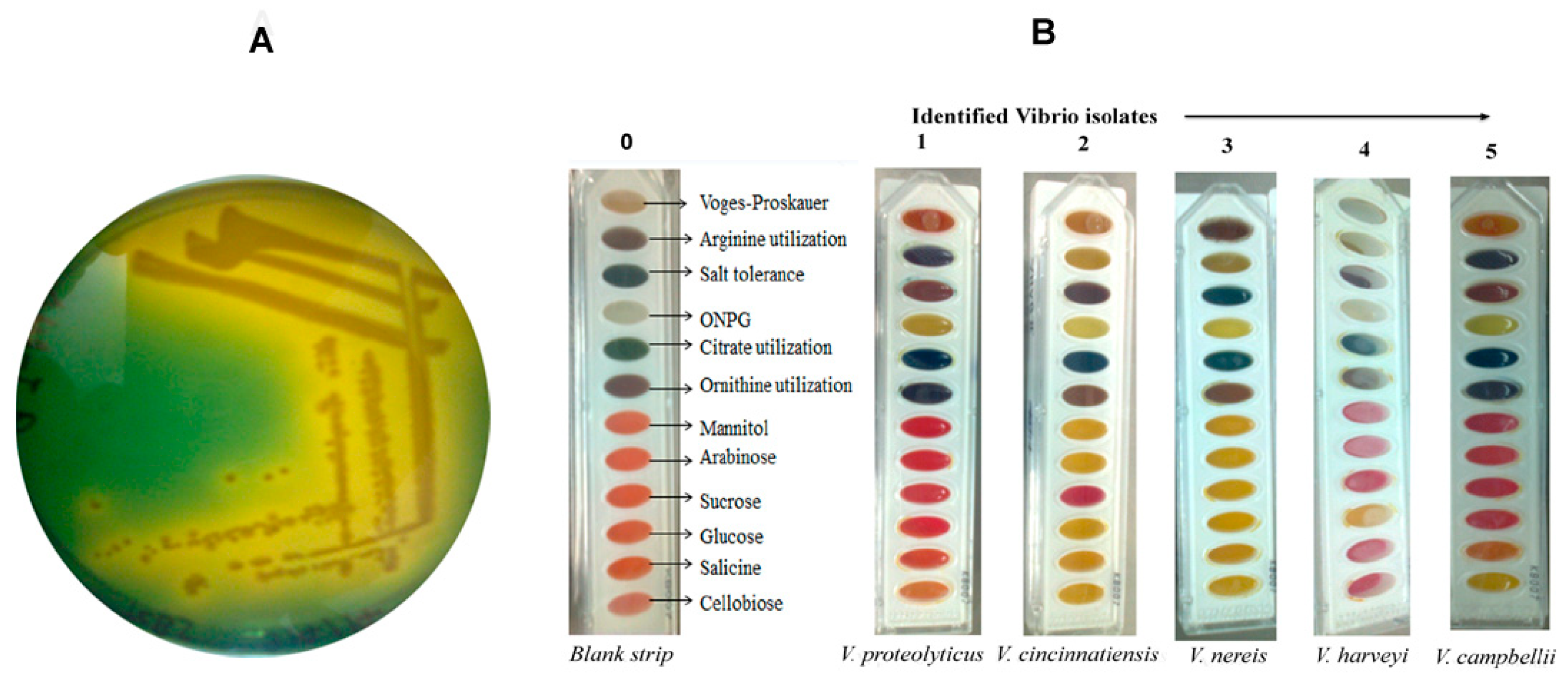
2.1. Isolation and Biochemical Identification of Vibrio Isolates
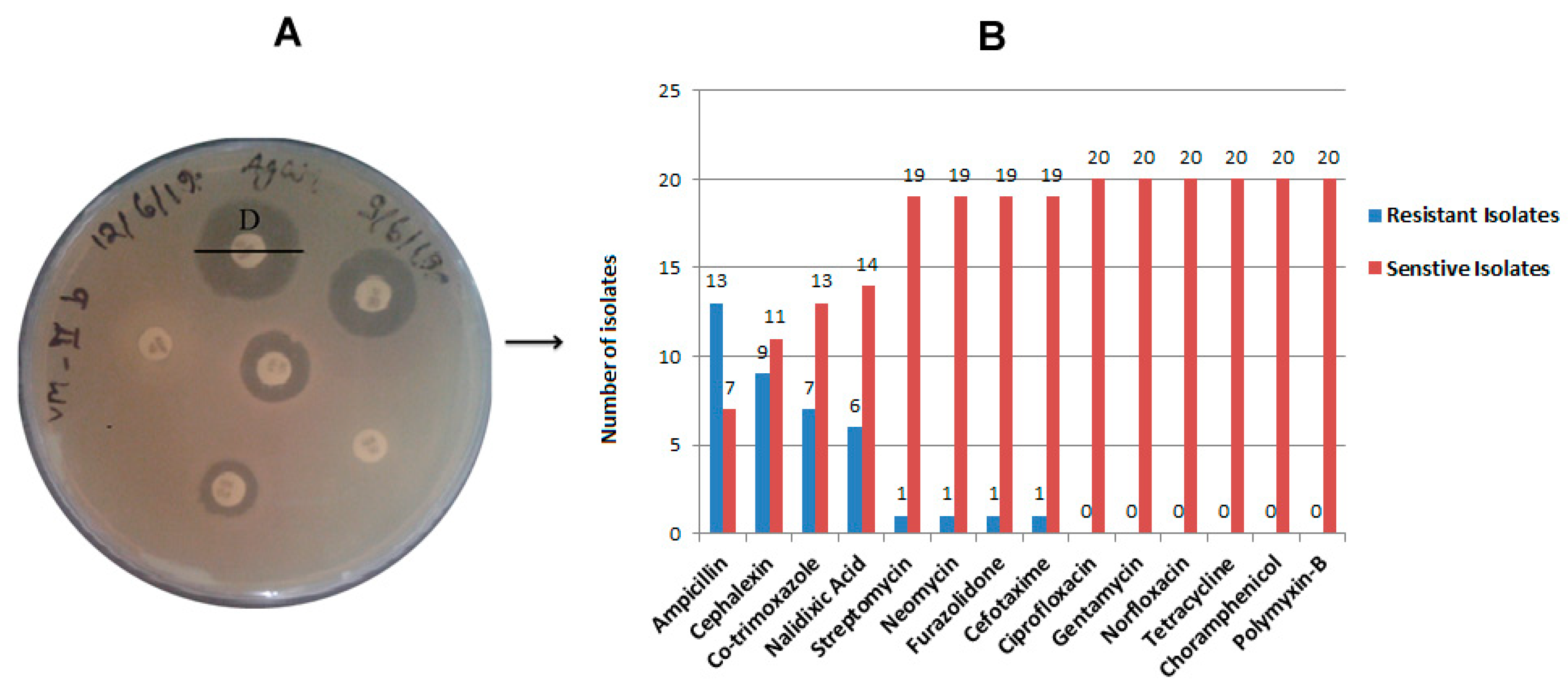
2.2. Antibiotic Resistance and Susceptibility Assay
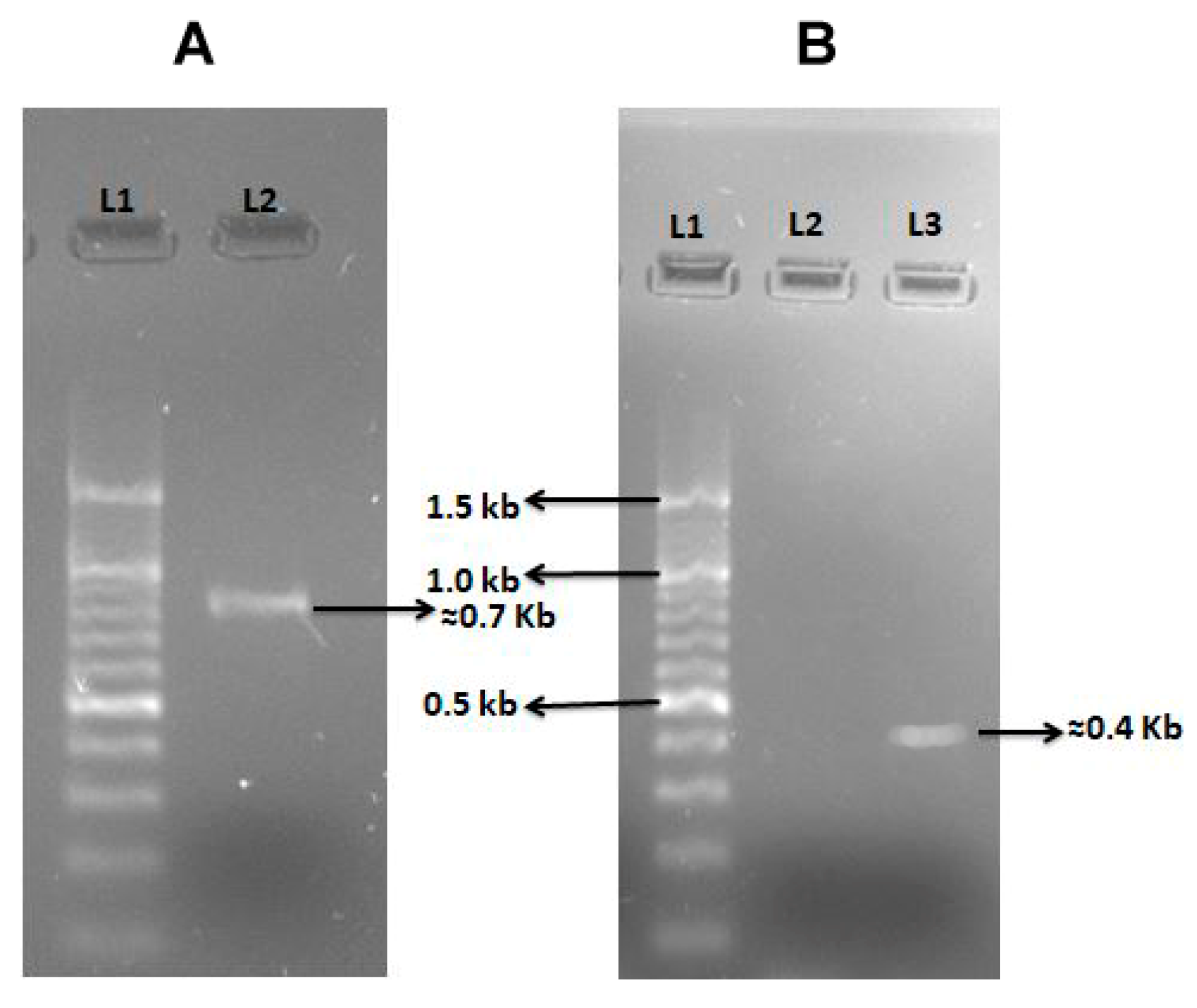
2.3. Virulence Profile of Vibrio Isolates
2.4. Phage Induction Assessment by Mitomycin C
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Isolation of Bacterial Colonies
4.2. Identification of Vibrio Isolates by Biochemical Tests Assay
4.3. Antibiotic Susceptibility Testing
4.4. Virulent Genes Amplification by Polymerase Chain Reaction (PCR)
4.5. Induction of Vibriophage by Mitomycin C
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzembo, B.A.; Kitahara, K.; Debnath, A.; Ohno, A.; Okamoto, K.; Miyoshi, S.I. Cholera Outbreaks in India, 2011–2020: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [PubMed]
- Brehm, T.T.; Dupke, S.; Hauk, G.; Fickenscher, H.; Rohde, H.; Berneking, L. Non-cholera Vibrio species-currently still rare but growing danger of infection in the North Sea and the Baltic Sea. Internist 2021, 62, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Nelson, K.; Morcrette, H.; Morcrette, C.; Preston, J.; Helmer, L.; Titball, R.W.; Butler, C.S.; Wagley, S. The increased prevalence of Vibrio species and the first reporting of Vibrio jasicida and Vibrio rotiferianus at UK shellfish sites. Water Res. 2022, 211, 117942. [Google Scholar] [CrossRef]
- Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and antibiotic resistance characteristics of Vibrio isolates from rustic environmental freshwaters. Front. Cell. Infect. Microbiol. 2021, 11, 765. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The complex relationship between virulence and antibiotic resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Soucy, S.M.; Huang, J.; Gogarten, J.P. Horizontal gene transfer: Building the web of life. Nat. Rev. Genet. 2015, 16, 472–482. [Google Scholar] [CrossRef]
- Hazen, T.H.; Pan, L.; Gu, J.-D.; Sobecky, P.A. The contribution of mobile genetic elements to the evolution and ecology of Vibrios. FEMS Microbiol. Ecol. 2010, 74, 485–499. [Google Scholar] [CrossRef]
- Ruwandeepika, H.; Defoirdt, T.; Bhowmick, P.; Shekar, M.; Bossier, P.; Karunasagar, I. Presence of typical and atypical virulence genes in vibrio isolates belonging to the Harveyi clade. J. Appl. Microbiol. 2010, 109, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Z.; Yu, K.; Wang, M.; Gao, H.; Bai, X.; Jiang, M.; Wang, D. Distribution and Molecular Characteristics of Vibrio Species Isolated from Aquatic Environments in China, 2020. Microorganisms 2022, 10, 2007. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Diagnosis and Treatment. 2023. Available online: https://www.cdc.gov/vibrio/diagnosis.html#:~:text=Treatment%20is%20not%20necessary%20in,in%20severe%20or%20prolonged%20illnesses (accessed on 8 January 2023).
- World Health Organization (WHO). Cholera. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/cholera?gclid=CjwKCAjwpuajBhBpEiwA_ZtfhRtJtsyyYxOn61c8unDkw_YvdjmCsJjbTBh_dC3OKsFrUUhC3CcFjhoCN-sQAvD_BwE (accessed on 8 January 2023).
- Yuan, X.H.; Li, Y.M.; Vaziri, A.Z.; Kaviar, V.H.; Jin, Y.; Jin, Y.; Maleki, A.; Omidi, N.; Kouhsari, E. Global status of antimicrobial resistance among environmental isolates of Vibrio cholerae O1/O139: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 62. [Google Scholar] [CrossRef]
- Gao, H.; Xu, J.; Lu, X.; Li, J.; Lou, J.; Zhao, H.; Diao, B.; Shi, Q.; Zhang, Y.; Kan, B. Expression of hemolysin is regulated under the collective actions of HapR, Fur, and HlyU in Vibrio cholerae El Tor serogroup O1. Front. Microbiol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Sharma, A.; Chaturvedi, A.N. Prevalence of virulence genes (ctxA, stn, OmpW and tcpA) among non-O1 Vibrio cholerae isolated from fresh water environment. Int. J. Hyg. Environ. Health 2006, 209, 521–526. [Google Scholar] [CrossRef]
- Keasler, S.P.; Hall, R.H. Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet 1993, 341, 1661. [Google Scholar] [CrossRef]
- Ogawa, A.; Takeda, T. The gene encoding the heat-stable enterotoxin of Vibrio cholerae is flanked by 123-base pair direct repeats. Microbiol. Immunol. 1993, 37, 607–616. [Google Scholar] [CrossRef]
- Madhusudana, R.B.; Surendran, P.K. Detection of ctx gene positive non-O1/non-O139 V. cholerae in shrimp aquaculture environments. J. Food Sci. Technol. 2013, 50, 496–504. [Google Scholar] [CrossRef]
- Nandi, B.; Nandy, R.K.; Mukhopadhyay, S.; Nair, G.B.; Shimada, T.; Ghose, A.C. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 2000, 38, 4145–4151. [Google Scholar] [CrossRef]
- Rivera, I.N.; Chun, J.; Huq, A.; Sack, R.B.; Colwell, R.R. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 2001, 67, 2421–2429. [Google Scholar] [CrossRef]
- Singh, D.V.; Isac, S.R.; Colwell, R.R. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae and Vibrio mimicus. J. Clin. Microbiol. 2002, 40, 4321–4324. [Google Scholar] [CrossRef] [PubMed]
- Adesiyan, I.M.; Bisi-Johnson, M.A.; Okoh, A.I. Incidence of antibiotic resistance genotypes of Vibrio species recovered from selected freshwaters in Southwest Nigeria. Sci. Rep. 2022, 12, 18912. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Ho, H.C.; Shi, L.; Zeng, Y.; Yang, X.; Huang, Q.; Pei, Y.; Huang, C.; Yang, L. Association between antibiotic resistance and increasing ambient temperature in China: An ecological study with nationwide panel data. Lancet Reg. Health-West. Pac. 2023, 30, 100628. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kinch, L.N.; Santos, M.D.S.; Grishin, N.V.; Orth, K.; Salomon, D. Proteomics analysis reveals previously uncharacterized virulence factors in Vibrio proteolyticus. mBio 2016, 7, e01077-16. [Google Scholar] [CrossRef]
- Paek, J.; Shin, J.H.; Shin, Y.; Park, I.-S.; Kim, H.; Kook, J.-K.; Kang, S.-S.; Kim, D.-S.; Park, K.-H.; Chang, Y.-H. Vibrio injenensis sp. nov., isolated from human clinical specimens. Antonie Leeuwenhoek 2017, 110, 145–152. [Google Scholar] [CrossRef]
- Kang, H.; Yu, Y.; Liao, M.; Wang, Y.; Yang, G.; Zhang, Z.; Li, B.; Rong, X.; Wang, C. Physiology, metabolism, antibiotic resistance, and genetic diversity of Harveyi Glade bacteria isolated from coastal mariculture system in China in the last two decades. Front. Mar. Sci. 2022, 9, 932255. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic resistance: Moving from individual health norms to social norms in one health and global health. Front. Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef]
- Heng, S.P.; Letchumanan, V.; Deng, C.Y.; Ab Mutalib, N.S.; Khan, T.M.; Chuah, L.H.; Chan, K.G.; Goh, B.H.; Pusparajah, P.; Lee, L.H. Vibrio vulnificus: An environmental and clinical burden. Front. Microbiol. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.M.; Dong, C.F.; Weng, S.P.; He, J.G. The high prevalence of pathogenic Vibrio harveyi with multiple antibiotic resistance in scale drop and muscle necrosis disease of the hybrid grouper, Epinephelus fuscoguttatus (♀)× E. lanceolatus (♂), in China. J. Fish Dis. 2018, 41, 589–601. [Google Scholar] [CrossRef]
- Fernández-Delgado, M.; Suárez, P.; Giner, S.; Sanz, V.; Peña, J.; Sánchez, D.; García-Amado, M.A. Occurrence and virulence properties of Vibrio and Salinivibrio isolates from tropical lagoons of the southern Caribbean Sea. Antonie Leeuwenhoek 2017, 110, 833–841. [Google Scholar] [CrossRef]
- Zanetti, S.; Spanu, T.; Deriu, A.; Romano, L.; Sechi, L.; Fadda, G. In vitro susceptibility of Vibrio spp. isolated from the environment. Int. J. Antimicrob. Agents 2001, 17, 407–409. [Google Scholar] [CrossRef]
- Schirmeister, F.; Dieckmann, R.; Bechlars, S.; Bier, N.; Faruque, S.M.; Strauch, E. Genetic and phenotypic analysis of Vibrio cholerae non-O1, non-O139 isolated from German and Austrian patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 767–778. [Google Scholar] [CrossRef]
- Cohen, H.; Baram, N.; Edry-Botzer, L.; Munitz, A.; Salomon, D.; Gerlic, M. Vibrio pore-forming leukocidin activates pyroptotic cell death via the NLRP3 inflammasome. Emerg. Microbes Infect. 2020, 9, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Jalajakumari, M.B.; Manning, P.A. Nucleotide sequence of the gene, ompW, encoding a 22kDa immunogenic outer membrane protein of Vibrio cholerae. Nucleic Acids Res. 1990, 18, 2180. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, J.; Li, T.; Zhang, M.; Li, J.; Kan, B. The outer membrane protein OmpW enhanced V. cholerae growth in hypersaline conditions by transporting carnitine. Front. Microbiol. 2018, 8, 2703. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Ren, H.; Lin, X.; Wu, L.; Peng, X. Proteomic analysis on the expression of outer membrane proteins of Vibrio alginolyticus at different sodium concentrations. Proteomics 2005, 5, 3142–3152. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, H.; Su, Y.; Liu, S.; Xu, L.; Guo, Z.; Wu, J.; Cheng, C.; Feng, J. Horizontal gene transfer contributes to virulence and antibiotic resistance of Vibrio harveyi 345 based on complete genome sequence analysis. BMC Genom. 2019, 20, 761. [Google Scholar] [CrossRef]
- Antonova, E.S.; Hammer, B.K. Genetics of natural competence in Vibrio cholerae and other vibrios. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef]
- Lo Scrudato, M.; Blokesch, M. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet. 2012, 8, e1002778. [Google Scholar] [CrossRef]
- Castillo, D.; Kauffman, K.; Hussain, F.; Kalatzis, P.; Rørbo, N.; Polz, M.F.; Middelboe, M. Widespread distribution of prophage-encoded virulence factors in marine Vibrio communities. Sci. Rep. 2018, 8, 9973. [Google Scholar] [CrossRef]
- Mishra, A.; Taneja, N.; Sharma, M. Environmental and epidemiological surveillance of Vibrio cholerae in a cholera-endemic region in India with freshwater environs. J. Appl. Microbiol. 2012, 112, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Castillo, D.; Andersen, N.; Kalatzis, P.G.; Middelboe, M. Large phenotypic and genetic diversity of prophages induced from the fish pathogen Vibrio anguillarum. Viruses 2019, 11, 983. [Google Scholar] [CrossRef] [PubMed]

| S/N | Isolate Code | Identified Species |
|---|---|---|
| 1 | V-Gan | V. proteolyticus |
| 2 | VR | |
| 3 | VH-I4 | |
| 4 | VH-II4 | |
| 5 | VP-IA | |
| 6 | VP-IB | |
| 7 | VM-IIA | |
| 8 | VHVB-I | |
| 9 | VH-I3 | V. campbellii |
| 10 | VH-II1 | |
| 11 | VHMC-A | |
| 12 | VM-IB | |
| 13 | VHMC-C | V. cincinnatiensis |
| 14 | VH-II2 | |
| 15 | VHVB-II | |
| 16 | VHMC-B | V. nereis |
| 17 | VHMC-D | |
| 18 | VM-IA | |
| 19 | VH-II3 | V. harveyi |
| 20 | VM-IIB | Not identifiable |
| S/N | Primers | Nucleotide Sequences (5′−3′) | Amplicon Size (bp) and Annealing Temperature in °C | Reference |
|---|---|---|---|---|
| 1 | ctxA-F | CTCAGACGGGATTTGTTAGGCACG | 302 (64 °C) | [21] |
| ctxA-R | TCTATCTCTGTAGCCCCTATTACG | |||
| 2 | ctxB-F | GATACACATAATAGAATTAAGGATG | 460 (60 °C) | [22] |
| ctxB-R | GGTTGCTTCTCATCATCGAACCAC | |||
| 3 | O1 rfbF | GTTTCACTGAACAGATGGG | 192 (57 °C) | [23] |
| O1 rfbR | GGTCATCTGTAAGTACAAC | |||
| 4 | O139 rfbF | AGCCTCTTTATTACGGGTGG | 449 (57 °C) | [23] |
| O139 rfbR | GTCAAACCCGATCGTAAAGG | |||
| 5 | ompW-F | CACCAAGAAGGTGACTTTATTGTG | 304 (56 °C) | [24] |
| ompW-R | GGTTTGTCGAATTAGCTTCACC | |||
| 6 | tcpA-F | CACGATAAGAAAACCGGTCAAGAG | 451 (El Tor) (60 °C) | [25] |
| tcpA-R | CGAAAGCACCTTCTTTCACACGTTG | |||
| tcpA-R | TTACCAAATGCAACGCCGAATG | 620 (Class) | ||
| 7 | zot-F | TCGCTTAACGATGGCGCGTTTT | 947 (60 °C) | [24] |
| zot-R | AACCCCGTTTCACTTCTACCCA | |||
| 8 | hlyA-F | GGCAAACAGCGAAACAAATACC | 481 (El Tor) | [24] |
| hlyA-F | GAGCCGGCATTCATCTGAAT | 738/727 (ET and Class) (56 °C) | ||
| hlyA-R | CTCAGCGGGCTAATACGGTTTA | |||
| 9 | toxR-F | CCTTCGATCCCCTAAGCAATAC | 779 (60 °C) | [24] |
| toxR-R | AGGGTTAGCAACGATGCGTAAG | |||
| 10 | ompU-F | ACGCTGACGGAATCAACCAAA | 869 (62 °C) | [26] |
| ompU-R | GCGGAAGTTTGGCTTGAAGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, R.; Sharma, S.; Sinha, K.K. Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics 2023, 12, 1062. https://doi.org/10.3390/antibiotics12061062
Pandey R, Sharma S, Sinha KK. Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics. 2023; 12(6):1062. https://doi.org/10.3390/antibiotics12061062
Chicago/Turabian StylePandey, Rajkishor, Simran Sharma, and Kislay Kumar Sinha. 2023. "Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species" Antibiotics 12, no. 6: 1062. https://doi.org/10.3390/antibiotics12061062
APA StylePandey, R., Sharma, S., & Sinha, K. K. (2023). Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics, 12(6), 1062. https://doi.org/10.3390/antibiotics12061062









