Multidrug-Resistant (MDR) Urinary Tract Infections Associated with Gut Microbiota in CoV and Non-CoV Patients in a Urological Clinic during the Pandemic: A Single Center Experience
Abstract
:1. Introduction
2. Results
3. Materials and Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagenlehner, F.M.; Naber, K.G. Treatment of bacterial urinary tract infections:presence and future. Eur Urol. 2006, 49, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Teixeira, J.C.; Marques-Pinto, A.; Vila, F.; Lindoro, J.; Fraga, A. How the COV-19 pandemic changed postoperative infections in urology wards: A retrospective cohort study from two urology departments. Can. Urol. Assoc. J. 2022, 16, E267–E273. [Google Scholar]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelleti, P. Reduction of Multidrug-Resistant (MDR) bacterial Infections during the COV-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Tham, N.; Fazio, T.; Johnson, D.; Skandarajah, A.; Hayes, I.P. Hospital Aquired Infections in Surgical Patients: Impact of COV-19-Related Infections Prevention Measures. World J. Surg. 2022, 46, 1249–1258. [Google Scholar] [CrossRef]
- Gasperini, B.; Cherubini, A.; Lucarelli, M.; Espinosa, E.; Prospero, E. Multidrug-Resistant Bacterial Infections in Geriatric Hospitalised Patients before and after the COV-19 Outbreak: Results from a Retrospective Observational Study in Two Geriatric Wards. Antibiotics 2021, 10, 95. [Google Scholar] [CrossRef]
- Miftode, I.L.; Leca, D.; Miftode, R.S.; Roşu, F.; Plesca, C.; Loghin, I.; Timpau, A.S.S.; Mitu, I.; Mititiuc, I.; Dorneanu, O.; et al. The Clash of the Titans: COVID-19, Carbapenem-Resistant Enterobacterales, and First mcr-1-Mediated Colistin Resistance in Humans in Romania. Antibiotics 2023, 12, 324. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant extensively drug-resistant and pandrug-resistant bacteria:An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef]
- Mohammadi, A.; Khatami, F.; Azimbeik, Z.; Khajavi, A.; Aloosh, M.; Aghamir, S.M. Hospital-aquired infections in a tertiary hospital in Iran before and during the COV-19 pandemic. Wien Med Wochenschr. 2022, 172, 220–226. [Google Scholar] [CrossRef]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016–2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef]
- Tyagi, V.; Sharma, A.K.; Bhandari, M. Urological implications of SARS-CoV-19. Can. J. Urol. 2020, 27, 10205–10212. [Google Scholar]
- Gul, M.; Kaymar, M.; Yildiz, M.; Batur, A.F.; Akrand, M.; Kilic, O.; Goktas, S. The Increased Risk of Complicated Ureteral Stones in the Era of COV-19 Pandemic. J. Endourol. 2020, 34, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed]
- Luciani, M.; Bentivegna, E.; Spuntareli, V.; Lamberti, P.A.; Cacioli, G.; del Potro, F.; Sesti, G.; Martalleti, P.; de Biase, L. Recurrent COV-19 pneumonia in the course of chemotherapy. Consequence of a weakened immune system? J. Med. Virol. 2021, 93, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Spinazzola, G.; Teofili, L.; Avolio, A.W.; Fiori, B.; Maresca, G.M.; Spanu, T.; Nicolotti, N.; De Pascuale, G. Protective Effect of SARS-CoV-2 preventive measures against ESKAPE and Escherichia coli infections. Eur. J. Clin. Investig. 2021, 51, e13689. [Google Scholar] [CrossRef]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COV-19 prevention measures on risk of healthcare-associated Clostridium difficile infection. Am. J. Infect. Control. 2021, 49, 640–642. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COV-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COV-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COV-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Kampmeier, S.; Tönnies, H.; Correa-Martinez, C.L.; Mellmann, A.; Schwierzeck, V. A nosocomial cluster of vancomycin- resistant Enterococci among COV-19 patients in an intensive care unit. Antimicrob. Resist Infect Control. 2020, 9, 154. [Google Scholar] [CrossRef]
- Medina-Polo, J.; Jimenes-Alcaide, E.; Garcia-Gonzales, L.; Guerrero-Ramos, F.; Perez-Cadavid, S.; Arre’bola-Pajares, A.; Sopena-Sutil, R.; Benites-Salas, R.; Diaz-Gonzales, R.; Tejido-Sanchez, A. Healthcare-associated infections in a department of urology: Incidence and patterns of antibiotic resistance. Scan. J. Urol. 2014, 48, 203–209. [Google Scholar] [CrossRef]
- Liew, Y.; Lee, W.H.L.; Tan, L.; Kwa, A.L.H.; Thien, S.Y.; Cherng, B.P.Z.; Chung, S.J. Antimicrobial stewardship programme: A vital resource for hospitals during the global outbreak of coronavirus disease 2019 (COV-19). Int. J. Antimicrob. Agents 2020, 56, 106145. [Google Scholar] [CrossRef]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Cheng Li, J.T.; Fung, K.H. Risk factors for aquiring multi-drug resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Milan, P.B.; Ivan, I.M. Catheter-associated and nosocomial urinary tract infections:antibiotic resistance and influence on commonly used antimicrobial therapy. Int. Urol. Nephrol. 2009, 41, 461–464. [Google Scholar] [CrossRef]
- Miftode, E.; Miftode, L.; Coman, I.; Prepeliuc, C.; Obreja, M.; Stamateanu, O.; Parânga, T.G.; Leca, D.; Plesca, C.E. Diabetes Mellitus—A Risk Factor for Unfavourable Outcome in COVID-19 Patients -The Experience of an Infectious Diseases Regional Hospital. Healthcare 2021, 9, 788. [Google Scholar] [CrossRef]
- Dezza, F.C.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco FCeccarelli, G.; Raponi, G.; Alessandri, F.; Mastroiani, C.M.; Venditti MOliva, A. Clinical Impact of COV-19 on Multi-Drug- Resistant, Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Settings: Two Pandemias Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Urinary Tract Infections. In Mandell, Douglas and Bennett’s Principles and Practice of Infectious Disease, 8th ed.; Mandell, G.L., Bennett, J.E., Eds.; Elsevier: Sanders, PA, USA, 2015; Volume 1, pp. 886–913. [Google Scholar]
- Bonkat, G.; Bartoletti, R.; Bruyere, F.; Cai, T.; Geerling, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2021. [Google Scholar]
- Wagenlehner, F.; Tandogdu, Z.; Bartoletti, R.; Cai, T.; Cek, M.; Kulchavenya, E.; Ko”ves, B.; Naber, K.; Perepanova, T.; Tenke, P.; et al. GPIU Investigators. The Global Prevalence of Infections in Urology(GPIU)Study: A Worldwide Surveillance Study in Urology Patients. Eur. Urol. Focus 2016, 2, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Medina-Polo, J.; Sopena-Sutil, R.; Benitez-Sala, R.; Lara-Isla, A.; Alonso-Isa, M.; Gil-Moradillo, J.; Justo- Quintas, J.; Garcia-Rojo, E.; Gonzales-Padilla, D.A.; Passas-Martinez, J.B.; et al. Prospective study analyzing risk factors and characteristics of healthcare-associated infections in a Urology ward. Investig. Clin. Urol. 2017, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Cek, M.; Wagenlehner, F.; Naber, K.; Tenke, P.; van Ostrum, E.; Johansen, T.B. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J. Urol. 2014, 32, 791–801. [Google Scholar] [CrossRef]
- Miftode, I.L.; Nastase, E.V.; Miftode, R.; Miftode, E.G.; Iancu, L.S.; Luncă, C.; Păduraru, D.A.; Costache, I.; Stafie, C.; Dorneanu, O. Insights into multidrug resistant K. pneumoniae urinary tract infections: From susceptibility to mortality. Exp. Ther. Med. 2021, 22, 1086. [Google Scholar] [CrossRef]
- Miftode, I.L.; Pasare, M.A.; Miftode, R.S.; Nastase, E.; Plesca, C.E.; Lunca, C.; Miftode, E.G.; Timpau, A.S.; Iancu, L.S.; Dorneanu, O.S. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Urinary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics 2022, 11, 548. [Google Scholar] [CrossRef]
| Non-COVID MDR UTI (n = 66) | COVID MDR UTI (n = 22) | p | ||
|---|---|---|---|---|
| Male | 35 (53%) | 12 (54.5%) | 0.902 F | |
| Female | 31 (47%) | 10 (45.5%) | ||
| Age (mean ± SD) | Male | 63.31 ± 13.29 | 67.92 ± 6.99 | 0.136 t |
| Female | 58.13 ± 18.69 | 65.20 ± 11.29 | 0.161 t | |
| Residence area (urban) | 35 (53%) | 10 (45.5%) | 0.538 F | |
| Comorbidities | ||||
| 1. Type 2 diabetes (DM) | 20 (30.3%) | 6 (27.3%) | 0.787 F | |
| 2. Neoplasia | 27 (40.9%) | 8 (36.4%) | 0.706 F | |
| 3. Kidney failure | 26 (39.4%) | 12 (54.5%) | 0.214 F | |
| 4. Heart failure | 17 (25.8%) | 3 (13.6%) | 0.240 F | |
| 5. Anemia | 21 (31.8%) | 7 (31.8%) | 1 F | |
| 6. Stroke sequelae | 10 (15.2%) | 3 (13.6%) | 0.990F | |
| 7. Hypertension | 28 (42.4%) | 9 (40.9%) | 0.901 F | |
| Urosepsis at the moment of admission | 14 (21.2%) | 12 (54.5%) | 0.003 F | |
| Urinary Catheters at the Time of Diagnosis | Non-COVID MDR UTI (n = 66) | COVID MDR UTI (n = 22) | p-Value for Chi-Square Test and Fisher’s Exact Test |
|---|---|---|---|
| Permanent urethral catheter | 15 (22.7%) | 5 (22.73%) | 1 |
| Permanent double-J ureteral catheter | 30 (45.5%) | 4 (18.2%) | 0.023 |
| Permanent nephrostomy catheter | 9 (13.6%) | 6 (27.3%) | 0.190 |
| Cutaneous ureterostomy catheter | 2 (3.0%) | 1 (4.5%) | 0.999 |
| Lumbar drain tube | 1 (1.5%) | 1 (4.5%) | 0.440 |
| Permanent cystostomy catheter | 3 (4.5%) | 0 (0.0%) | 0.507 |
| Total number of permanent urinary catheters | 60 catheters in 56 patients (4 patients had 2 catheter types at the same time) | 17 catheters in 17 patients | |
| Total number of patients with urinary catheters | 56 (84.85%) | 17 (77.27%) | 0.413 |
| Non-COVID MDR UTI (n = 66) | COVID MDR UTI (n = 22) | p-Value for Chi-Square Test and Fisher’s Exact Test | |
|---|---|---|---|
| Hospitalization in the last 180 days | 51 (77.3%) | 16 (72.7%) | 0.665 |
| Antibiotic therapy in the last 180 days | 58 (87.9%) | 17 (77.3%) | 0.297 |
| Hospitalization days (mean ± standard deviation) | 6.09 ± 4.87 | 9.27 ± 4.92 | 0.010 t |
| Types of urological interventions performed before the diagnosis of MDR infections | |||
| 1 TURP (transurethral resection of the prostate) | 1 (1.5%) | 3 (13.6%) | 0.047 |
| 2 TURBT (transurethral resection of bladder tumors) | 2 (3.0%) | 0 (0.0%) | 0.999 |
| 3 Percutaneous nephrostomy tube insertion | 6 (9.1%) | 5 (22.73%) | 0.093 |
| 4 Nephrectomy | 1 (1.5%) | 1 (4.5%) | 0.440 |
| 5 Urethral catheter replacement | 8 (12.1%) | 1 (4.5%) | 0.440 |
| 6 Urethrotomy | 3 (4.5%) | 1 (4.54%) | 0.440 |
| 7 Percutaneous nephrolithotomy (PCNL) | 3 (4.5%) | 0 (0.0%) | 0.570 |
| 8 Percutaneous lumbar drainage | 1(1.5%) | 1 (4.5%) | 0.440 |
| 9 Double-J catheter insertion | 18 (27.3%) | 2 (9.1%) | 0.078 |
| 10 Double-J catheter replacement | 14 (21.2%) | 2 (9.1%) | 0.338 |
| 11 Urethral catheter insertion | 3 (4.5%) | 2(9,1%) | 0.425 |
| 12 Urethral dilatation | 1 (1.5%) | 0 (0.0%) | 0.999 |
| 13 Percutaneous nephrostomy tube replacement | 2 (3.0%) | 2 (9.1%) | 0.259 |
| 14 Ureterostomy double-J catheter replacement | 1 (1.5%) | 1 (4.5%) | 0.440 |
| Total urological maneuvers before the occurrence of MDR | 64 (96.97%) | 21 (95.45%) | 0.734 |
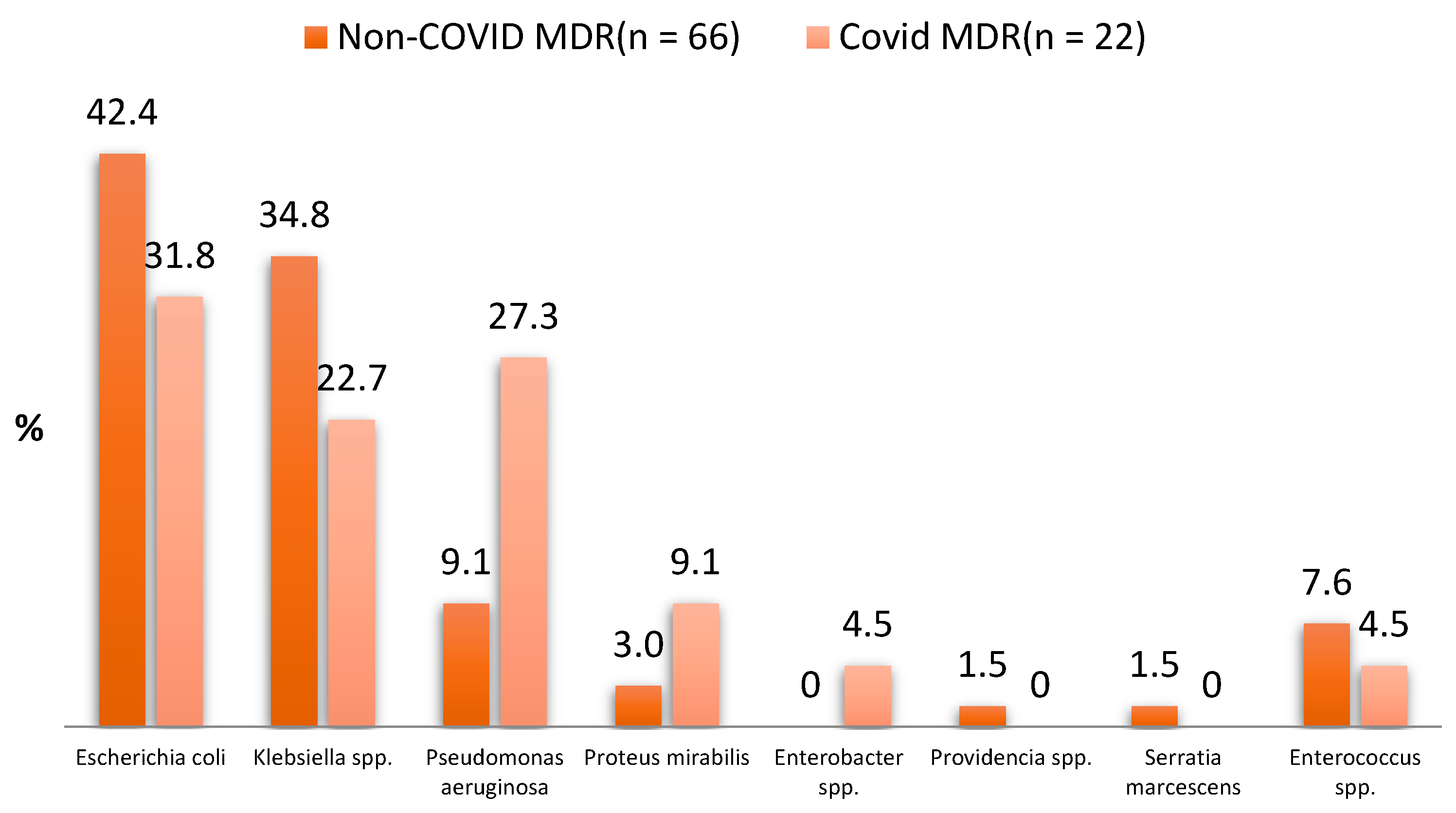
| Types of MDR Bacteria | Non-COVID MDR UTI (n = 66) | COVID MDR UTI (n = 22) | p-Value for Chi-Square Test and Fisher’s Exact Test |
|---|---|---|---|
| Escherichia coli | 28 (42.4%) | 7 (31.8%) | 0.379 |
| Klebsiella spp. | 23 (34.8%) | 5 (22.7%) | 0.290 |
| Pseudomonas aeruginosa | 6 (9.1%) | 6 (27.3%) | 0.066 |
| Proteus mirabilis | 2 (3.0%) | 2 (9.1%) | 0.259 |
| Enterobacter spp. | 0 (0.0%) | 1 (4.5%) | 0.250 |
| Providencia spp. | 1 (1.5%) | 0 (0.0%) | 0.999 |
| Serratia marcescens | 1 (1.5%) | 0 (0.0%) | 0.999 |
| Enterococcus spp. | 5 (7.6%) | 1 (4.5%) | 0.998 |
| Total MDR infections | 66 | 22 |
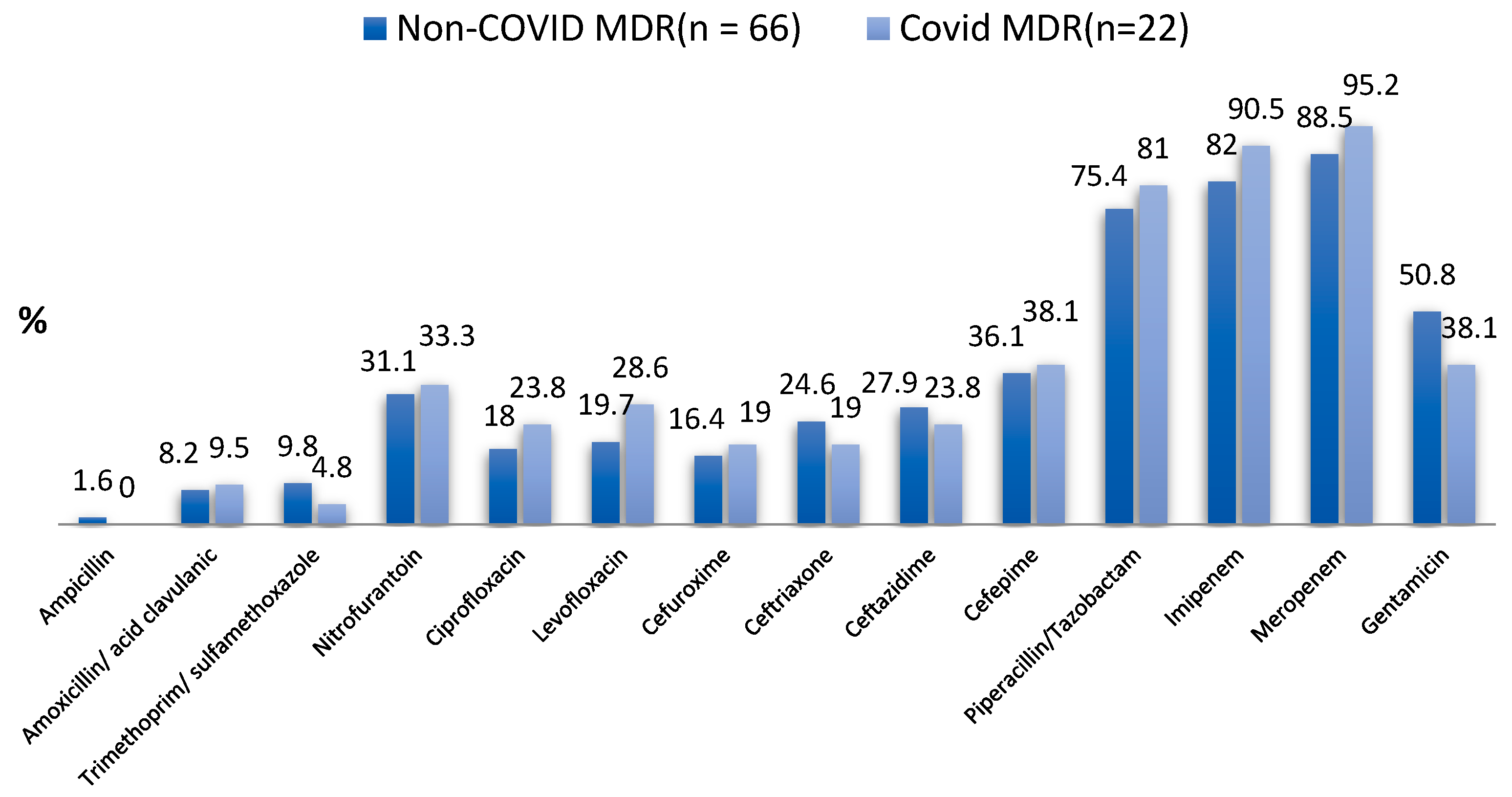
| Type of Tested Antibiotic | Non-COVID MDR UTI (n = 61) | COVID MDR UTI (n = 21) | p-Value for Chi-Square Test and Fisher’s Exact Test |
|---|---|---|---|
| Ampicillin | 1 (1.6%) | 0 (0.0%) | 0.999 |
| Amoxicillin/Acid clavulanic | 5 (8.2%) | 2 (9.5%) | 0.999 |
| Trimethoprim/Sulfamethoxazole | 6 (9.8%) | 1 (4.8%) | 0.671 |
| Nitrofurantoin | 19 (31.1%) | 7 (33.3%) | 0.853 |
| Ciprofloxacin | 11 (18.0%) | 5 (23.8%) | 0.541 |
| Levofloxacin | 12 (19.7%) | 6 (28.6%) | 0.542 |
| Cefuroxime | 10 (16.4%) | 4 (19.0%) | 0.747 |
| Ceftriaxone | 15 (24.6%) | 4 (19.0%) | 0.768 |
| Ceftazidime | 17 (27.9%) | 5 (23.8%) | 0.717 |
| Cefepime | 22 (36.1%) | 8 (38.1%) | 0.868 |
| Piperacillin/Tazobactam | 46 (75.4%) | 17 (81.0%) | 0.768 |
| Imipenem | 50 (82.0%) | 19 (90.5%) | 0.498 |
| Meropenem | 54 (88.5%) | 20 (95.2%) | 0.673 |
| Gentamicin | 31 (50.8%) | 8 (38.1%) | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, V.D.; Costache, R.C.; Onofrei, P.; Miftode, E.; Linga, I.; Ouatu, R.M.; Boiculese, L.; Bobeica, R.L.; Tanasa Vasilache, I.; Mititiuc, I.L. Multidrug-Resistant (MDR) Urinary Tract Infections Associated with Gut Microbiota in CoV and Non-CoV Patients in a Urological Clinic during the Pandemic: A Single Center Experience. Antibiotics 2023, 12, 973. https://doi.org/10.3390/antibiotics12060973
Radu VD, Costache RC, Onofrei P, Miftode E, Linga I, Ouatu RM, Boiculese L, Bobeica RL, Tanasa Vasilache I, Mititiuc IL. Multidrug-Resistant (MDR) Urinary Tract Infections Associated with Gut Microbiota in CoV and Non-CoV Patients in a Urological Clinic during the Pandemic: A Single Center Experience. Antibiotics. 2023; 12(6):973. https://doi.org/10.3390/antibiotics12060973
Chicago/Turabian StyleRadu, Viorel Dragos, Radu Cristian Costache, Pavel Onofrei, Egidia Miftode, Iacov Linga, Radu Mihaita Ouatu, Lucian Boiculese, Razvan Lucian Bobeica, Ingrid Tanasa Vasilache, and Irina Luanda Mititiuc. 2023. "Multidrug-Resistant (MDR) Urinary Tract Infections Associated with Gut Microbiota in CoV and Non-CoV Patients in a Urological Clinic during the Pandemic: A Single Center Experience" Antibiotics 12, no. 6: 973. https://doi.org/10.3390/antibiotics12060973






