Νanomaterial-Loaded Polymer Coating Prevents the In Vitro Growth of Candida albicans Biofilms on Silicone Biomaterials
Abstract
:1. Introduction
2. Methods
2.1. Biomaterials
2.2. MIC Determination
2.3. Mature Biofilm Production and Minimum Biofilm Inhibitory Concentration (MBIC) Determination
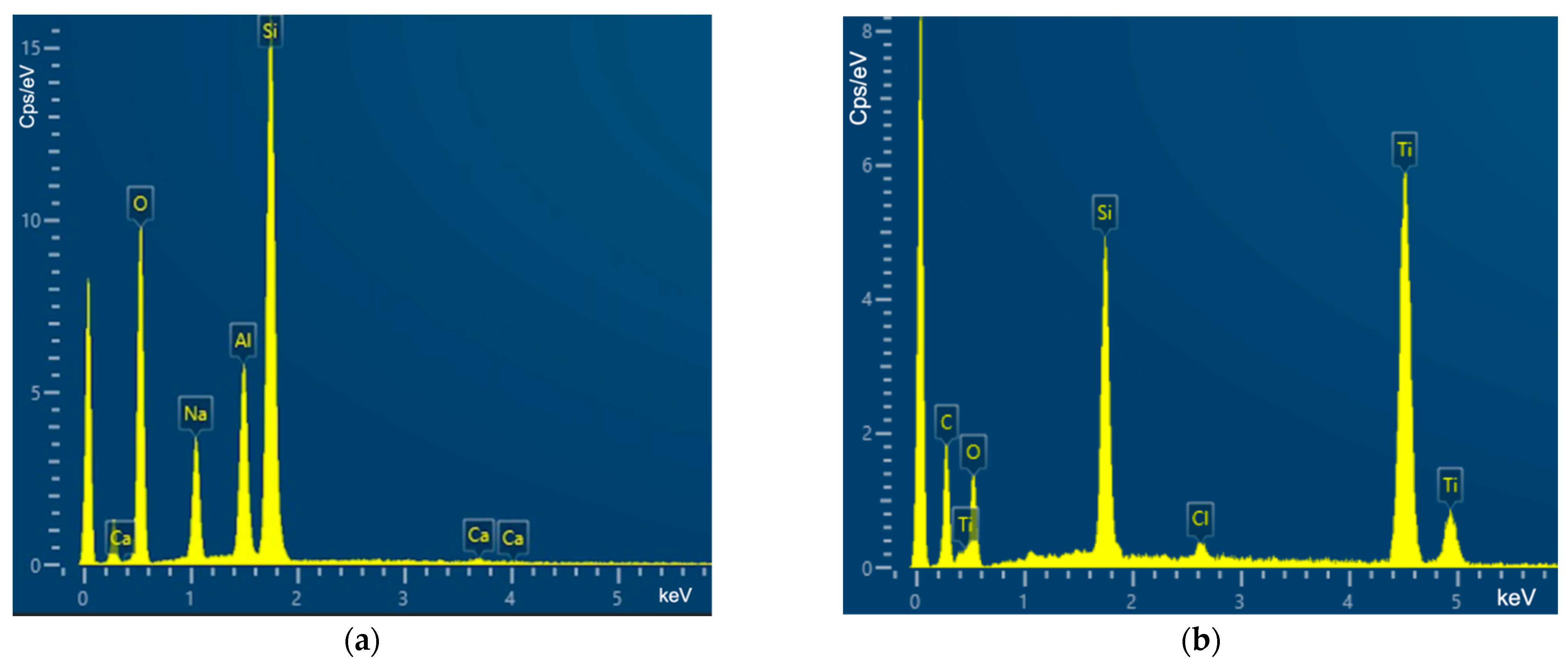
2.4. Scanning Electron Microscopy and Coating Assessment
2.5. Simultaneous Exposure of Biofilms and Planktonic Cells to Al2O3 and TiO2
2.6. Synergy Assessment between Al2O3 and TiO2
2.7. Coating Technique
2.8. Colorimetric Assessment
2.9. Statistical Analysis and Interpretation of the Results
2.10. Interpretation of the Results
3. Results
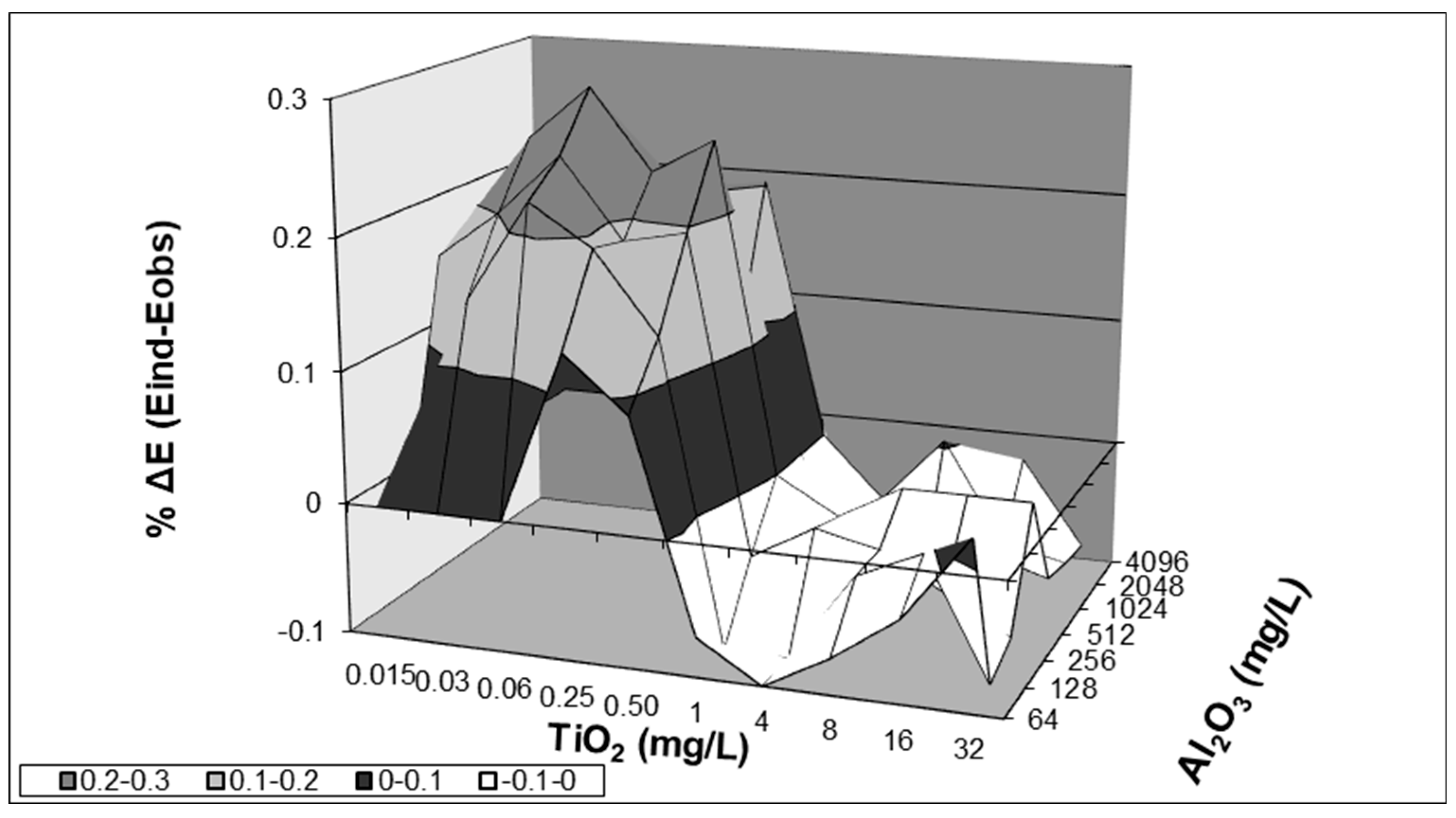
3.1. Synergy Assessment
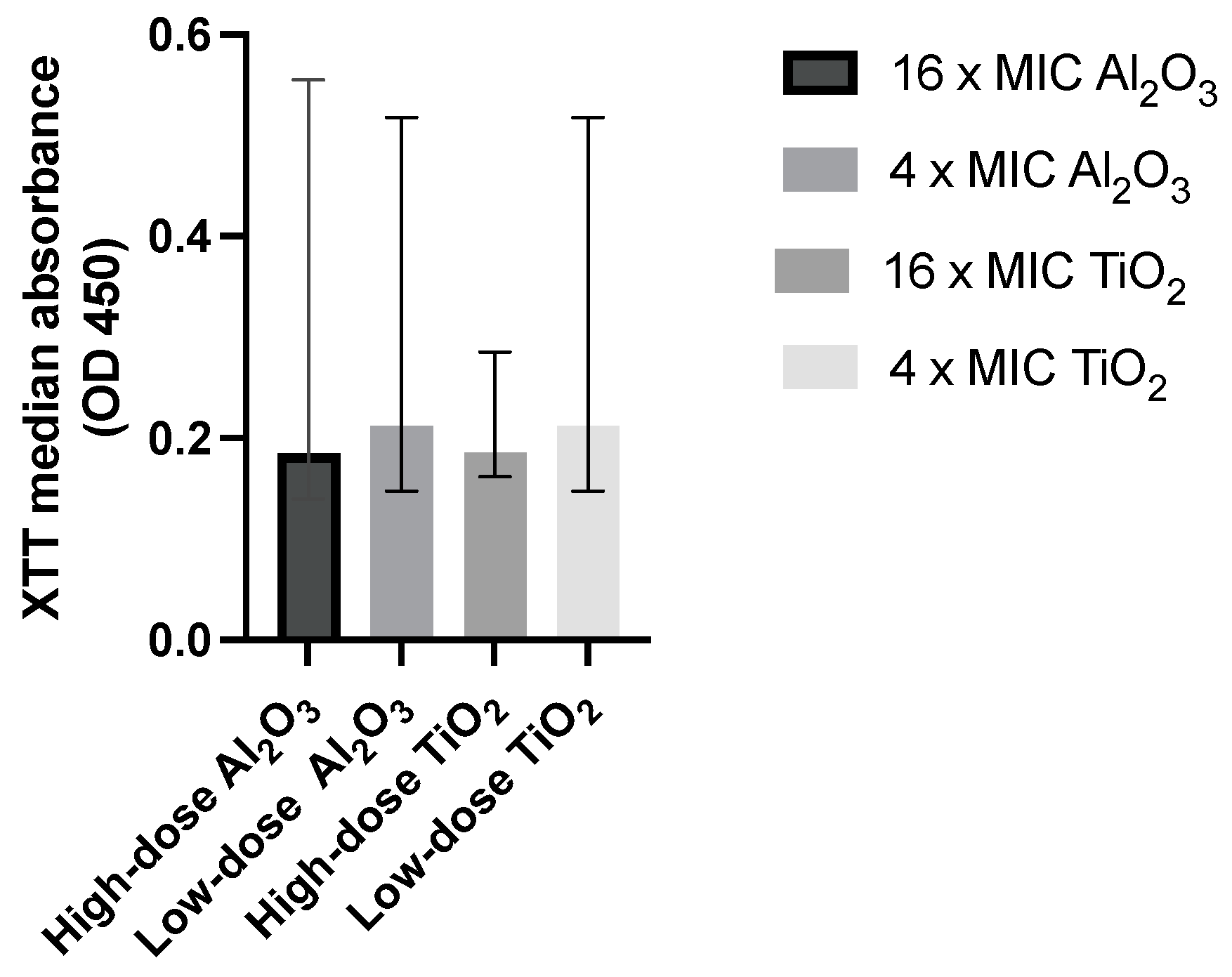
3.2. Impact of Al2O3- and TiO2- Resomer® Coating on Candida Biofilm Growth
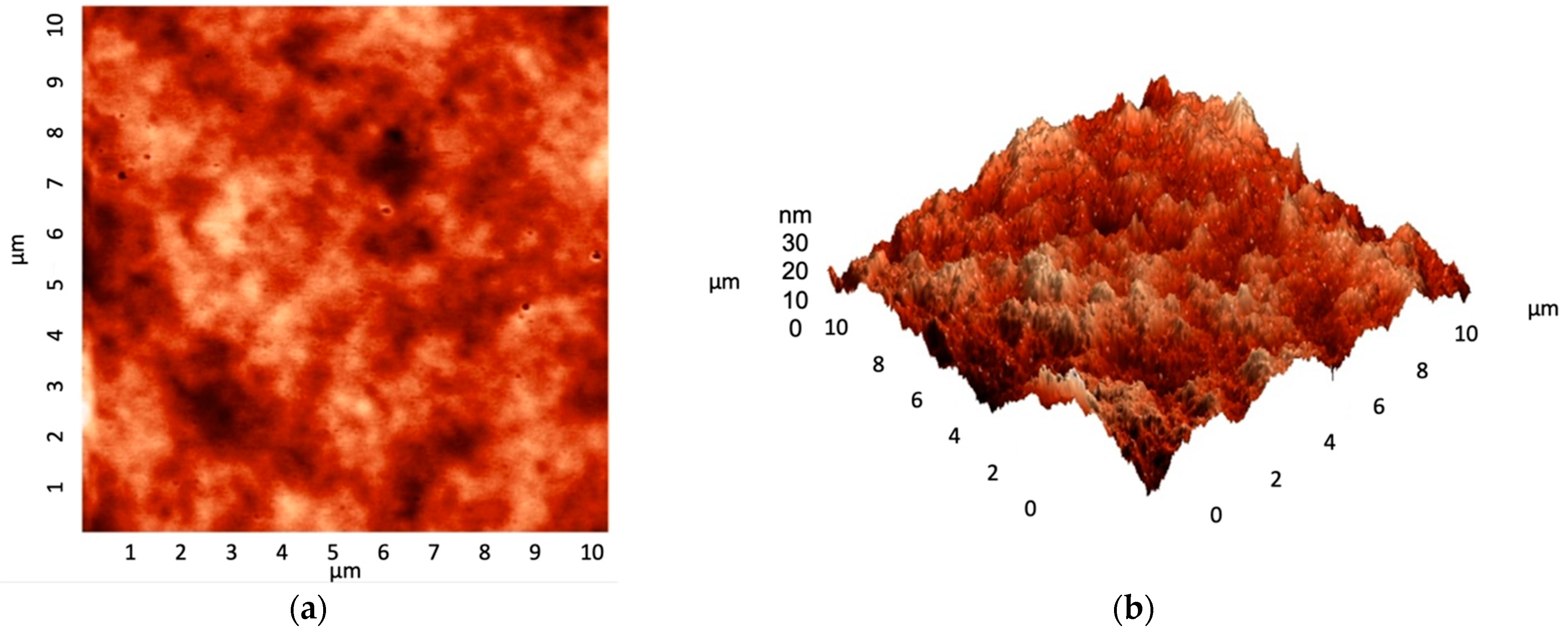
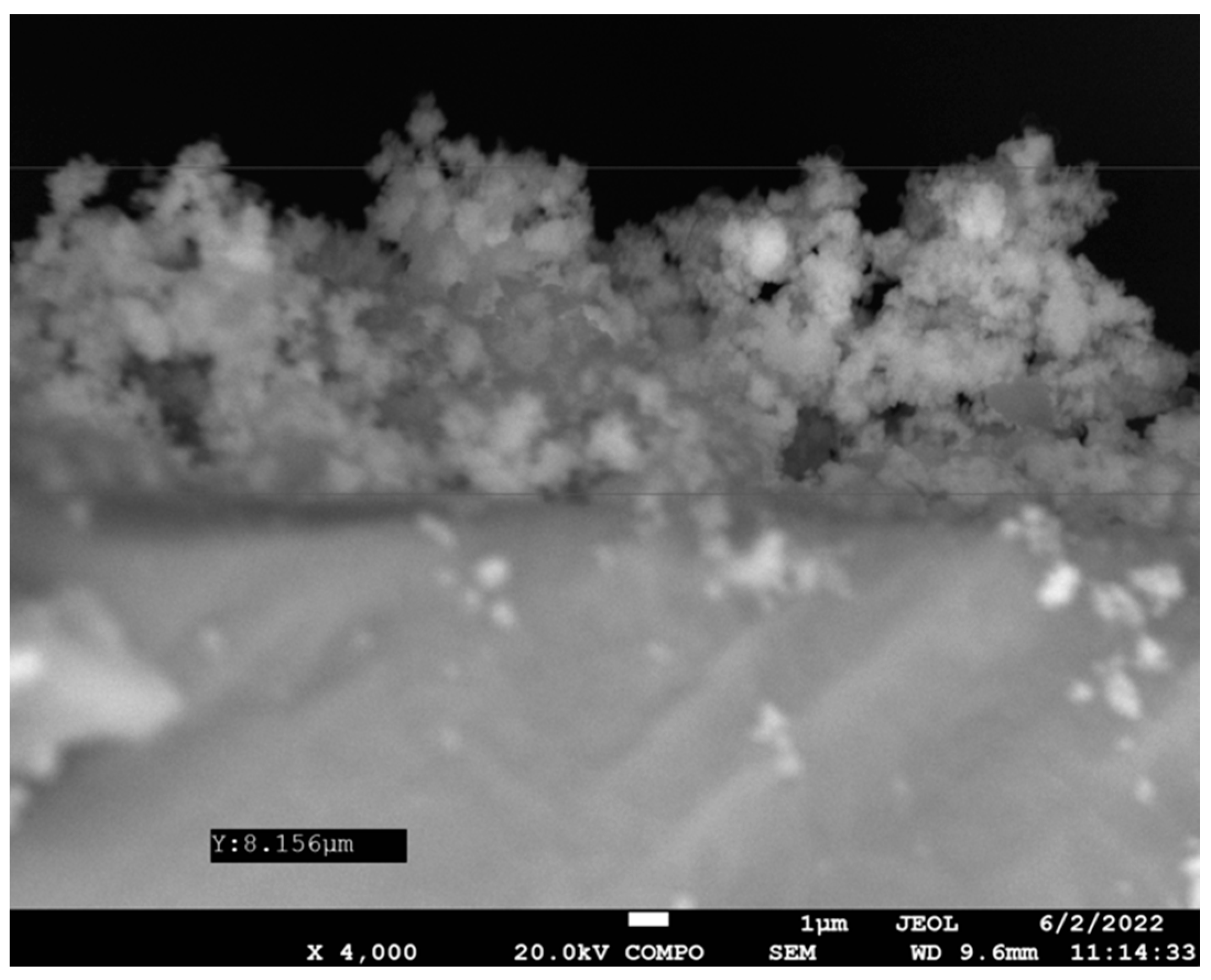
3.3. Coating Assessment and Characterization Data
4. Discussion
4.1. Toxicity Concerns
4.2. Coating Remarks
4.3. Coating Considerations
4.4. Study Limitations and Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef]
- Groome, P.A.; O’Sullivan, B.; Irish, J.C.; Rothwell, D.M.; Schulze, K.; Warde, P.R.; Schneider, K.M.; MacKenzie, R.G.; Hodson, D.I.; Hammond, J.A.; et al. Management and Outcome Differences in Supraglottic Cancer Between Ontario, Canada, and the Surveillance, Epidemiology, and End Results Areas of the United States. J. Clin. Oncol. 2003, 21, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current Role of Total Laryngectomy in the Era of Organ Preservation. Cancers 2020, 12, 584. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.G.R.; Santos, I.C.; Bergmann, A.; Thuler, L.C.S.; Freitas, A.S.; Freitas, E.Q.; Dias, F.L. Quality of life after total laryngectomy: Impact of different vocal rehabilitation methods in a middle income country. Health Qual. Life Outcomes 2020, 18, 92. [Google Scholar] [CrossRef]
- Schuster, M.; Lohscheller, J.; Kummer, P.; Hoppe, U.; Eysholdt, U.; Rosanowski, F. Quality of Life in Laryngectomees after Prosthetic Voice Restoration. Folia Phoniatr. Logop. 2003, 55, 211–219. [Google Scholar] [CrossRef]
- Balm, A.; Brekel, M.V.D.; Tan, I.; Hilgers, F. The indwelling voice prosthesis for speech rehabilitation after total laryngectomy: A safe approach. Otolaryngol. Pol. 2011, 65, 402–409. [Google Scholar] [CrossRef]
- Chen, H.; Brook, M.A.; Sheardown, H. Silicone elastomers for reduced protein adsorption. Biomaterials 2004, 25, 2273–2282. [Google Scholar] [CrossRef]
- Neu, T.; Verkerke, G.; Herrmann, I.; Schutte, H.; Mei, H.V.; Busscher, H. Microflora on explanted silicone rubber voice prostheses: Taxonomy, hydrophobicity and electrophoretic mobility. J. Appl. Bacteriol. 1994, 76, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.; Van der Mei, H.; Busscher, H.; Dijk, F.; Verkerke, G. Biodeterioration of medical-grade silicone rubber used for voice prostheses: A SEM study. Biomaterials 1993, 14, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Attieh, A.Y.; Searl, J.; Shahaltough, N.H.; Wreikat, M.M.; Lundy, D.S. Voice restoration following total laryngectomy by tracheoesophageal prosthesis: Effect on patients’ quality of life and voice handicap in Jordan. Health Qual. Life Outcomes 2008, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Weissenbruch, R.; Albers, F.W.J.; Bouckaert, S.; Nelis, H.J.; Criel, G.; Remon, J.P.; Sulter, A.M. Deterioration of the Provox™ Silicone Tracheoesophageal Voice Prosthesis: Microbial Aspects and Structural Changes. Acta Otolaryngol. 1997, 117, 452–458. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Nett, J.; Andes, D. Candida albicans biofilm development, modeling a host–pathogen interaction. Curr. Opin. Microbiol. 2006, 9, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, A.; Petinaki, E.; Festas, C.; Tsikopoulos, K.; Meroni, G.; Drago, L.; Skoulakis, C. In vitro Inhibition of Biofilm Formation on Silicon Rubber Voice Prosthesis: A Systematic Review and Meta-Analysis. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Kucharíková, S.; Neirinck, B.; Sharma, N.; Vleugels, J.; Lagrou, K.; Van Dijck, P. In vivo Candida glabrata biofilm development on foreign bodies in a rat subcutaneous model. J. Antimicrob. Chemother. 2015, 70, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- de Coul, B.M.R.O.; Hilgers, F.J.M.; Balm, A.J.M.; Tan, I.B.; Hoogen, F.J.A.V.D.; van Tinteren, H. A Decade of Postlaryngectomy Vocal Rehabilitation in 318 Patients. Arch. Otolaryngol. Neck Surg. 2000, 126, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Elving, G.J.; van der Mei, H.C.; van Weissenbruch, R.M.; Albers, F.W.M.; Busscher, H.J. Effect of antifungal agents on indwelling voice prosthetic biofilms. Curr. Opin. Otolaryngol. Head Neck Surg. 2000, 8, 165–168. [Google Scholar] [CrossRef]
- Denning, D. Can we prevent azole resistance in fungi? Lancet 1995, 346, 454–455. [Google Scholar] [CrossRef]
- Chen, J.; Qin, G.; Wang, J.; Yu, J.; Shen, B.; Li, S.; Ren, Y.; Zuo, L.; Shen, W.; Das, B. One-step fabrication of sub-10-nm plasmonic nanogaps for reliable SERS sensing of microorganisms. Biosens. Bioelectron. 2013, 44, 191–197. [Google Scholar] [CrossRef]
- Aslam, S. Effect of antibacterials on biofilms. Am. J. Infect. Control 2008, 36, S175.e9–S175.e11. [Google Scholar] [CrossRef]
- Elving, G.J.; van der Mei, H.C.; van Weissenbruch, R.; Busscher, H.J.; Albers, F.W.J. Comparison of the microbial composition of voice prosthesis biofilms from patients requiring frequent versus infrequent replacement. Ann. Otol. Rhinol. Laryngol. 2002, 111, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, F.; Abdollahi, S.; Jelvehgari, M.; Valizadeh, H.; Hassan, M.; Milani, M. Study of Antimicrobial Effects of Vancomycin Loaded PLGA Nanoparticles Against Enterococcus Clinical Isolates. Drug Res. 2014, 64, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Quality Manual, 3rd ed.; Wayne, P.A., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Zhao, W.; Sachsenmeier, K.; Zhang, L.; Sult, E.; Hollingsworth, R.E.; Yang, H. A New Bliss Independence Model to Analyze Drug Combination Data. SLAS Discov. Adv. Sci. Drug Discov. 2014, 19, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Faggion, C.M. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Èvid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Opperman, T.J.; Kwasny, S.M.; Williams, J.D.; Khan, A.R.; Peet, N.P.; Moir, D.T.; Bowlin, T.L. Aryl Rhodanines Specifically Inhibit Staphylococcal and Enterococcal Biofilm Formation. Antimicrob. Agents Chemother. 2009, 53, 4357–4367. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilyy, R.; Paryzhak, S.; Turcheniuk, K.; Dumych, T.; Barras, A.; Boukherroub, R.; Wang, F.; Yushin, G.; Szunerits, S. Aluminum oxide nanowires as safe and effective adjuvants for next-generation vaccines. Mater. Today 2019, 22, 58–66. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Sasaki, J.-I.; Imazato, S. Investigation of the cytotoxicity of aluminum oxide nanoparticles and nanowires and their localization in L929 fibroblasts and RAW264 macrophages. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Croll, S. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Balabanava, N.; Wierzbicki, R.; Zielecka, M.; Rymuza, Z. Effect of roughness on adhesion of polymeric coatings used for microgrippers. Microelectron. Eng. 2007, 84, 1227–1230. [Google Scholar] [CrossRef]
- Chuang-Smith, O.N.; Wells, C.L.; Henry-Stanley, M.J.; Dunny, G.M. Acceleration of Enterococcus faecalis Biofilm Formation by Aggregation Substance Expression in an Ex Vivo Model of Cardiac Valve Colonization. PLoS ONE 2010, 5, e15798. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Antifungal Agent | MIC (μg/mL) | MBIC (μg/mL) |
|---|---|---|
| TiO2 nanoparticles | 1024 | 4096 |
| Al2O3 nanowires | 512 | 2048 |
| Fluconazole | 0.25 | 2048 |
| Al2O3 | TiO2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.015 mg/L | 0.03 mg/L | 0.06 mg/L | 0.25 mg/L | 0.50 mg/L | 1 mg/L | 4 mg/L | 8 mg/L | 16 mg/L | 32 mg/L |
| 64 mg/L | IND | IND | IND | IND | SYN | IND | IND | IND | IND | IND |
| 128 mg/L | IND | SYN | SYN | SYN | SYN | IND | IND | IND | IND | IND |
| 256 mg/L | SYN | SYN | SYN | SYN | SYN | IND | IND | IND | IND | IND |
| 512 mg/L | SYN | SYN | SYN | SYN | SYN | IND | IND | IND | IND | IND |
| 1024 mg/L | IND | IND | IND | IND | IND | IND | IND | SYN | SYN | IND |
| 2048 mg/L | IND | IND | IND | IND | IND | IND | IND | IND | IND | IND |
| 4096 mg/L | IND | IND | IND | IND | IND | IND | IND | IND | IND | IND |
| Treatment Group | Median Absorbance (IQR) | p Value | % Biofilm Reduction | |
|---|---|---|---|---|
| Intervention Group | Biofilm Control Group | |||
| High-dose Al2O3-enhanced polymer coating | 0.176 (0.207) | 0.805 (0.381) | 0.003 | 98% |
| Low-dose Al2O3-enhanced polymer coating | 0.25 (0.161) | 0.805 (0.381) | 0.002 | 87% |
| High-dose TiO2-enhanced polymer coating | 0.186 (0.024) | 0.766 (0.458) | <0.001 | 93% |
| Low-dose TiO2-enhanced polymer coating | 0.213 (0.152) | 0.766 (0.458) | <0.001 | 89% |
| Polymer coating alone | 0.246 (0.098) | 0.766 (0.458) | <0.001 | 84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikopoulos, A.; Tsikopoulos, K.; Meroni, G.; Gravalidis, C.; Soukouroglou, P.; Chatzimoschou, A.; Drago, L.; Triaridis, S.; Papaioannidou, P. Νanomaterial-Loaded Polymer Coating Prevents the In Vitro Growth of Candida albicans Biofilms on Silicone Biomaterials. Antibiotics 2023, 12, 1103. https://doi.org/10.3390/antibiotics12071103
Tsikopoulos A, Tsikopoulos K, Meroni G, Gravalidis C, Soukouroglou P, Chatzimoschou A, Drago L, Triaridis S, Papaioannidou P. Νanomaterial-Loaded Polymer Coating Prevents the In Vitro Growth of Candida albicans Biofilms on Silicone Biomaterials. Antibiotics. 2023; 12(7):1103. https://doi.org/10.3390/antibiotics12071103
Chicago/Turabian StyleTsikopoulos, Alexios, Konstantinos Tsikopoulos, Gabriele Meroni, Christoforos Gravalidis, Prodromos Soukouroglou, Athanasios Chatzimoschou, Lorenzo Drago, Stefanos Triaridis, and Paraskevi Papaioannidou. 2023. "Νanomaterial-Loaded Polymer Coating Prevents the In Vitro Growth of Candida albicans Biofilms on Silicone Biomaterials" Antibiotics 12, no. 7: 1103. https://doi.org/10.3390/antibiotics12071103






