Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing
Abstract
:1. Introduction
2. Results
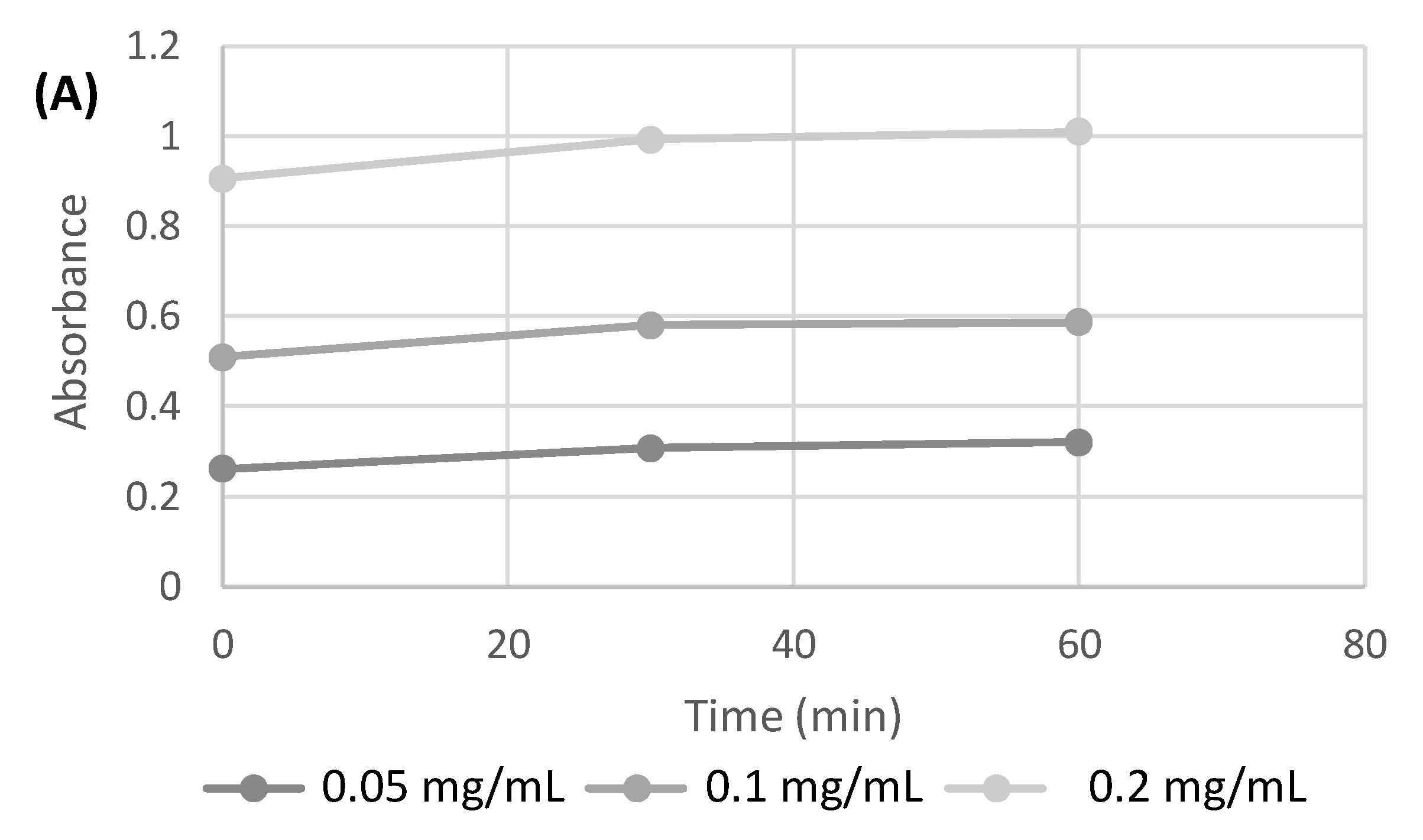
2.1. 3D-Printed Scaffold Preparation and Characterization
2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.3. Antimicrobial Activity Assay
2.4. In-Vitro Cytocompatibility Tests
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Methods
Ink Preparation for 3D Printing
5.3. 3D Printing and Scaffold Production
5.4. Scaffold Characterization
5.5. SEM and SEM-EDS Analysis
5.6. Antimicrobial Activity Tests
5.7. Cell Viability Test
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of Chronic Wounds in Germany: Analysis of Statutory Health Insurance Data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health Economic Burden That Wounds Impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue Engineering and Regenerative Medicine. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 108, pp. 1–33. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.G.; Dolman, J. Antimicrobial Activity of Silver-Containing Dressings on Wound Microorganisms Using an In Vitro Biofilm Model. Int. Wound J. 2007, 4, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.; Savina, I.; Mikhalovsky, S. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seal, B. Polymeric Biomaterials for Tissue and Organ Regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Jodar, K.S.P.; Balcao, V.M.; Chaud, M.V.; Tubino, M.; Yoshida, V.M.H.; Oliveira, J.M.; Vila, M.M.D.C. Development and Characterization of a Hydrogel Containing Silver Sulfadiazine for Antimicrobial Topical Applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef] [Green Version]
- Farazin, A.; Shirazi, F.A.; Shafiei, M. Natural biomarocmolecule-based antimicrobial hydrogel for rapid wound healing: A review. Int. J. Biol. Macromol. 2023, 16, 125454. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Lopes, L.C.; Caleare, A.O.; Britta, E.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Silver Sulfadiazine Loaded Chitosan/Chondroitin Sulfate Films for a Potential Wound Dressing Application. Mater. Sci. Eng. C 2013, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced Antibacterial and Wound Healing Activities of Microporous Chitosan-Ag/ZnO Composite Dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A Bilayer Composite Composed of TiO2 -Incorporated Electrospun Chitosan Membrane and Human Extracellular Matrix Sheet as a Wound Dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Wang, R.; Yuan, P.; Fan, Z.; Yang, S. Preparation and Properties of ZnO/Sodium Alginate Bi-Layered Hydrogel Films as Novel Wound Dressings. N. J. Chem. 2019, 43, 8684–8693. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of Novel Alginate Based Hydrogel Films for Wound Healing Applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Sudheesh Kumar, P.T.; Divyarani, V.V.; Srinivasan, S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V. Fabrication of Chitin–Chitosan/Nano ZrO2 Composite Scaffolds for Tissue Engineering Applications. Int. J. Biol. Macromol. 2011, 49, 274–280. [Google Scholar] [CrossRef]
- Seo, S.Y.; Lee, G.H.; Lee, S.G.; Jung, S.Y.; Lim, J.O.; Choi, J.H. Alginate-Based Composite Sponge Containing Silver Nanoparticles Synthesized in Situ. Carbohydr. Polym. 2012, 90, 109–115. [Google Scholar] [CrossRef]
- Boateng, J.; Burgos-Amador, R.; Okeke, O.; Pawar, H. Composite Alginate and Gelatin Based Bio-Polymeric Wafers Containing Silver Sulfadiazine for Wound Healing. Int. J. Biol. Macromol. 2015, 79, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Wal, P.; Pathak, K.; Khandai, M.; Behl, T.; Alhazmi, H.A.; Khuwaja, G.; Khalid, A. Nanosilver-functionalized polysaccharides as a platform for wound dressing. Environ. Sci. Pollut. Res. Int. 2023, 30, 54385–54406. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambala, V.S.R.; Naidu, R. Disinfection Studies on TiO2 Thin Films Prepared Bya Sol–Gel Method. J. Biomed. Nanotechnol. 2009, 5, 121–129. [Google Scholar] [CrossRef]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, B.; Ekdahl, K.N.; Vinogradov, V.V.; Kessler, V.G. Dispersion of TiO2 Nanoparticles Improves Burn Wound Healing and Tissue Regeneration through Specific Interaction with Blood Serum Proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef] [Green Version]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 Composite Membrane Improves Proliferation and Survival of L929 Fibroblast Cells: Application in Wound Dressing and Skin Regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-Printed Chitosan Scaffold Features after Different Post-Printing Gelation Processes. Sci. Rep. 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An In Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Gorham, J.M.; MacCuspie, R.I.; Klein, K.L.; Fairbrother, D.H.; Holbrook, R.D. UV-Induced Photochemical Transformations of Citrate-Capped Silver Nanoparticle Suspensions. J. Nanopart. Res. 2012, 14, 1139. [Google Scholar] [CrossRef]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly Defined 3D Printed Chitosan Scaffolds Featuring Improved Cell Growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Tiwari, A. (Eds.) Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-29032-3. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Bin Ahmad, M.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y. Synthesis of Silver Nanoparticles in Chitosan, Gelatin and Chitosan/Gelatin Bionanocomposites by a Chemical Reducing Agent and Their Characterization. Molecules 2011, 16, 7237–7248. [Google Scholar] [CrossRef] [Green Version]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The Effect of Chitin and Chitosan on the Proliferation of Human Skin Fibroblasts and Keratinocytes In Vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Majima, T.; Funakosi, T.; Iwasaki, N.; Yamane, S.-T.; Harada, K.; Nonaka, S.; Minami, A.; Nishimura, S.-I. Alginate and Chitosan Polyion Complex Hybrid Fibers for Scaffolds in Ligament and Tendon Tissue Engineering. J. Orthop. Sci. 2005, 10, 302–307. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D Printed Chitosan Scaffolds: A New TiO2 Support for the Photocatalytic Degradation of Amoxicillin in Water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Basile, R.; Bergamonti, L.; Fernandez, F.; Graiff, C.; Haghighi, A.; Isca, C.; Lottici, P.P.; Pizzo, B.; Predieri, G. Bio-Inspired Consolidants Derived from Crystalline Nanocellulose for Decayed Wood. Carbohydr. Polym. 2018, 202, 164–171. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Bettini, R.; Elviri, L. 3D Printed Chitosan/Alginate Hydrogels for the Controlled Release of Silver Sulfadiazine in Wound Healing Applications: Design, Characterization and Antimicrobial Activity. Micromachines 2023, 14, 137. [Google Scholar] [CrossRef]
- Godebo, G.; Kibru, G.; Tassew, H. Multidrug-Resistant Bacterial Isolates in Infected Wounds at Jimma University Specialized Hospital, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biemer, J.J. Antimicrobial Susceptibility Testing by the Kirby-Bauer Disc Diffusion Method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar] [PubMed]
- Stanley, B.A.; Neverova, I.; Brown, H.A.; Van Eyk, J.E. Optimizing Protein Solubility for Two-Dimensional Gel Electrophoresis Analysis of Human Myocardium. Proteomics 2003, 3, 815–820. [Google Scholar] [CrossRef] [PubMed]







| SCAFFOLD (Ø 6 mm) | Staphylococcus aureus | Pseudomonas aeruginosa | ||
|---|---|---|---|---|
| Ø Inhibition Diameter (mm) | ||||
| CH 6% w/v | 6 | 6 | 6 | 6 |
| CH 6% w/v + AgNP 10 μg/mL | 7 | 7 | 7 | 7 |
| CH 6% w/v + AgNP 100 μg/mL | 8 | 8 | 8 | 8 |
| CH 6% w/v + AgNP 100 μg/mL + TiO2 1% w/v | 8 | 8 | 6 | 6 |
| CH 6% w/v + TiO2 1% w/v | 6 | 6 | 6 | 6 |
| ALG 6% w/v | 0 | 0 | 0 | 0 |
| ALG 6% w/v + AgNP 100 μg/mL | 6 | 6 | 8 | 8 |
| ALG 6% w/v + AgNP 10 μg/mL | 6 | 6 | 8 | 8 |
| ALG 6% w/v + AgNP 5 μg/mL | 0 | 0 | 6 | 6 |
| ALG 6% w/v + AgNP 1 μg/mL | 0 | 0 | 0 | 0 |
| ALG 6% w/v + AgNP 100 μg/mL + TiO2 1% w/v | 6 | 6 | 6 | 6 |
| ALG 6% w/v + TiO2 1% w/v | 6 | 6 | 6 | 6 |
| Ink | Polysaccharide (w/v) | AgNPs (μg/mL) | TiO2 (w/v) |
|---|---|---|---|
| 1 | Chitosan 6% (ctrl) | - | - |
| 2 | Chitosan 6% | 10 | - |
| 3 | Chitosan 6% | 100 | - |
| 4 | Chitosan 6% | 100 | 1% |
| 5 | Chitosan 6% | - | 1% |
| 6 | Alginate 6% (ctrl) | - | - |
| 7 | Alginate 6% | 1 | - |
| 8 | Alginate 6% | 5 | - |
| 9 | Alginate 6% | 10 | - |
| 10 | Alginate 6% | 100 | - |
| 11 | Alginate 6% | 100 | 1% |
| 12 | Alginate 6% | - | 1% |
| Ink | Polysaccharide (w/v) | AgNPs (μg/mL) | TiO2 (w/v) |
|---|---|---|---|
| 1 | Chitosan 6% (ctrl) | - | - |
| 2 | Chitosan 6% | 10 | - |
| 3 | Chitosan 6% | 100 | - |
| 4 | Chitosan 6% | 100 | 1% |
| 5 | Chitosan 6% | - | 1% |
| 6 | Alginate 6% (ctrl) | - | - |
| 10 | Alginate 6% | 100 | - |
| 11 | Alginate 6% | 100 | 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remaggi, G.; Bergamonti, L.; Graiff, C.; Ossiprandi, M.C.; Elviri, L. Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing. Antibiotics 2023, 12, 1104. https://doi.org/10.3390/antibiotics12071104
Remaggi G, Bergamonti L, Graiff C, Ossiprandi MC, Elviri L. Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing. Antibiotics. 2023; 12(7):1104. https://doi.org/10.3390/antibiotics12071104
Chicago/Turabian StyleRemaggi, Giulia, Laura Bergamonti, Claudia Graiff, Maria Cristina Ossiprandi, and Lisa Elviri. 2023. "Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing" Antibiotics 12, no. 7: 1104. https://doi.org/10.3390/antibiotics12071104
APA StyleRemaggi, G., Bergamonti, L., Graiff, C., Ossiprandi, M. C., & Elviri, L. (2023). Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing. Antibiotics, 12(7), 1104. https://doi.org/10.3390/antibiotics12071104








